Inhalation of Microaerosolized Hypochlorous Acid (HOCl): Biochemical, Antimicrobial, and Pathological Assessment
Article Information
Eric D Rasmussen1*, Lori I Robins2, Jeremy Stone R1, Jeffrey F Williams1
1Briotech Inc., Woodinville, USA
2Department of Physical Sciences, University of Washington Bothell, WA, USA
*Corresponding author: Eric Rasmussen, Briotech Inc., 14120 NE 200th St, Woodinville, WA 98072, USA
Received: 17 June 2022; Accepted: 28 June 2022; Published: 08 July 2022
Citation: Eric D Rasmussen, Lori I Robins, Jeremy Stone R, Jeffrey F Williams. Inhalation of Microaerosolized Hypochlorous Acid (HOCl): Biochemical, Antimicrobial, and Pathological Assessment. Archives of Internal Medicine Research 5 (2022): 311-318.
View / Download Pdf Share at FacebookAbstract
Evidence is emerging of the beneficial effects of inhaling microaerosolized hypochlorous acid (HOCl) as an intervention in the prevention and treatment of respiratory virus infections, including SARS CoV-2. However, little information is available about the safety and efficacy of exogenous HOCl solutions in laboratory cellular evaluations or experimental animals. In this report, we establish through independent laboratories that the SARS-CoV-2 virus is rapidly inactivated by exposure to HOCl, as is poliovirus – a far more difficult virus to inactivate and often the gold standard for virucidal assessment. Inhalation of a microaerosolized fog of that same virucidal HOCl solution by rodents using the US EPA’s acute 4-hour inhalation toxicity protocol later provided observational, physiological, gross pathological, and histopathological evidence that showed pulmonary exposure of 52ppm HOCl to respiratory epithelium did not result in any difference when compared to control animals. Also reported are the chemical and antimicrobial characterizations of the HOCl microaerosols reportedly used as SARS-CoV-2 prophylaxis within the United States and elsewhere during the pandemic lockdown. Results are discussed in relation to (1) additional peer-reviewed articles describing HOCl inhalational preparations and both their documented pulmonary safety and their efficacy against respiratory pathogens, and (2) the potential for rational intervention in infections arising from respiratory pathogens using inhalation of a microaerosolized fog as a safe, effective, inexpensive, and broadly accessible method for the administration of HOCl.
Keywords
<p>Hypochlorous, HOCl, Innate Immune System, Antiseptic, Lower Respiratory Tract, Inhalation, Virucidal Activity, Bactericidal Activity, SARS-Cov-2, Volunteers, Pandemic, Lockdown, Chemical Characterization</p>
Hypochlorous articles; HOCl articles; Innate Immune System articles; Antiseptic articles; Lower Respiratory Tract articles; Inhalation articles; Virucidal Activity articles; Bactericidal Activity articles; SARS-Cov-2 articles; Volunteers articles; Pandemic articles; Lockdown articles; Chemical Characterization articles
Article Details
1. Introduction
Amid the sudden onset of the COVID-19 pandemic and early uncertainties about the mode of transmission, regulatory authorities globally were pressed to make recommendations about environmental disinfection measures. They searched for biocidal formulations already registered and approved that might inactivate coronaviruses and many were found. The global scale and pervasive protocols rapidly reached unprecedented levels. Janitorial staff, healthcare professionals, and members of the public have now been, sometimes involuntarily, exposed to aerosolized biocides at concentrations and dispensing frequencies far beyond conventional use patterns [1, 2]. . . In keeping with that expansion, hypochlorous acid (HOCl), a highly effective virucide [3] was entered into the EPA’s List N for use in disinfection against the pandemic coronavirus. That inclusion led to significantly increased human exposures to aerosolized HOCl. There were no reports in the literature about such HOCl inhalation exposure, and that gap led to this research.
We describe here the results of such exposures in laboratory rats and rabbits, and by others reported to us during the pandemic lockdown. Based on those reports from other cities we reproduced the described fogging systems within our laboratory for physical, biochemical, and antimicrobial characterization. We then contracted for a national GLP lab to generate a similar fog with the same HOCl solution for an EPA toxicity study on rats, and later an ocular study on rabbits. For each, we used the same well-characterized and homogenous HOCl preparations proven to inactivate SARS-CoV-2 viruses rapidly and to high levels in vitro. We note the relevance of the appeal from the International Society for Aerosols in Medicine that advocated for the increased use of the inhalational route of administration for prophylactic and therapeutic interventions against SARS-CoV-2, its variants, and other infectious respiratory diseases [4].
2. Materials and Methods
The physical, chemical, microbiological, and animal safety characteristics of the fogging system described to us (HOCl solution, tubing, nozzle, and tent) were determined using methods detailed in the following test systems.
2.1 HOCl preparation and characteristics/titration of FAC method
Reagents for the iodometric titrations were purchased from Hach (dissolved oxygen powder pillows, potassium iodide powder pillows, sodium thiosulfate digital titrator cartridge, and starch indicator solution). Samples were titrated following our previous procedure [5]. Briefly, iodometric titrations using sodium thiosulfate (0.113 N) were completed using HACH reagent kits for total (active and free) chlorine (Hach Company, Loveland, CO) following HACH method 8,209. The pH was measured using an Accumet pH meter. The oxidation reduction potential (ORP) measurements were made using an ORP sensor (ORP-BTA) and analyzed using the LabQuest®2 (LABQ2) apparatus from Vernier software and technology.
2.2 SARS-CoV-2 inactivation efficacy of the HOCl preparation
A test for efficacy of the pure HOCl solution versus mature infectious SARS-CoV-2 coronavirus was performed under Good Laboratory Practice (GLP) protocol at Bioscience Laboratories, 1755 South 19th Ave, Bozeman, Montana. Stocks of an isolate of SARS-CoV-2 (strain USA-WA1/2020, BEI Resources, #NR52281) were propagated in Vero E6 cells (ATCC #CRL-1586) with 5% heat-inactivated fetal bovine serum. Briotech HOCl with 180 ppm of titratable Cl was used to inactivate samples of virus suspensions exposed for 120 seconds, compared to virus recoveries from aliquots exposed to culture medium alone or to 45% ethanol as a positive control. Exposures were conducted at a temperature of 22oC and at a relative humidity of 24.7%-25.7%.
2.3 Endotoxin content determination of the HOCl preparation
The endotoxin content of triplicate samples of the HOCl used for aerosolization was determined using the Limulus Amebocyte Lysate assay at Nelson Laboratories, Fairfield, NJ. The test was conducted in accordance with GLP standards on an HOCl solution containing 170 ppm FAC. The procedure complied with current USP method <85> using the LAL Kinetic Chromogenic Inhibition/Enhancement test and a standard curve ranging from 0.005 EU/mL to 50 EU/mL.
2.4 Acute inhalation exposure of rodents to HOCl
This test was conducted in accordance with published guidelines (US EPA OSCPP 870.1300) and GLP standards by Stillmeadow Incorporated, Sugarland, TX 77478, and in compliance with the Animal Welfare Act. Two groups of 5 male and two groups of 5 female adult Sprague Dawley rats were exposed to an aerosol generated from a solution of HOCl containing 176 ppm of free available chlorine (Cl), at a rate of 2 mg/L of air flowing at 218 L/min, equal to 26 changes per hour. Aerosols were created using a Spraying Systems Air Atomizer. The particle size distribution and Mass Median Aerodynamic Diameter (MMAD) of the droplets were determined using a Sierra Cascade Impactor Model 21-B. A third group of 10 adult rats (5 males, 5 females) was exposed to aerosolized isotonic saline solution for the same period. Observations on the appearance, behavior, food intake, and body weight were made over the following 14 days before necropsy, and the lungs and other internal organs were then examined for any grossly visible changes. Five male rats and 5 female rats were sacrificed serially at intervals after the HOCl exposure, beginning at 6 hours and subsequently at 1, 3, 4, and 14 days. The complete pulmonary systems, including head, trachea, and lungs, were removed, then fixed in 10% buffered formalin for histological section preparation and for staining with hematoxylin and eosin for microscopic assessment of any pathological changes.
2.5 Acute ocular exposure of rabbits to HOCl
This test was conducted in accordance with published guidelines (US EPA OSCPP 870.2400) and GLP standards by Stillmeadow Incorporated, Sugarland, TX 77478, and in compliance with the Animal Welfare Act. Undiluted 180ppm HOCl solution in 0.9% saline (0.1mL) was placed into the conjunctival sac of nine albino rabbits and the animals were observed for any signs of irritation or alterations in the cornea or conjunctiva at 1 hour, 4 hours, 24 hours, and 72 hours post-exposure.
2.6 Remote human exposure to HOCl microaerosols
A self-selected cohort, that had been classed as “essential workers” and were still in their aircraft metalworking factory in remote city, chose to be exposed to aerosolized HOCl in an enclosed single-person tent for periods averaging 2 minutes prior to entering their workplace. They used emails and sketches to describe their system to the authors and that system was replicated within our laboratory.
2.7 The tent
The tent those employees used, a beach cabana, was shaped as a trapezoidal prism, square at the base, 120 cm on a side, and 18 cm per side at the top (plus a vented screen), 200 cm tall, and with a volume of 1.7 m3. A commercial 60 psi compressor fed air to a container of ~180 ppm HOCl suspended in saline (0.9% sodium chloride, NaCl). That pressurized HOCl was then expelled from a downward-facing reflective-disc steel fogging nozzle suspended from the peak of the tent. That system generated a substantial volume of pressurized droplets, 0.5-20 microns in size, filling the tent within 15 seconds with a billowing fog dense enough to obscure a conventional smartphone screen at 6 inches (15 cm). Figure 1 below shows the tent fogging system as replicated in the author’s laboratory, and Figure 2 shows the characteristics of the fog after measuring the droplet concentration.
2.8 The employees
A subset of the employees at the shop who chose to enter the tent for a 2-minute exposure also agreed to anonymously describe their impressions to the authors. The nature of the pandemic lockdown, and the constraints of a survey protocol that had no IRB and for which we were not willing to violate Helsinki or COPES standards, limited the information that could be collected. The authors have no personally identifiable information about the employees and have never met or directly talked with them.
2.9 Composition of the HOCl fog at inhalational distances
The machine shop fogging tent was replicated in a laboratory. The composition of the HOCl fog inhaled was studied at milestone distances, and the results can be found in Table 1. With an average respiratory rate of 12-16 breaths/minute and an average tidal volume of roughly 500 ml (7 ml/kg), the minute ventilation for each tent exposure is near 7 liters/minute, averaging 14 liters of respiratory exchange for the 2 minutes in the tent. The fog can be assumed to achieve 100% humidity and was found to contain 52 ppm (0.0052%) HOCl at 75 cm from the nozzle (approximate nose height).
With 14 liters inhaled over 2 minutes and 0.0052% of that calculated to be HOCl, the HOCl volume delivered to airways would be a total of about 0.72 ml of pure HOCl, or about 0.36 ml per minute in the tent, inhaled as droplets of between 0.5 microns and 20 microns. Very small droplets of less than 1 micron are readily carried to the most distant alveoli, and droplets larger than 10 microns can be captured by vibrissae located on epithelia in the anterior vestibule of the nose, so droplets spanning this fogging range can be deposited anywhere in the respiratory tree.
2.10 Determination of HOCl concentration in condensed moisture in a tent identical to that in which remote employees chose to be exposed.
Samples of the HOCl fog were collected for 5 minutes and the concentration of HOCl was measured following the iodometric titration, pH, and ORP measurements previously described.
2.11 Antimicrobial effects of the aerosols of HOCl generated in the replication tent
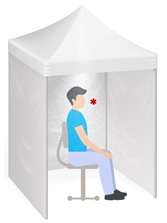
The antimicrobial properties of the aerosolized HOCl in the tent were determined using a carrier test. Bacteria-loaded glass coupons were placed on a flat shelf at the level of the nose of a seated person, 75cm from the fogging nozzle, for the 2-minute minimum time described for each tent exposure. The red asterisk represents the site of the glass coupon sampling (Figure 3) and no humans were exposed in this laboratory evaluation. Cultures of E. coli and Staphylococcus aureus were grown up overnight for use as challenge organisms, and 25 µl of appropriate dilutions of the bacterial suspensions were dried down on 25x50 mm coverslips. To determine loading outcomes, samples of dried coupons were subjected to 0.05N sodium thiosulphate quenching before vortexing with sterile glass beads, and the resulting eluted suspensions were evaluated using the plate count method and serial dilution into TSA medium. Test coupons in triplicate for each bacterial species and contact time were removed from the shelf at the end of the 2-minute exposure. Quenching was used to determine the antibacterial efficacy at this 2-minute time point, while other coupons were allowed additional contact times of either 5 minutes or 10 minutes post-removal before being processed similarly for plate count determinations. The experimental procedure was completed twice.
|
Distance (cm) |
HOCl (ppm) |
|
0 (nozzle) |
112 |
|
18 |
77 |
|
38 |
66 |
|
75* (inhalation) |
52 |
Table 1: Characterization of aerosolized HOCl at various distances from the nozzle.
Figure 3: Graphic representation of the reported exposure of essential workers to aerosolized HOCl in a confined space of approximately 1.7m3. The front of the tent was zipped closed for the duration of the exposure. For the replication, a microaerosolized HOCl fog was introduced at the apex of the tent, and droplet samples were collected at the point indicated by the asterisk for analysis of the active Cl content at the estimated point of inhalation. Coupons bearing bacteria were also introduced at this point to determine the antimicrobial efficacy of HOCl delivered at the site of inhalation.
3. Results
3.1 Efficacy of pure HOCl solutions versus SARS-CoV-2 virus
Coronavirus suspensions exposed to HOCl for 120 seconds showed a log reduction value (LRV) of 4.25log10, equivalent to a 99.994% inactivation compared to control recoveries.
3.2 Endotoxin content of HOCl
All three samples assayed for endotoxin in the LAL assay satisfied the test criteria and were found in compliance with the USP specification of <0.4 EU per mg. Briotech’s HOCl is therefore classified as endotoxin-free and pyrogen-free.
3.3 Exposure of experimental animals to pure HOCl solutions – rats and rabbits
There were no adverse effects on behavior or appearance observed in groups of rats exposed to aerosolized HOCl continuously for 4 hours. No behavioral changes were observed, and no gross pathological changes were seen in the respiratory system on necropsy at 14 days post exposure. Furthermore, no acute histopathological changes were present on microscopic examination of turbinate, bronchial, tracheal, or pulmonary parenchyma tissue sectioned from the animals that were sacrificed serially post-exposure. The GLP and academic evaluation of the HOCl inhalation effect within the pulmonary system was rated as “no different from controls” and showed no evidence of toxicity. Similarly, there were no adverse effects noted following ocular exposure of rabbits to solutions of the same HOCl via the conjunctival sac. The GLP EPA toxicity test result was classed “ Not Irritating”.
3.4 Human tent exposure
During the early months of the pandemic, some workplaces were classified as “essential” by local public health authorities and remained open during the lockdown. Several of those workplaces elected to make exposure to an HOCl mist, in concert with other prophylactic measures that included masks, physical distancing, and handwashing, an option when arriving at work. Between 16 April and 24 June of 2020, information on a total of 102 aerosolized HOCl inhalation exposures were submitted to the authors. The exposures documented a cumulative total of 191 minutes (3 hours, 11 minutes) of adults inhaling a dense 0.9% saline fog containing HOCl in a closed space. Ages ranged from 18 to 71, and 87% were male. The total volume of HOCl entering the pulmonary tree in those 102 exposures was roughly 72 ml. With that volume of pure hypochlorous acid in contact with delicate respiratory epithelium, zero serious issues were declared. All minor issues reported (6.9% of reports describing “nose tickle”, “runny nose”, and the like) were transient and resolved in less than 1 minute after leaving the tent. The authors note that this set of exposures was not a clinical study in any sense. It was unwitnessed, unmonitored, and unevenly reported from a remote location during pandemic lockdown and is included here only for context. No conclusions are drawn.
3.5 HOCl preparation and characteristics within the tent
The authors had no direct access to the tent, so we chose to replicate the scenario remotely. We learned what tent was being used, and what compressor, tubing and nozzle, and replicated it all in our laboratory to assess the likely effect on anyone sitting in a tent breathing a fog of HOCl on arrival to their shift and then proceeding to their work in the shop. Once replicated locally, the characteristics of the HOCl fog were delineated. The initial concentration of liquid HOCl in the original HDPE container was 139 ppm at pH 5.0 and the ORP was 1022 mV. After travel through approximately 1 meter of plastic tubing and then through the fogging nozzle, the concentration of HOCl decreased as distance from the nozzle increased, as seen in Table 1. The concentration of HOCl at 75 cm (inhalation point) was measured as 52 ppm and so had decreased by more than 60% from the initial concentration.
3.6 Antibacterial properties of fogged HOCl at the point of tent inhalation
We designed a protocol where coupons coated with E. coli were mounted in our tent at nostril-height and exposed to the aerosolized HOCl fog in the tent for the same 2 minutes. Then, 10 minutes post-removal, the coupons were neutralized with sodium thiosulfate and assayed. Analysis of the coupons showed a bacterial reduction of more than 4.5 LRV (99.997%). To shorten the overall contact time to just 2 minutes (assuming, as an exercise, that all antimicrobial activity from the inhalation stopped when the subject left the tent), we mounted and exposed coupons in the tent using the same protocol but with S. aureus as the bacterial inoculant. We then neutralized the coupons at the 2-minute mark instead of the ten-minute mark. Analysis still showed more than 4 LRV (99.99% reduction) compared to control coupons.
4. Discussion
There is increasing evidence that hypochlorous acid (HOCl) can play a role in the protection of respiratory system epithelia against viral infection. Rhinovirus replication in primary cultured nasal epithelial cells was markedly inhibited by the exposure of infected cultures to HOCl [6], suggesting that exogenous sources of HOCl may be able to intervene in the maturation of intracellular virions. Furthermore, inhibition of coronavirus infection within cultured epithelial cell lines has been shown coincident with intracellular production of HOCl [7]. Those each point to the possible operation of innate resistance mechanisms in mucosal epithelia mediated by HOCl, independent of the need for phagocytosis by neutrophils and tissue macrophages. Similar protective contributions by reactive oxygen species (ROS) have been proposed to occur in alveolar cells in the lower respiratory tract [8]. A sprayable HOCl has been shown effective at inactivating 99.8% of SARS-CoV-2 in less than 1 minute in vitro in pre-clinical trials [9], and the topical nasal application of HOCl-containing solutions has been proposed as a useful protective approach to avoiding common cold viruses [6, 10]. Inhalation of HOCl-containing aerosols was recently shown to be an effective therapeutic intervention in the treatment of COVID-19 virus-infected patients, halting the progression of symptoms and speeding their return to normal [11]. In 2021 a clinical trial was conducted to test using inhaled HOCl as a prophylactic regimen for the protection of healthcare workers in a dedicated COVID-19 hospital. The results show a statistically significant reduction in COVID-19 conversion [12]. Despite these advances and their obvious relevance to both prophylaxis and therapy opportunities in the ongoing pandemic of SARS-CoV-2 virus infections, little is known about the responsiveness of either the upper or lower respiratory tract to exposure to aerosolized HOCl in experimental animals and that is the focus of this paper.
In our research described herein, none of the animals exposed to microaerosolized HOCl by inhalation for 4 hours showed any adverse signs in behavior, nor any gross or microscopic changes upon necropsy. It appears that even prolonged inhalation of HOCl generates neither acute nor lingering after-effects. Such findings satisfy the standard required by US federal regulatory agencies for determination of the acute toxicity of inhaled drugs or device solutions. Acute exposure of ocular tissues of rabbits also proved entirely uneventful and without evidence of any irritant effects or adverse consequences. Humans whose eyes were exposed to microaerosolized HOCl in their tent also reported no acute or subsequent eye irritation from the fog. The amount of active chlorine declined markedly in aerosolized HOCl droplets expressed through the microaerosolizing nozzle, falling by more than 60% by the time they reached the inhalation point for seated subjects and the sample point for antimicrobial efficacy. Despite that reduction in concentration, the HOCl solutions used in these exposures were clearly antimicrobial and displayed high-level inactivation of S. aureus and E. coli in laboratory suspension tests. Some concentration attrition is inevitable in the administration of microaerosolized HOCl by these methods, regardless of whether the creation and dispersion of droplets is affected by pressurized air or via the ultrasonic oscillation of membranes typical of nebulizing devices [13]. The results of measurements of the antibacterial properties of the microaerosolized HOCl arriving at the surface of coupons placed within the enclosure and used to mimic essential worker exposure make it clear that, despite the attrition, there remains a high level of disinfecting power at the point of droplet deposition, again in accord with prior reports [14, 15]. The dissipation of active Cl in air with distance from the aerosol source of HOCl has been reported previously in publications when establishing efficacy against norovirus or against several bacterial pathogens in vegetative and spore forms [14, 15]. How this comes about and what the reaction products are of the oxidative chlorine with air during transit remains to be determined.
A recent report on systemic administration of HOCl as a part of the therapeutic intervention in COVID-19 patients halted the progression of symptoms [11]. The authors attributed the success of their HOCl treatment of COVID-19 patients to its antiviral properties. Published evidence of the value of HOCl in vitro in protecting nasal mucosal epithelial cells against respiratory viral infections supports that interpretation [6]. The changes experienced by healthcare workers who inhaled nebulized HOCl within the study conducted by Rafael et al. [12] indicate that there are systemic effects resulting from exposure to HOCl in this way, some of which are clearly beneficial and do not seem to be simply antimicrobial. Whether or not those are affected by HOCl directly or through reaction products arising at the point of mucosal contact remains to be determined. There is a high likelihood that HOCl rapidly modifies a variety of constituents of both extracellular and intracellular fluids, with the interaction with taurine being one of the most prominent, resulting in the formation of N-chlorotaurine (NCT) [16, 17]. Taurine is present in body fluids in amounts that can total 0.1% of total body weight and is particularly likely to be involved in the biological effects of HOCl exposure [18]. Previous work indicates that HOCl and NCT have anti-inflammatory properties [19, 20] and, while beyond the scope of this evaluation, it seems feasible that the anti-inflammatory properties of HOCl and NCT might also lead to reduced local inflammation, lower infectivity through reduced permeability, and perhaps lower morbidity and mortality overall.
Our recent observations on the efficacy of HOCl as an anti-inflammatory agent via inactivation of interleukin 6 (IL-6) [in press] suggest that it can intervene in the pathogenic pathways of COVID-19 beyond its antiviral efficacy, possibly by downregulating mediators of the cytokine storm that characterizes clinical deterioration in this disease [21]. Our observations here are particularly relevant considering recent developments involving human exposure to HOCl. Solutions of HOCl have been shown to rapidly inactivate the SARS- CoV-2 virus in test systems comparable to those used in this study [22]. Based on those results, commercial products containing HOCl have been placed on the recommended pandemic coronavirus disinfectant list (List N) by the US Environmental Protection Agency [23].
More broadly, we note that widespread, high-volume use of disinfectants has come about as a response to the current pandemic, and it is becoming increasingly apparent that many of these are inappropriate for such use patterns, never having been designed or intended for intensive applications around - and sometimes upon – humans [1, 24]. Toxicological evidence of serious untoward effects of repeated exposures to commonplace disinfectants, especially by inhalation of aerosols, are emerging [2]. Such findings have brought HOCl to the fore as a highly effective alternative to quaternary ammonium formulations, hypochlorite solutions, ozone, peroxide, and alcohols of various types [25, 26]. We undertook this research because humans are being exposed to HOCl at an unprecedented rate, sometimes via aerosols that they are obliged to encounter as a condition of entry into public buildings to control the COVID-19 virus [27]. Some of these HOCl exposure patterns offer unproven benefits, but the overall safety profile of HOCl, the recognition of its in vitro efficacy, and popular understanding of HOCl as a natural product of human cellular defense, have each encouraged these new patterns of use. Further expansion of HOCl as an environmental disinfectant is inevitable, and studies such as ours that begin to provide evidence of the safety profile associated with HOCl are timely. While further work is needed on longer term exposures, the data from our experiments to date have not raised issues of concern and we think our results should encourage further experimental evaluation.
Regardless of the possible mechanisms involved, the potential significance of the use of HOCL in the management of COVID-19 is substantial, though more data is needed. Current approaches using monoclonal antibodies directed against the SARS-CoV-2 virus, or against IL-6, or against viral receptors on mucosal epithelial cells are costly, cumbersome, and scarce [28, 29]. The practical complications of the specificity, manufacture, transport, administration, and expense of such measures preclude their ever becoming broadly available to the great majority of people around the world. In contrast, homogenous and stable forms of HOCL can now be produced in industrial quantities and readily distributed to local pharmacies and clinics without special requirements for transport or storage conditions [30]. Were further research to delineate potential avenues of respiratory care in viral illness using microaerosolized HOCl, the advantages in delivery, simplicity of administration, and in cost might be broadly substantial. It is also worth noting that the specific amino acid residues in the SARS-CoV-2 spike protein against which the redox mechanism of action for HOCl is active are conserved in all variants evaluated to date. That argues that HOCl may continue to be an effective molecule for inactivation of other strains of SARS-CoV-2 as each appears. The persistent effectiveness of HOCl against increasingly variable SARS-CoV-2 strains, therefore, presents an opportunity worthy of more extensive clinical evaluation.
Acknowledgements
Our thanks to Briotech’s microbiology laboratory director, Jose Santiago, for all quantitative microbiology performed for this paper, and to Briotech’s Product Chemist, Joseph McKinley, for the chemical assessments of the tent mist characteristics.
Conflicts of Interest
This study received funding from Briotech, Inc. Briotech had the following involvement with the study: Funding for quantitative microbiology, laboratory tent assessments, and independent GLP laboratory rodent and rabbit testing. Briotech was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. ER and JW are Chief Medical Officer and Chief Science Officer respectively of Briotech, Inc. LR is tenured faculty within the Department of Physical Sciences of the University of Washington and a consulting biophysical chemist to Briotech. RJS is a consultant physician to Briotech. All authors declare no other competing interests.
Availability of Data
All data are available from the corresponding author on request.
Authors' Contributions
ER collected the external tent data and wrote substantial sections of the text. LR supervised all laboratory assessments and edited the text. JW wrote substantial sections of the text, reviewed all references for rigor and fit, and edited the entire manuscript. RJS served as a consultant on clinical questions and contributed to discussions that shaped our laboratory design and evaluations.
Ethics Approval and Consent to Participate
Neither approval nor consent were required. The human tent exposures during the pandemic lockdown were reported to the authors from an unrelated business entity in another city and are not a formal part of any study. We have (still) never met them, we did not coordinate them, and the HOCl they were using is commercially available on Amazon. They are an aircraft engineering firm and the fogging system they devised for the tent was entirely their own invention. The evaluation presented within this paper was of a replicated system with no humans inside and studied only for biochemical characterization and antimicrobial efficacy. The rodent inhalation safety study, the rabbit ocular irritation test, and the endotoxin assessments were performed, and documented to meet or exceed ethical standards, by the independent and Federally certified GLP laboratories that performed the tests. (Nelson Laboratories and Stillmeadow Laboratories)
Patient Consent for Publication
Not applicable
References
- Zheng G, Filippelli GM, Salamova A. Increased Indoor Exposure to Commonly Used Disinfectants during the COVID-19 Pandemic. Environmental science & technology letters 7(2020): 760-765.
- Dewey HM, Jones JM, Keating MR, et al. Increased Use of Disinfectants During the COVID-19 Pandemic and Its Potential Impacts on Health and Safety. ACS Chemical Health & Safety (2021).
- Kim HJ, Lee JG, Kang JW, et al. Effects of a low concentration hypochlorous Acid nasal irrigation solution on bacteria, fungi, and virus. Laryngoscope 118 (2008): 1862-1867.
- Mitchell JP, Berlinski A, Canisius S, et al. Urgent Appeal from International Society for Aerosols in Medicine (ISAM) During COVID-19: Clinical Decision Makers and Governmental Agencies Should Consider the Inhaled Route of Administration: A Statement from the ISAM Regulatory and Standardization Issues Networking Group. Journal of aerosol medicine and pulmonary drug delivery 33 (2020): 235-238.
- Hughson AG, Race B, Kraus A, et al. Inactivation of Prions and Amyloid Seeds with Hypochlorous Acid. PLoS Pathog 12 (2016): e1005914.
- Yu MS, Park HW, Kwon HJ, et al. The effect of a low concentration of hypochlorous acid on rhinovirus infection of nasal epithelial cells. Am J Rhinol Allergy 25 (2011): 40-44.
- Bradley Field B, Amy Lynn D, Thomas H, et al. Enhancement of Innate Immunity to COVID-19 with Natural Measures. Immunome Research 16 (2020): 1-4.
- Evans SE, Xu Y, Tuvim MJ, et al. Inducible innate resistance of lung epithelium to infection. Annual review of physiology 72 (2010): 413-435.
- Giarratana N, Rajan B, Kamala K, et al. A sprayable Acid-Oxidizing solution containing hypochlorous acid (AOS2020) efficiently and safely inactivates SARS-Cov-2: a new potential solution for upper respiratory tract hygiene. European archives of oto-rhino-laryngology (2021): 1-5.
- Ramalingam S, Cai B, Wong J, et al. Antiviral innate immune response in non-myeloid cells is augmented by chloride ions via an increase in intracellular hypochlorous acid levels. Sci Rep 8 (2018): 13630.
- Delgado-Enciso I, Paz-Garcia J, Barajas-Saucedo CE, et al. Safety and efficacy of a COVID-19 treatment with nebulized and/or intravenous neutral electrolyzed saline combined with usual medical care vs. usual medical care alone: A randomized, open-label, controlled trial. Experimental and Therapeutic Medicine 22 (2021): 1-16.
- Rafael G-G, Juan Carlos De la C-Á, Ariana C-L, et al. Neutral Electrolyzed Water (SES) for Nasopharyngeal and Oropharyngeal Rinses Prevented COVID-19 in Front-Line Health Professionals: A Randomized, Open-Label, Controlled Trial in a General Hospital in Mexico City. BMC Infectious Diseases Pre-print, (2021).
- Zhao Y, Xin H, Zhao D, et al. Free chlorine loss during spraying of membraneless acidic electrolyzed water and its antimicrobial effect on airborne bacteria from poultry house. Annals of Agricultural and Environmental Medicine 21 (2014): 249-255.
- Park GW, Boston DM, Kase JA, et al. Evaluation of liquid- and fog-based application of Sterilox hypochlorous acid solution for surface inactivation of human norovirus. Appl Environ Microbiol 73 (2007): 4463-4468.
- Fertelli D, Jennifer LC, Michelle MN, et al. Effectiveness of an Electrochemically Activated Saline Solution for Disinfection of Hospital Equipment. Infection control and hospital epidemiology 34 (2013): 543-544.
- Gottardi W, Nagl M. Chemical Properties of N-Chlorotaurine Sodium, a Key Compound in the Human Defence System. Archiv der Pharmazie (Weinheim) 335 (2002): 411-421.
- Zgliczynski JM, Stelmaszynska T, Domanski J, et al. Chloramines as intermediates of oxidation reaction of amino acids by myeloperoxidase. BBA - Enzymology 235 (1971): 419-424.
- Huxtable RJ. Physiological Actions of Taurine. Physiological reviews 72 (1992): 101-163.
- Fukuyama T, Martel BC, Linder KE, et al. Hypochlorous acid is antipruritic and anti-inflammatory in a mouse model of atopic dermatitis. Clin Exp Allergy 48 (2018): 78-88.
- Nagl M, Arnitz R, Lackner M. N-Chlorotaurine, a Promising Future Candidate for Topical Therapy of Fungal Infections. Mycopathologia 183 (2018): 161-170.
- de la Rica R, Borges M, Gonzalez-Freire M. COVID-19: In the Eye of the Cytokine Storm. Frontiers in immunology 11 (2020): 558898-558898.
- Rasmussen ED, Williams J, Robins L. Final GLP testing results for Briotech HOCl against SARS- CoV-2 Briotech (2021).
- EPA: Briotech’s Aquavert Entered on List N for activity against SARS-CoV-2 (COVID-19). Agency EP (ed.) EPA, Washington, DC (2020).
- Dindarloo K, Aghamolaei T, Ghanbarnejad A, et al. Pattern of disinfectants use and their adverse effects on the consumers after COVID-19 outbreak. Journal of environmental health science and engineering (2020): 1-10.
- Block MS, Rowan BG: Hypochlorous Acid: A Review. Journal of oral and maxillofacial surgery: official journal of the American Association of Oral and Maxillofacial Surgeons (2020).
- Nguyen K, Bui D, Hashemi M, et al. The Potential Use of Hypochlorous Acid and a Smart Prefabricated Sanitising Chamber to Reduce Occupation-Related COVID-19 Exposure. Risk management and healthcare policy 14 (2021): 247-252.
- Ozaki S. Facilities in Japan cautioned against hypochlorous acid misting to fight COVID-19. In: The Mainichi, Tokyo, Japan, (2020).
- Chakraborty C, Sharma AR, Bhattacharya M, et al. COVID-19: Consider IL-6 receptor antagonist for the therapy of cytokine storm syndrome in SARS-CoV-2 infected patients. Journal of medical virology 92 (2020): 2260-2262.
- Antwi-Amoabeng D, Kanji Z, Ford B, et al. Clinical outcomes in COVID-19 patients treated with tocilizumab: An individual patient data systematic review. Journal of medical virology 92 (2020): 2516-2522.
- Trust H. HOCl Trust for Hygiene and Safer Water (2021).