Research on the development of Value-added Vinegar using Subcritical Treated Water
Article Information
Shoji Hirayama1,2, Daigo Murakami1, Yuri Fukagusa3, Yuriko Hoshino2, Kazuharu Yamato4, Munehiro Hoshino4, Mitsuru Sasaki5,6,7*
1Graduate School of Science and Technology (GSST), Kumamoto University, 2-39-1 Kurokami, Chuo-ku, Kumamoto 860-8555, Japan
2M&A Food Technology and Biology of Technical Center (M.A.F.T), 2400-1 Tabara, Kawasaki-Machi, Tagawa-Gun, Fukuoka 827-0004, Japan
3Department of Materials Science and Applied Chemistry, Faculty of Engineering, Kumamoto University, 2-39-1 Kurokami, Chuo-ku, Kumamoto 860-8555, Japan
4Maruboshi vinegar, 2425 Tabara, Kawasaki-Machi, Tagawa-Gun, Fukuoka 827-0004, Japan
5Institute of Industrial Nanomaterials (IINa), Kumamoto University,2-39-1 Kurokami, Chuo-ku, Kumamoto 860-8555, Japan
6Faculty of Advanced Science and Technology (FAST), Kumamoto University, 2-39-1 Kurokami, Chuo-ku, Kumamoto 860-8555, Japan
7International Research Organization for Advanced Science and Technology (IROAST), Kumamoto University, 2-39-1 Kurokami, Chuo-ku, Kumamoto 860-8555, Japan
*Corresponding Author: Mitsuru Sasaki, Institute of Industrial Nanomaterials (IINa), Kumamoto University, Faculty of Advanced Science and Technology (FAST), Kumamoto
University, and International Research Organization for Advanced Science and Technology (IROAST), Kumamoto University, 2-39-1 Kurokami, Chuo-ku, Kumamoto 860-8555, Japan
Received: 21 July 2023; Accepted: 31 July 2023; Published: 06 September 2023
Citation: Shoji Hirayama, Daigo Murakami, Yuri Fukagusa, Yuriko Hoshino, Kazuharu Yamato, Munehiro Hoshino, Mitsuru Sasaki. Research on the development of Value-added Vinegar using Subcritical Treated Water. Journal of Food Science and Nutrition Research. 6 (2023): 111-116.
View / Download Pdf Share at FacebookAbstract
Sake lees and rice bran, which are residues from food processing, were liquefied by subcritical water treatment to effectively produce new vinegar. Our previous studies reported that high concentrations of amino acids can be recovered from sake lees treated with subcritical water at 120°C for 240 min [1,2]. In this study, the conditions for subcritical water treatment of rice bran were examined, and a liquefied product with a high liquefaction rate and high mineral content was obtained at 180°C for 30 min. Acetic acid fermentation was then conducted with a ratio of sake lees liquefied product to rice bran liquefied product of 1:1. The resulting vinegar had a high nutritional value similar to commercially available black vinegar.
Keywords
Biomass, Green chemistry, Subcritical water extraction, Vinegar
Biomass articles; Green chemistry articles; Subcritical water extraction articles; Vinegar articles
Biomass articles Biomass Research articles Biomass review articles Biomass PubMed articles Biomass PubMed Central articles Biomass 2023 articles Biomass 2024 articles Biomass Scopus articles Biomass impact factor journals Biomass Scopus journals Biomass PubMed journals Biomass medical journals Biomass free journals Biomass best journals Biomass top journals Biomass free medical journals Biomass famous journals Biomass Google Scholar indexed journals Green chemistry articles Green chemistry Research articles Green chemistry review articles Green chemistry PubMed articles Green chemistry PubMed Central articles Green chemistry 2023 articles Green chemistry 2024 articles Green chemistry Scopus articles Green chemistry impact factor journals Green chemistry Scopus journals Green chemistry PubMed journals Green chemistry medical journals Green chemistry free journals Green chemistry best journals Green chemistry top journals Green chemistry free medical journals Green chemistry famous journals Green chemistry Google Scholar indexed journals Subcritical water extraction articles Subcritical water extraction Research articles Subcritical water extraction review articles Subcritical water extraction PubMed articles Subcritical water extraction PubMed Central articles Subcritical water extraction 2023 articles Subcritical water extraction 2024 articles Subcritical water extraction Scopus articles Subcritical water extraction impact factor journals Subcritical water extraction Scopus journals Subcritical water extraction PubMed journals Subcritical water extraction medical journals Subcritical water extraction free journals Subcritical water extraction best journals Subcritical water extraction top journals Subcritical water extraction free medical journals Subcritical water extraction famous journals Subcritical water extraction Google Scholar indexed journals Vinegar articles Vinegar Research articles Vinegar review articles Vinegar PubMed articles Vinegar PubMed Central articles Vinegar 2023 articles Vinegar 2024 articles Vinegar Scopus articles Vinegar impact factor journals Vinegar Scopus journals Vinegar PubMed journals Vinegar medical journals Vinegar free journals Vinegar best journals Vinegar top journals Vinegar free medical journals Vinegar famous journals Vinegar Google Scholar indexed journals subcritical water treatment articles subcritical water treatment Research articles subcritical water treatment review articles subcritical water treatment PubMed articles subcritical water treatment PubMed Central articles subcritical water treatment 2023 articles subcritical water treatment 2024 articles subcritical water treatment Scopus articles subcritical water treatment impact factor journals subcritical water treatment Scopus journals subcritical water treatment PubMed journals subcritical water treatment medical journals subcritical water treatment free journals subcritical water treatment best journals subcritical water treatment top journals subcritical water treatment free medical journals subcritical water treatment famous journals subcritical water treatment Google Scholar indexed journals carbohydrates articles carbohydrates Research articles carbohydrates review articles carbohydrates PubMed articles carbohydrates PubMed Central articles carbohydrates 2023 articles carbohydrates 2024 articles carbohydrates Scopus articles carbohydrates impact factor journals carbohydrates Scopus journals carbohydrates PubMed journals carbohydrates medical journals carbohydrates free journals carbohydrates best journals carbohydrates top journals carbohydrates free medical journals carbohydrates famous journals carbohydrates Google Scholar indexed journals rice bran articles rice bran Research articles rice bran review articles rice bran PubMed articles rice bran PubMed Central articles rice bran 2023 articles rice bran 2024 articles rice bran Scopus articles rice bran impact factor journals rice bran Scopus journals rice bran PubMed journals rice bran medical journals rice bran free journals rice bran best journals rice bran top journals rice bran free medical journals rice bran famous journals rice bran Google Scholar indexed journals amino acids articles amino acids Research articles amino acids review articles amino acids PubMed articles amino acids PubMed Central articles amino acids 2023 articles amino acids 2024 articles amino acids Scopus articles amino acids impact factor journals amino acids Scopus journals amino acids PubMed journals amino acids medical journals amino acids free journals amino acids best journals amino acids top journals amino acids free medical journals amino acids famous journals amino acids Google Scholar indexed journals monosaccharides articles monosaccharides Research articles monosaccharides review articles monosaccharides PubMed articles monosaccharides PubMed Central articles monosaccharides 2023 articles monosaccharides 2024 articles monosaccharides Scopus articles monosaccharides impact factor journals monosaccharides Scopus journals monosaccharides PubMed journals monosaccharides medical journals monosaccharides free journals monosaccharides best journals monosaccharides top journals monosaccharides free medical journals monosaccharides famous journals monosaccharides Google Scholar indexed journals
Article Details
1. Introduction
Rice bran and sake lees, which are residues of the vinegar production process, contain large amounts of proteins and minerals. However, they are discarded in large quantities every year, thereby incurring significant costs. Against this background, previous studies have shown that these food production residues contain useful components [3-6]. Therefore, the authors focused on rice bran and sake lees, and the application of subcritical water treatment for their liquefaction to produce a product rich in amino acids and minerals, which can be used to produce new vinegar. The subcritical water used in this study refers to water in a liquid state at high temperature and high pressure, and it has the characteristics of being non-toxic, non-flammable, and non-explosive. In particular, the ionic product constant of subcritical water is high and acts like an acid or alkali catalyst, making it applicable to the hydrolysis of proteins in biomass, as reported in previous studies [7-9]. Sake lees, which is the raw material that is the focus of this study, is characterized by high protein and water contents, while rice bran is high in carbohydrates and minerals. In addition, previous studies have reported that high concentrations of amino acids can be recovered from sake lees by processing at relatively low temperatures of 120-140°C [1,2]. However, previous studies on subcritical water treatment of rice bran are limited; thus, this study focused on subcritical water treatment of rice bran. As a result, the authors conducted solid-liquid separation of the treated material after subcritical water treatment in the range of 120-160°C; however, successful separation was not achieved. It is possible that the polysaccharides were not hydrolyzed sufficiently and could not be separated by filtration. This indicates that rice bran may require treatment at a higher temperature because of its low water content and robust carbohydrate structure.
2. Experimental
2.1 Materials and Standard reagents
2.1.1 Materials: Rice bran and sake lees provided by Maruboshi Vinegar Co. (Fukuoka, Japan) were used as raw materials, as shown in figure 1.

Figure 1: Raw materials used in this study
2.1.2 Standard reagents: Amino acid mixture standard solution (Type H). Calibration result is traceable to Primary Measurement Standards (National Standards) based on Measurement Law. Ammonium nitrogen standard solution (for water analysis). These were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Standard solutions for calcium, magnesium, potassium, and sodium were purchased from FUJIFILM Wako Pure Chemical Corporation. Calibration result is traceable to Primary Measurement Standards (National Standards) based on Measurement Law.
2.2 Experimental apparatus and procedure
The experimental equipment and methods are published in our previous paper [1,2]. An autoclave was to generate high temperature and high pressure (shown in figure 2). The reactor was constructed from SS 316 steel and has an internal volume of 500mL. The reactor was charged with 45 g of raw material and 300 mL of distilled water, mixed with a stirrer, and then sealed. Thereafter, the temperature was raised to a predetermined setpoint (180-220°C) by a band heater installed in the reactor. The heating time was 15 to 30 min. After reaching the predetermined temperature, the contents were reacted for 30-240 min while stirring at 300 rpm. The pressure in the reactor varied from 1.3 to 2.6 MPa depending on the vapor pressure of water and the product gas evolved during processing. After the subcritical water treatment, the band heater was removed from the reactor and a fan was used to quickly quench the reactor. After the reaction solution was sufficiently cooled (hereinafter this solution will be referred to as a sub-critical water treatment solution) was collected and separated into filtrate and water-insoluble components by suction filtration.
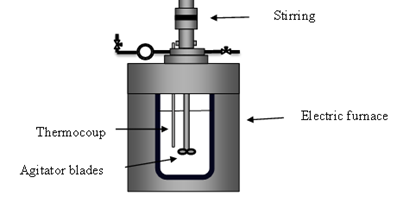
Figure 2: Diagram of the batch reactor used in subcritical treatment
2.3 Analysis
The following sections describe how each obtained filtration residue and aqueous solution was analyzed, and the methods used to quantitatively analyze the concentrations of amino acids, nitrogen, phosphorus, and minerals contained in the subcritical water treatment solution.
2.3.1 Amino acids: Amino acids were derivatized with 3-mercaptopropionic acid [MPA] and o-phthalaldehyde [OPA] and separated by a column [2.6 µ Kinetex EVO C18 100×3 mm; SHIMADZU GLC (Tokyo, Japan)] for ultra high-speed analysis, then analyzed by high performance liquid chromatography (HPLC) with a fluorescence detector (NEXERA X 2; SHIMADZU (Kyoto, Japan). Eighteen amino acids were isolated, as shown in table 1.
Table 1: Amino acids quantitatively analyzed in this study.
|
1. Aspartic acid |
2. Glutamic acid |
3. Serine |
4. Histidine |
|
5. Glycine |
6. Threonine |
7. Arginine |
8. Alanine |
|
9. GABA |
10. Tyrosine |
11. Valine |
12. Methionine |
|
13. Cystine |
14. Tryptophan |
15. Phenylalanine |
16. Isoleucine |
|
17. Leucine |
18. Lysine |
*GABA: 4-aminobutyric acid
Total amino acid content (mg/L) = Total of 18 amino acids (mg/L)
2.3.2 Total nitrogen concentration: Total nitrogen (mg/L) was analyzed using the Kjeldahl method [10].
2.3.3 Ammonia nitrogen concentration: Ammonia nitrogen (mg/L) was analyzed using the indophenol method [11].
2.3.4 Phosphorus concentration: Phosphorus (mg/L) was analyzed using the molybdenum-blue method [12].
2.3.5 Mineral concentration: Potassium, calcium, and magnesium (mg/L) were quantitatively determined by atomic absorption spectrometry (AA-7000; SHIMADZU).
2.3.6 Liquefaction rate (%): The liquefaction rate was calculated according to the following formula:
Liquefaction rate [%] = ((Preparation weight of raw material [g] × (100 - moisture content) [%] - Dry weight of residue [g]) / (Preparation weight of raw material [g] × (100 - moisture content) [%]) × 100
2.3.7 Total organic carbon (TOC): TOC was determined using a TOC meter [TOC-V SCH; SHIMADZU (Kyoto Japan).]. The method is based on a 50-fold dilution of the liquefied material (1 mL of liquefied material in a measuring flask is metered up to 50 mL with distilled water).
2.3.8 Protein concentration (g/100 mL): Total nitrogen (mg/L) was analyzed using the Kjeldahl method. The nitrogen conversion factor was 6.25.
2.3.9 Lipid concentration (g/100 mL): Lipid concentration was analyzed by the liquid-liquid extraction method [13].
2.3.10 Carbohydrate concentration (g/100 mL) Calculation Formula: Total mass = Moisture - Protein - Lipid - Ash
2.3.11 Ash concentration (g/100 mL): Ash concentration was determined using the direct ashing method [14].
2.3.12 Acidity concentration (g/100 mL): Acidity concentration was determined using the titration method [15].
2.3.13 Caloric value (kcal/100 mL): Caloric value was determined using the modified Atwater method [16].
3. Results and Discussion
3.1 Subcritical water liquefaction of rice bran
For rice bran, we aimed to elute high concentrations of various components by treatment at temperatures higher than 180°C. The liquefaction rate and pH results are shown in table 2. The highest liquefaction rate was achieved by treatment at 180°C. The liquefaction rate decreased as the treatment temperature increased. The reason for the decrease in liquefaction rate at 200 and 220°C, at which the treatment capacity was originally higher, can be attributed to the carbonization of cellulose, which is abundantly contained in rice bran. The pH also decreased at higher temperatures. The decomposition of the abundant carbohydrates in rice bran, from monosaccharides to organic acids, is thought to have caused the decrease in pH.
Table 2: Liquefaction rate and pH of subcritical water liquefied rice bran
|
Raw materials |
Liquefaction rate [%] |
pH |
||||
|
180°C |
200°C |
220°C |
180°C |
200°C |
220°C |
|
|
30 g |
68.2 |
66.74 |
55.74 |
4.78 |
3.57 |
3.53 |
|
45 g |
69.44 |
64.19 |
54.67 |
4.62 |
3.41 |
3.63 |
|
60 g |
67.17 |
56.8 |
48.58 |
4.34 |
3.26 |
3.74 |
Figure 3 shows the carbon content in the solid residue and subcritical water liquefied product at 200 and 220°C. The organic carbon concentration in the aqueous phase decreased with increasing temperature. On the other hand, the carbon concentration in the solid residue increased in a temperature-dependent manner. From these two results, it can be inferred that the carbohydrates in rice bran, especially amorphous polysaccharides and cellulose in particular, underwent hydrolysis to saccharification and further aromatization (formation of furan compounds) during the treatment process, and became insoluble due to recombination (Maillard reaction and cross-linking) in the reactor [17-20]. In the future, we will conduct quantitative analysis of sugars, organic acids, and furan compounds to elucidate the reaction pathway.
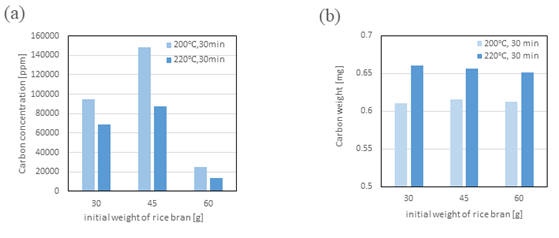
Figure 3: Carbon contents in the solid residue and subcritical water fractions
(a) Carbon concentration of liquid fraction
(b) Carbon weight of insoluble fraction
Next, the results of amino acid analysis per unit mass are shown in figure 4. Although no significant differences were observed, slightly more efficient results were achieved at lower raw material weights.
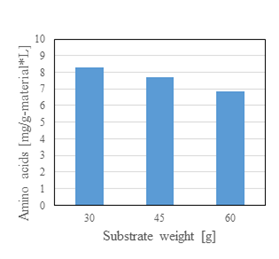
Figure 4: Results of amino acid analysis per unit mass
Next, figure 5 shows the results of phosphorus and mineral components per unit mass. Here as well, no significant differences were observed, and slightly more efficient results were achieved at lower substrate weights.
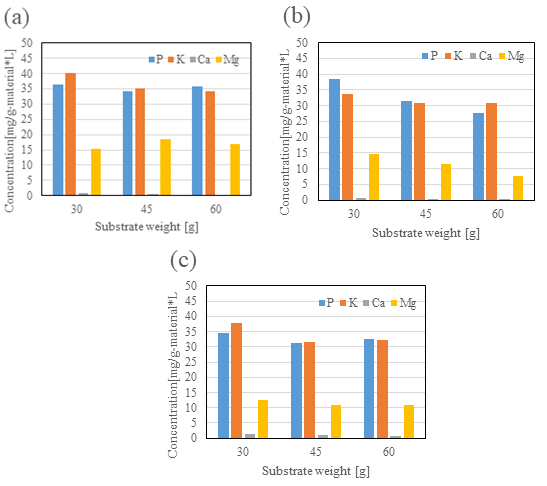
Figure 5: Phosphorus and mineral components per unit mass. (a) 180°C, 30min (b) 200°C, 30min (c) 220°C, 30min
From these results, it was concluded that treatment at 180°C was the most suitable from the standpoint of processing efficiency; the water solubilization rate decreased under temperature conditions of 200°C or higher. The highest elution amount per unit mass was obtained at 30 g substrate weight. However, “45 g substrate weight” was selected as the optimal condition, as this condition ensured the elution of phosphorus and minerals comparable to the target “black vinegar” and had a relatively high elution efficiency.
3.2 Acetic acid fermentation of the subcritical water treated solution
In previous studies [1,2], the authors performed subcritical water treatment of sake lees (temperature 120°C, treatment time 240 min) and acetic acid fermentation of the liquefied product. The results showed a greater concentration of total amino acids compared to commercial black vinegar, yet the amounts of phosphorus and mineral components were inferior. In this experiment, we aimed to produce vinegar containing high concentrations of phosphorus and mineral components as well as amino acids by using rice bran as a source of phosphorus and mineral components. Specifically, subcritical water-treated sake lees and rice bran under their respective optimal conditions (sake lees: 120°C, 240 min; rice bran: 180°C, 30 min) were mixed 1:1 on a volume basis, and vinegar was produced by static fermentation for 3 days (hereafter referred to as mixed vinegar). Various analyses were then performed and the results are shown in table 3. The acidity of the mixed vinegar was 4.68%, which cleared the acidity specified in the JAS standard, and vinegar was successfully produced, albeit at the laboratory level.
Table 3: Nutritional analysis of the prepared vinegar (per 100mL)
|
Energy (kcal) |
Protein (g) |
Lipids (g) |
Carbohydrate (g) |
Mineral content (g) |
Acidity (g) |
|
|
Mixed sample (1:1) |
34 |
1.4 |
0 |
8.3 |
0.4 |
4.68 |
|
Sake lees 120°C,240min |
25 |
1.7 |
0 |
5.7 |
0.1 |
4.39 |
|
Grain vinegar |
26 |
0.4 |
0 |
7.1 |
0.1 |
4.26 |
Other analyses were also performed on this mixed vinegar in comparison to commercial products and sake lees vinegar (Figure 6). The amino acid content of the mixed vinegar was higher than that of the commercial products, which is a positive result. The mineral content was significantly higher compared to sake lees vinegar, but was not as high as black vinegar. Through a series of acetic acid fermentation processes, it was confirmed that the subcritical water-treated products made from sake lees and rice bran did not interfere with each other even when mixed, and each of them retained high concentrations of nutrients.
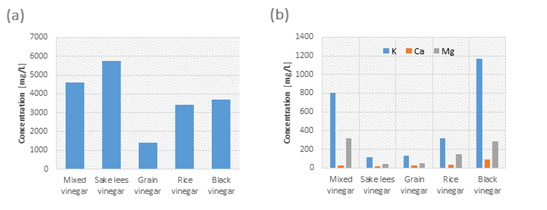
Figure 6: Comparison of the components of various vinegars. (a) Comparison of contained amino acids (b) Comparison of contained minerals
4. Conclusions
The purpose of this study was to liquefy sake lees and rice bran by subcritical water treatment to produce a liquefied product rich in amino acids and minerals, and apply the liquefied product to the production of a new vinegar. In their previous studies[1,2], the authors found that subcritical water treatment of sake lees at 120°C for 240 min produced a liquefied product rich in amino acids. In the present study, subcritical treatment of rice bran at 180°C for 30 min produced a liquefied product rich in mineral components. The subcritical water liquefied rice bran was mixed with the subcritical water liquefied sake lees at a ratio of 1:1, and acetic acid fermentation was carried out. As a result, a vinegar with high nutritional value, similar to commercially-available black vinegar, was produced. This experiment also suggested that the nutrient content of the vinegar could be controlled by changing the mixing ratio of sake lees and rice bran subcritical water-treated samples. We aim to conduct future experiments with different mixing ratios.
Acknowledgments
This research was carried out with the support of Graduate School of Science and Technology (GSST), Kumamoto University and M&A Food Technology and Biology of Technical Center (M.A.F.T).
Conflict of interests
The authors declare that they have no conflict of interests.
References
- Yamato K, Minami K, Hirayama S, et al. Recovery and liquefaction of nitrogen-containing component and minerals from food processing wastes of vinegar using subcritical water. SN Applied Science 2 (2020): 2081.
- Yamato K, Murakami D, Hirayama S, et al. Food-Grade Vinegar Production from the Extract of Sake Lees Obtained by Subcritical Water Treatment, Journal of Food and Nutrition 7 (2021): 1-12.
- Abaide ER, Tres MV, Zabot GL, et al. Reasons for processing of rice coproducts: reality and expectations. Biomass Bioenergy 120 (2019): 240-256.
- Abaide ER, Mortari SR, Ugalde G, et al. Subcritical water hydrolysis of rice straw in a semi-continuous mode. J Clean Prod 209 (2019): 386-397.
- Garrote G, Falque E, Domínguez H, et al. Autohydrolysis of agricultural residues. study of reaction byproducts. Bioresour Technol 98 (2007): 1951-1957.
- Ministry of Economy, Tread and Industry Agency for Natural Resources and Energy: Annual Report on Energy (2007).
- Mochidzuki K, Kobayashi S, Wang H, et al. Effect of Rice Bran as a Nitrogen and Carbohydrate Source on Fed-Batch Simultaneous Saccharification and Fermentation for the Production of Bioethanol from Rice Straw. Journal of the Japan Institute of Energy 94 (2015): 151-158.
- Nojiro K, Kozaki M, Yoshii H, et al. Revision Zymurgy. Kodansha 11 (2001): 74-75.
- Qian L, Wang S, Savage PE. Hydrothermal liquefaction of sewage sludge under isothermal and fast conditions. Bioresour Technol 232 (2017): 27-34.
- Marshall W, Franck E. Ion product of water substance, 0-1000°C, 1-10000 bars - new international formulation and its Background. J Phys Chem 10 (1981): 295-304.
- Arakawa Y, Akagi II, Yamamoto K. Determination of ammonium nitrogen in KCl extracts of cropland soils by using 2-hydroxybiphenyl sodium salt. J Soc Soil Sci Plant Nut 75 (2003): 657-659.
- Ran Y, Wang YZ., Liao Q, et al. Effects of operation conditions on enzymatic hydrolysis of high-solid rice straw, International Journal of Hydrogen Energy 37 (2012): 13660-13666.
- Liu FZ, Yamada I, Mori H, et al. A Method of Calculating Multistage Liquid-Liquid Extraction for Ternary Systems. Journal of Chemical Engineering of Japan 24 (1991): 261-264.
- Kubo A. Degradation Methods of Biological Samples for Inorganic Analysis - Wet Oxidation and Focusing on dry incineration. Bunseki Kagaku 11 (1962): 964-871?
- Japanese Industrial Standards. Sodium carbonate (2019).
- Report of the Subdivision on Resources the Council for Science and technology Ministry of Education, Culture, Sports, Science and Technology, Japan. Standard tables of food composition in Japan (Eighth Revised Edition) (2023).
- Wakita Y, Harada O, Kuwata M, et al. Sub-and Supercritical Water Treatment of Sake (Japanese Rice Wine) Cake and Physiological Functions of Soluble Components. J Brew Soc Japan 100 (2005): 49-55.
- Pramote K, Kumutakan W, Shuji A. Carbohydrate content and composition of product from subcritical water treatment of coconut meal. Journal of Industrial and Engineering Chemistry 18 (2012): 225-229.
- Hee-Jeong Lee, Periaswamy SS, Yong-NamCho, et al. Extraction of bioactive compounds from oyster (Crassostrea gigas) by pressurized hot water extraction. The Journal of Supercritical Fluids 141 (2018) 120-127.
- Yu-Rin J, Jin-Seok P, David N, et al. Valorization of blue mussel for the recovery of free amino acids rich products by subcritical water hydrolysis The Journal of Supercritical Fluids 169 (2020): 105-135.
