Colourless Agar for Enhancing Colour Contrast between Microbial Colonies and Agar
Article Information
Wenfa Ng*
Department of Chemical and Biomolecular Engineering, National University of Singapore, Singapore
*Corresponding Author: Wenfa Ng, Department of Chemical and Biomolecular Engineering, National University of Singapore, Singapore
Received: 16 October 2021; Accepted: 25 October 2021; Published: 02 November 2021
Citation:
Wenfa Ng. Colourless Agar for Enhancing Colour Contrast between Microbial Colonies and Agar. Journal of Food Science and Nutrition Research 4 (2021): 270-285.
View / Download Pdf Share at FacebookAbstract
Lack of colour contrast hampers automated identification of colonies on agar, given that many microbial colonies are of the same colour as the background colour of most agar: beige colour. On the other hand, a colourless agar could increase the colour contrast between the agar background and microbial colonies. But, the challenge in preparing a colourless agar comes from the formation of coloured compounds when sugars and ammonium compounds are sterilized together in an autoclave. Hence, by separating glucose and ammonium compounds into different solutions for autoclave sterilization, a method was developed for preparing colourless agar that remained colourless even with 1 g/L of yeast extract supplementation. Specifically, three separate solutions were used in reconstituting the colourless agar at ~48oc after individual sterilization. Solution A comprised glucose, MgSO4 and agar powder; Solution B contained NH4Cl, K2HPO4, KH2PO4, and NaCl; and Solution C was yeast extract solution for providing vitamins and trace elements needed by microorganisms unable to grow in minimal medium. Reconstituted colourless agar could be poured into agar plates using standard techniques and had a viscosity similar to many commercial agar. Composition of the colourless agar medium was [g/L]: D-Glucose, 2.0; NH4Cl, 0.5; K2HPO4, 0.5; KH2PO4, 0.1; NaCl, 0.5; MgSO4.7H2O, 1.0; Yeast extract, 1.0; Agar, 15.0. On observation against varied background images, the formulated colourless agar lacked the yellow tinge present in LB Lennox agar and had greater optical transparency. Good growth of common bacteria such as Escherichia coli DH5α (ATCC 53868), Bacillus subtilis NRS-762 (ATCC 8473) and Pseudomonas protegens Pf-5 (ATCC BAA-477) was observed in both liquid and solid versions of the formulated colourless agar medium. Specifically, colonies of E. coli DH5α, B. subtilis NRS762 and P. protegens Pf-5 exhibited similar morphology and characteristics compa
Keywords
Colourless agar, Glucose, Ammonium compounds, Coloured compounds, Autoclave sterilization, Escherichia coli, Bacillus subtilis, Pseudomonas protegens, Yeast extract, Colour contrast
Colourless agar articles; Glucose articles; Ammonium compounds articles; Coloured compounds articles; Autoclave sterilization articles; Escherichia coli articles; Bacillus subtilis articles; Pseudomonas protegens articles; Yeast extract articles; Colour contrast articles
Colourless agar articles Colourless agar Research articles Colourless agar review articles Colourless agar PubMed articles Colourless agar PubMed Central articles Colourless agar 2023 articles Colourless agar 2024 articles Colourless agar Scopus articles Colourless agar impact factor journals Colourless agar Scopus journals Colourless agar PubMed journals Colourless agar medical journals Colourless agar free journals Colourless agar best journals Colourless agar top journals Colourless agar free medical journals Colourless agar famous journals Colourless agar Google Scholar indexed journals Glucose articles Glucose Research articles Glucose review articles Glucose PubMed articles Glucose PubMed Central articles Glucose 2023 articles Glucose 2024 articles Glucose Scopus articles Glucose impact factor journals Glucose Scopus journals Glucose PubMed journals Glucose medical journals Glucose free journals Glucose best journals Glucose top journals Glucose free medical journals Glucose famous journals Glucose Google Scholar indexed journals Ammonium compounds articles Ammonium compounds Research articles Ammonium compounds review articles Ammonium compounds PubMed articles Ammonium compounds PubMed Central articles Ammonium compounds 2023 articles Ammonium compounds 2024 articles Ammonium compounds Scopus articles Ammonium compounds impact factor journals Ammonium compounds Scopus journals Ammonium compounds PubMed journals Ammonium compounds medical journals Ammonium compounds free journals Ammonium compounds best journals Ammonium compounds top journals Ammonium compounds free medical journals Ammonium compounds famous journals Ammonium compounds Google Scholar indexed journals Coloured compounds articles Coloured compounds Research articles Coloured compounds review articles Coloured compounds PubMed articles Coloured compounds PubMed Central articles Coloured compounds 2023 articles Coloured compounds 2024 articles Coloured compounds Scopus articles Coloured compounds impact factor journals Coloured compounds Scopus journals Coloured compounds PubMed journals Coloured compounds medical journals Coloured compounds free journals Coloured compounds best journals Coloured compounds top journals Coloured compounds free medical journals Coloured compounds famous journals Coloured compounds Google Scholar indexed journals Autoclave sterilization articles Autoclave sterilization Research articles Autoclave sterilization review articles Autoclave sterilization PubMed articles Autoclave sterilization PubMed Central articles Autoclave sterilization 2023 articles Autoclave sterilization 2024 articles Autoclave sterilization Scopus articles Autoclave sterilization impact factor journals Autoclave sterilization Scopus journals Autoclave sterilization PubMed journals Autoclave sterilization medical journals Autoclave sterilization free journals Autoclave sterilization best journals Autoclave sterilization top journals Autoclave sterilization free medical journals Autoclave sterilization famous journals Autoclave sterilization Google Scholar indexed journals Escherichia coli articles Escherichia coli Research articles Escherichia coli review articles Escherichia coli PubMed articles Escherichia coli PubMed Central articles Escherichia coli 2023 articles Escherichia coli 2024 articles Escherichia coli Scopus articles Escherichia coli impact factor journals Escherichia coli Scopus journals Escherichia coli PubMed journals Escherichia coli medical journals Escherichia coli free journals Escherichia coli best journals Escherichia coli top journals Escherichia coli free medical journals Escherichia coli famous journals Escherichia coli Google Scholar indexed journals Bacillus subtilis articles Bacillus subtilis Research articles Bacillus subtilis review articles Bacillus subtilis PubMed articles Bacillus subtilis PubMed Central articles Bacillus subtilis 2023 articles Bacillus subtilis 2024 articles Bacillus subtilis Scopus articles Bacillus subtilis impact factor journals Bacillus subtilis Scopus journals Bacillus subtilis PubMed journals Bacillus subtilis medical journals Bacillus subtilis free journals Bacillus subtilis best journals Bacillus subtilis top journals Bacillus subtilis free medical journals Bacillus subtilis famous journals Bacillus subtilis Google Scholar indexed journals Pseudomonas protegens articles Pseudomonas protegens Research articles Pseudomonas protegens review articles Pseudomonas protegens PubMed articles Pseudomonas protegens PubMed Central articles Pseudomonas protegens 2023 articles Pseudomonas protegens 2024 articles Pseudomonas protegens Scopus articles Pseudomonas protegens impact factor journals Pseudomonas protegens Scopus journals Pseudomonas protegens PubMed journals Pseudomonas protegens medical journals Pseudomonas protegens free journals Pseudomonas protegens best journals Pseudomonas protegens top journals Pseudomonas protegens free medical journals Pseudomonas protegens famous journals Pseudomonas protegens Google Scholar indexed journals Yeast extract articles Yeast extract Research articles Yeast extract review articles Yeast extract PubMed articles Yeast extract PubMed Central articles Yeast extract 2023 articles Yeast extract 2024 articles Yeast extract Scopus articles Yeast extract impact factor journals Yeast extract Scopus journals Yeast extract PubMed journals Yeast extract medical journals Yeast extract free journals Yeast extract best journals Yeast extract top journals Yeast extract free medical journals Yeast extract famous journals Yeast extract Google Scholar indexed journals Colour contrast articles Colour contrast Research articles Colour contrast review articles Colour contrast PubMed articles Colour contrast PubMed Central articles Colour contrast 2023 articles Colour contrast 2024 articles Colour contrast Scopus articles Colour contrast impact factor journals Colour contrast Scopus journals Colour contrast PubMed journals Colour contrast medical journals Colour contrast free journals Colour contrast best journals Colour contrast top journals Colour contrast free medical journals Colour contrast famous journals Colour contrast Google Scholar indexed journals
Article Details
Graphical Abstract
Short Description
By separately sterilizing glucose and ammonium compounds, a method was developed for creating colourless agar, that helped improve colour contrast between microbial colonies and agar. With no generation of growth inhibitory compounds during agar preparation, colourless agar was able to support the growth of a wide variety of microbial species including common bacteria such as Escherichia coli DH5α, Bacillus subtilis NRS-762, and Pseudomonas protegens Pf-5. Additionally, the formulated colourless agar was able to support a cell density useful for profiling microbial diversity in different environments. Show here is the microbial flora from deionized water cultivated on formulated colourless agar at 30oc after spread plate inoculation, where small colonies are more easily identified due to high optical transparency of the colourless agar.
Subject areas: Biotechnology, Microbiology, Biochemistry, Ecology, Cell Biology

Importance of the work
Lack of colour contrast hampers automated colony identification and counting given the difficulty of discerning small yellowish microbial colonies against beige colour, which is the common colour of agar background. Colourless agar, on the other hand, could enhance the colour contrast between colonies and agar background. However, preparation of colourless agar is hampered by heat induced reaction between sugars and ammonium compounds that generates chromogenic compounds. By separately sterilizing glucose and ammonium compounds, this study reports a simple method for the preparation of colourless agar that could help enhance the colour contrast between agar background and colonies of any colour. Experiment results revealed that colourless agar remained colourless even with addition of yeast extract at 1 g/L. Growth inhibitory compounds are likely not generated during agar preparation given good growth of a variety of common bacteria in both liquid and solid versions of the colourless agar. Overall, a simple method for preparing colourless agar was validated with good growth of a variety of microorganisms from deionized water that indicated growth inhibitory compounds were not generated during agar preparation. The formulated colourless agar could find use in a wide variety of applications such as in microbial ecology studies.
Introduction
Most commercially available agars are beige in colour, which is also the colour of many microbial colonies. Thus, there is a lack of colour contrast between microbial colonies and agar, which hampers the identification of small colonies by visual observation as well as automated cell counting software able to differentiate a colony from an agar background during cell counting experiments.1 2 3 To ameliorate the problem, a colourless agar would be preferable given that it provides good colour contrast between microbial colonies and agar background.
However, reaction between sugars and ammonium compounds during autoclave sterilization generates coloured compounds that lend a reddish colour to the agar; thereby, preventing the formation of a colourless agar. In this study, an approach of separately sterilizing glucose and ammonium compounds, followed by reconstitution after the solutions have cooled to ~48 oC was used in preparing colourless agar. Additionally, given that many microorganisms could not survive in environments without an input of vitamins and growth factors, the prepared colourless agar must be constituted with small amounts of yeast extract while retaining optical transparency. Experiments revealed that the colourless agar remained colourless even with 1 g/L of yeast extract supplementation. Besides visual observations of the optical transparency of the reconstituted agar, growth experiments were also used in assessing the ability of the formulated agar medium in cultivating a variety of common bacteria (i.e., Escherichia coli DH5α (ATCC 53868), Bacillus subtilis NRS-762 (ATCC 8473), and Pseudomonas protegens Pf-5 (ATCC BAA477)) in both liquid and solid medium format.
Materials and methods
Materials
LB Lennox medium was purchased from Difco and used as is. Composition of LB Lennox medium is [g/L]: Tryptone, 10.0; Yeast extract, 5.0; NaCl, 5.0. Composition of formulated colourless agar medium is [g/L]: D-Glucose, 2.0; NH4Cl, 0.5; K2HPO4, 0.5; KH2PO4, 0.1; NaCl, 0.5; MgSO4.7H2O, 1.0; Yeast extract, 1.0; Agar, 15.0. Composition of M9 medium is [g/L]: DGlucose, 4.0; NH4Cl, 1.0; Na2HPO4, 6.78; NaH2PO4, 3.0; NaCl, 0.5. Composition of LB Lennox agar is [g/L]: Tryptone, 10.0; Yeast extract, 5.0; NaCl, 5.0; Agar, 15.0. R2A agar was purchased from Merck and used as is. Composition of R2A agar was [g/L]: yeast extract, 0.5; Proteose Peptone, 0.5; Casein hydrolysate, 0.5; Glucose, 0.5; Starch soluble, 0.5; Sodium pyruvate, 0.3; K2HPO4, 0.3; MgSO4, 0.024; Agar, 15.0.
Preparation of colourless agar medium
Solution A comprised 0.4g of D-Glucose, 0.2g of MgSO4.7H2O and 3.0g of agar powder in 200 mL of deionized water in a 500 mL glass conical flask. Solution B contained 0.5g of K2HPO4, 0.5g of NH4Cl, 0.1g of KH2PO4 and 0.5g of NaCl in 50 mL of deionized water in a 100 mL Schott Duran glass bottle. Solution C contained 1g of yeast extract in 50 mL of deionized water in a 100 mL Schott Duran glass bottle. Solution A, B and C were sterilized separately at 121 oC for 20 minutes.
After cooling to ~48 oC, 10 mL of the sterilized Solution B and C was added to 200 mL of Solution A under aseptic conditions to reconstitute the colourless agar medium, which could be poured into agar plates using standard techniques. The cooling step is important for preventing heat induced reactions between sugars and ammonium compounds after reconstitution of the agar medium.
Growth of bacteria in liquid versions of formulated colourless agar medium
Stock cultures of Escherichia coli DH5α, Bacillus subtilis NRS-762 and Pseudomonas protegens Pf-5 were prepared in 40% glycerol and stored at -70oc prior to use. E. coli DH5α and B. subtilis NRS-762 were activated by inoculating a glycerol stock culture in 100 mL of LB Lennox medium in 250 mL glass shake flask with cotton plugs at 37oc and 30oc, respectively with 230 rpm rotational shaking in a temperature controlled incubator (orbital diameter: 25 mm, Yih Der LM570RD, Taiwan). On the other hand, P. protegens Pf-5 was activated similarly, but in M9 medium given its ability to grow in minimal salts medium. After 24 hours of incubation, 1 mL of seed cultures was used in inoculating experiment cultures in the respective media used for the study. Incubation conditions were as follows: E. coli DH5α (37oc and 230 rpm rotational shaking), B. subtilis NRS-762 (30oc and 230 rpm rotational shaking), P. protegens Pf-5 (30oc and 230 rpm rotational shaking). Three biological replicates were performed for each experiment.
Measurement of optical density and pH
At appropriate time points, 5 mL of culture was withdrawn from the shake flasks for optical density and pH measurement. Optical density was measured via a UV-Visible spectrophotometer (Shimadzu Bio-spec Mini) with a quartz cuvette of 10 mm pathlength (volume: 3.5 ml). Dilution with deionized water was performed if the optical density at 600 nm exceeded 1. pH was measured with an Orion 9156 BNWP pH probe outfitted to a Mettler Toledo Delta 320 pH meter.
Growth of bacteria on solid formulated colourless agar medium
- coli DH5α, B. subtilis NRS-762, and P. protegens Pf-5 were activated in 100 mL of LB Lennox medium in 250 mL glass shake flask by inoculating glycerol stock cultures of the bacteria, and incubated at the following conditions: E. coli DH5α (37oc and 230 rpm rotational shaking), B. subtilis NRS-762 (30oc and 230 rpm rotational shaking), P. protegens Pf-5 (30oc and 230 rpm rotational shaking). After 24 hours of incubation, inoculating loops were used aseptically to inoculate experiment cultures on colourless agar medium via the streak plate technique. Incubation conditions for the different bacteria were as follows: E. coli DH5α (37oc), B. subtilis NRS-762 (30oc), P. protegens Pf-5 (30oc) in a temperature controlled incubator.
Profiling the microbial flora of deionized water
Deionized water was collected using a pre-sterilized 20 mL plastic bottle after allowing 5 minutes for flow through of deionized water collected in the deionized water production system. Thus, the deionized water used as inoculum was freshly produced. Spread plate technique was used in inoculating 0.1 mL of deionized water on formulated colourless agar and R2A agar. Agar plates were incubated at 30oc in a temperature controlled incubator and observed daily.
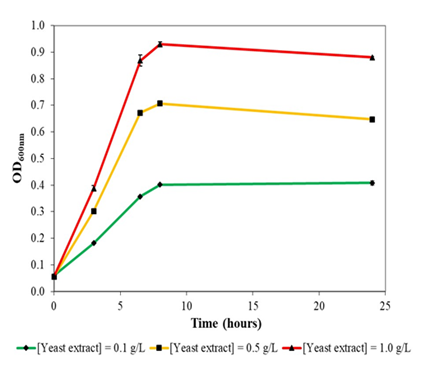
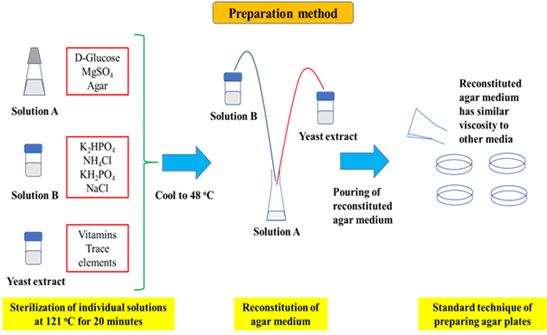
Figure 1: Method for preparing colourless agar. Specifically, the constituents of the agar are separated into three solutions that contain key elements of the agar. Solution A, for example, contains glucose, agar powder and MgSO4, Solution B contains phosphate salts, ammonium chloride and sodium chloride, while Solution C is yeast extract which provides the trace elements and vitamins needed for growth.
Given that heat induced reaction between sugars and ammonium compounds would result in the formation of coloured compounds that lend a colour to the agar, glucose and ammonium compounds must be separately sterilized. Specifically, glucose could not be sterilized with ammonium chloride and yeast extract. However, it could be paired with agar powder in Solution A, which also contains MgSO4 as the sulphur source. Solution B would be constituted by ammonium chloride as the nitrogen source of the colourless agar medium. This was combined with K2HPO4, KH2PO4 and sodium chloride. Yeast extract is Solution C as it contains important vitamins and trace elements necessary for growth of microorganisms unable to grow in minimal salts medium. However, since yeast extract contains sugars and ammonium compounds, it could not be sterilized with glucose or ammonium chloride, given the formation of coloured compounds when sugars react with ammonium compounds.
After separate sterilization, Solutions A, B and C were cooled to approximately ~48oc and subsequently mixed to reconstitute the colourless agar medium (Figure 1). The reconstituted solution was swirled repeatedly prior to pouring of agar plates using standard techniques. Viscosity of the colourless agar medium was similar to those of commercial agar media. Finally, yeast extract could be added up to a concentration of 1 g/L in the colourless agar medium formulation without affecting the optical transparency of the medium.

Figure 2: Colourless agar delivers higher optical transparency and colour rendition compared to a commercial LB Lennox agar, which is beige in colour
Compared to beige LB Lennox agar, the formulated colourless agar provided higher optical transparency and colour rendition (Figure 2). Specifically, tinge of yellow was absent in the colourless agar even if it was supplemented with yeast extract to a concentration of 1 g/L. Furthermore, colourless agar was able to render a background image’s colours better than a beige LB Lennox agar. Finally, greater optical transparency was apparent in images observed through the colourless agar compared to a beige LB Lennox agar.
|
Components |
Concentration (g/L) |
|
K2HPO4 |
0.5 |
|
KH2PO4 |
0.1 |
|
D-Glucose |
2 |
|
NH4Cl |
0.5 |
|
Yeast extract |
1 |
|
NaCl |
0.5 |
|
MgSO4.7H2O |
1 |
|
Agar |
15 |
Table 1: Composition of formulated colourless agar
Composition of formulated colourless agar medium is shown in Table 1. To allow sufficient capacity for supporting the growth of myriad microbes, a glucose concentration of 2 g/L was used. Ammonium chloride concentration was designed to maintain a 1:1 concentration ratio with glucose on a molar basis. Concentration of yeast extract, which is important to provide a source of vitamins and growth factors for the growth of microbes unable to synthesize vitamins, could be increased and decreased for understanding the effect of yeast extract in inducing the growth of different microbes. Buffering capacity of the agar as well as a source of potassium and phosphate comes from K2HPO4 and KH2PO4. MgSO4.7H2O, on the other hand, provides sulphate and magnesium for the microorganisms. Finally, NaCl adjusts the osmolarity of the medium.
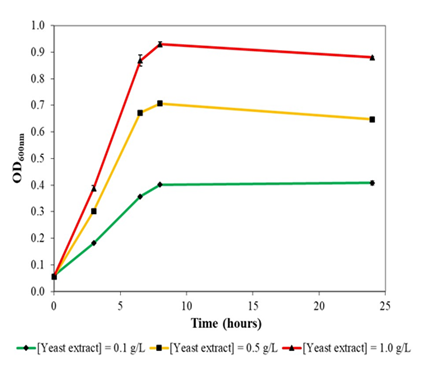
Figure 3a: Growth of Escherichia coli DH5α at 37oc and 230 rpm rotational shaking in liquid version of formulated colourless agar with different concentrations of yeast extract supplementation. E. coli DH5α could not grow in minimal salts medium without yeast extract supplementation.
Escherichia coli DH5α could not grow in liquid version (without agar powder) of the colourless agar medium without yeast extract supplementation. With increasing concentration of yeast extract supplementation, E. coli DH5α demonstrated better growth and biomass formation, with maximal optical density obtained of 0.4, 0.7 and 0.9 at 0.1 g/L, 0.5 g/L and 1.0 g/L yeast extract supplementation, respectively (Figure 3a). Growth rates of E. coli DH5α also increased with yeast extract concentration in a concentration-dependent manner. In general, E. coli DH5α grew reasonably well in yeast extract supplemented colourless agar medium with no drastic decline in optical density.
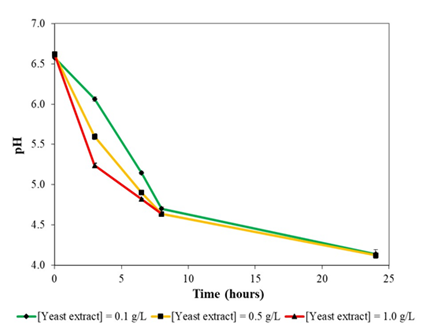
Figure 3b: Variation of pH during growth of E. coli DH5α in liquid version of formulated colourless agar medium at 37oc and 230 rpm rotational shaking.
At the onset of growth in liquid version of colourless agar medium, E. coli DH5α secreted significant amounts of acidic metabolites into the culture broth, which resulted in a decrease of pH from 6.6 to 4.6 at the start of stationary phase in the cultures (Figure 3b). Following which, pH declined further to 4.1 at the end of cultivation. What likely transpired was rapid secretion of acidic metabolites in a yeast extract concentration-dependent manner where the higher the yeast extract supplementation, the greater would be the amount of acidic metabolites secreted. pH of culture broth continued to decrease during stationary phase.
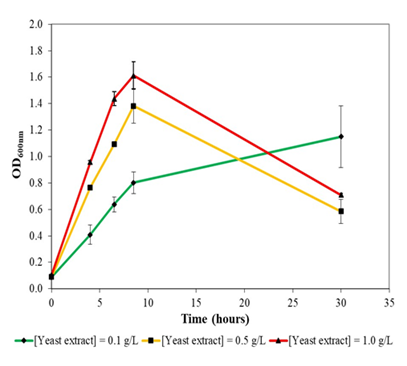
Figure 4a: Growth of Bacillus subtilis NRS-762 in liquid version of formulated colourless agar medium at different concentrations of yeast extract supplementation at 30oc and 230 rpm rotational shaking. Drastic decline in optical density after stationary phase likely resulted from cannibalism of non resistant cells. B. subtilis NRS-762 could not grow in minimal salts medium without yeast extract supplementation.
Similar to the case with E. coli DH5α, B. subtilis NRS-762 exhibited higher growth rates with greater amount of yeast extract supplementation in the formulated colourless agar medium (Figure 4a). Specifically, maximal optical density obtained was 1.4 and 1.6 for yeast extract concentration of 0.5 and 1.0 g/L, respectively. On the other hand, growth of B. subtilis NRS-762 at low yeast extract supplementation of 0.1 g/L extended beyond 30 hours given the slow rate of cell growth. Drastic decline in optical density after onset of stationary phase in cells cultivated in 0.5 and 1.0 g/L yeast extract supplementation was likely due to cannibalism rather than prophage induced cell lysis. Specifically, in delaying entry into the sporulation programme, subpopulations of B. subtilis NRS-762 likely secreted cell lysis factors that lysed non resistant cells for releasing cellular content needed for sustaining the remaining population. Prophage induced cell lysis, on the other hand, would have resulted in precipitous decline of the optical density rather than the gradual decline observed.
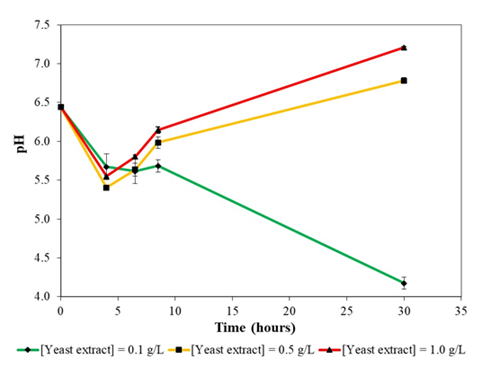
Figure 4b: Variation of pH during growth of B. subtilis NRS-762 in liquid version of formulated colourless agar medium with different concentrations of yeast extract supplementation at 30oc and 230 rpm rotational shaking.
Growth of B. subtilis NRS-762 in colourless agar medium with 0.1 g/L yeast extract supplementation might be using a different set of metabolic pathways compared to that in colourless agar medium with 0.5 and 1.0 g/L yeast extract, given the distinctly different pH profiles during growth (Figure 4b). Specifically, in the case of growth in colourless agar medium with 0.5 and 1.0 g/L yeast extract, pH decreased followed by an increase. On the other hand, growth of the bacterium in colourless agar medium with 0.1 g/L of yeast extract exhibited a continuous decrease in pH.
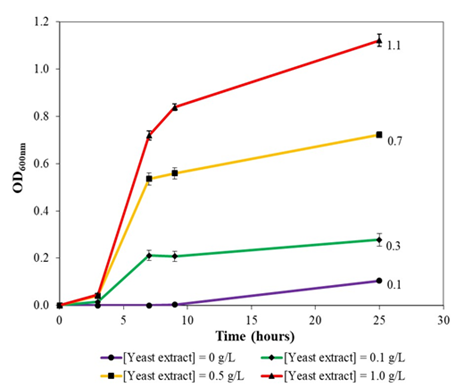
Figure 5a: Growth of Pseudomonas protegens Pf-5 in colourless agar medium supplemented with different concentrations of yeast extract at 30oc and 230 rpm rotational shaking. Note that growth rates and maximal optical density increased with yeast extract concentration in a concentrationdependent manner.
Growth rates and maximal optical density of P. protegens Pf-5 in formulated colourless agar medium positively correlated with amount of yeast extract supplementation. Specifically, optical density increased from 0.1 to 0.3 and 0.7 and finally 1.1 with increasing yeast extraction supplementation from 0, 0.1, 0.5 and 1.0 g/L, respectively (Figure 5a). Hence, given that yeast extract is a source of vitamins and growth factors, rates of cell division and biomass formation increased with increasing yeast extract supplementation as P. protegens Pf-5 cells do not need to expend metabolic energy from nutrients in synthesizing vitamins and other building blocks for growth. On the other hand, the bacterium could grow slowly in liquid version of colourless agar medium without yeast extract supplementation where an intense green colour broth was observed. With increasing concentrations of yeast extract supplementation, the green colouration of the broth decreased (0.1 g/L yeast extract) and turned to beige coloured broth (at 0.5 and 1.0 g/L yeast extract). Overall, this likely suggested that secretion of a green colour siderophore by P. protegens Pf-5 was suppressed with increasing concentration of yeast extract since yeast extract was able to supply the iron necessary for growth, where in the case of no yeast extract supplementation, large amount of siderophore would need to be synthesized for sequestrating iron needed for biomass formation.
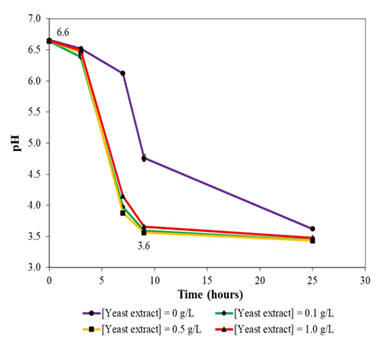
Figure 5b: Variation of pH during growth of P. protegens Pf-5 in liquid version of formulated colourless agar medium with different concentrations of yeast extract supplementation. Note that the bacterium likely utilized similar metabolic pathways for growth in colourless agar medium irrespective of amount of yeast extract supplementation (0.1, 0.5, and 1.0 g/L).
pH profile for P. protegens Pf-5 growth in liquid version of formulated colourless agar medium under different concentrations of yeast extract supplementation (0.1, 0.5 and 1.0 g/L) revealed that irrespective of the concentrations of yeast extract used, similar pH profile was obtained, which suggested that similar sets of metabolic pathways were used for the growth of P. protegens Pf-5 in the medium (Figure 5b). Specifically, pH decreased from 6.6 at the onset of growth to 3.6 after 9 hours of incubation. pH continued to decrease thereafter. On the other hand, a different pH profile was observed for growth of P. protegens Pf-5 in liquid version of colourless agar medium without yeast extract supplementation, which suggested that the bacterium was likely using different sets of metabolic pathways for growth. Finally, pH profile studies revealed that P. protegens Pf-5 could grow at a pH of 3.6; for example, Figure 5a clearly indicated that optical density continued to increase for the bacterium after 9 hours of incubation in liquid versions of formulated colourless agar medium with 0.5 and 1.0 g/L yeast extract supplementation.
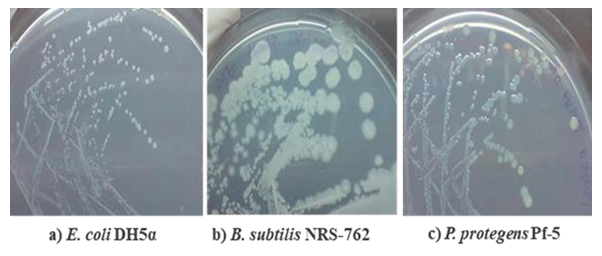
Figure 6: Growth of E. coli DH5α, B. subtilis NRS-762 and P. protegens Pf-5 in solid formulated colourless agar medium after streak plate inoculation.
Good growth of E. coli DH5α, B. subtilis NRS-762 and P. protegens Pf-5 on solid formulated colourless agar medium was observed with colony morphologies similar to those obtained during growth on LB Lennox agar medium, which suggested that growth inhibitory compounds were not produced during agar preparation (Figure 6). Specifically, small, round, white colonies were obtained for E. coli DH5α growth on colourless agar medium, which was similar to the morphology obtained during growth on LB Lennox agar. However, colonies of E. coli DH5α on colourless agar medium lacked the beige colour typical of the bacterium’s colonies on LB Lennox agar. In the case of B. subtilis NRS-762, white, wrinkled, round colonies were obtained during growth on colourless agar medium, which compared well with the beige, round and wrinkled colonies on LB Lennox agar. But, the mechanisms underlying the loss of pigmentation of E. coli DH5α and B. subtilis NRS-762 colonies during growth on formulated colourless agar remain unknown. Finally, P. protegens Pf-5 colonies on colourless agar medium were greenish, small and round, which were similar to those obtained on LB Lennox agar. However, there was no secretion of green siderophore by P. protegens Pf-5 during growth on colourless agar medium with yeast extract supplementation at 1 g/L.
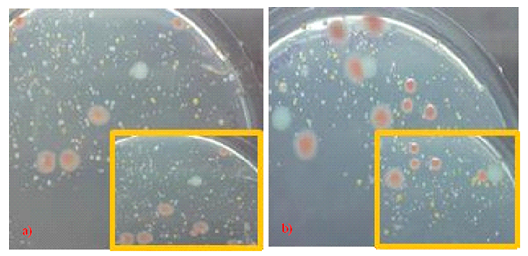
Figure 7: Growth of microbial flora in deionized water on a) R2A agar, and b) formulated colourless agar incubated at 30oc. Insets showed a close-up of the original photograph. Note that many types of microbial species were present in deionized water, and colourless agar was able to support the growth of many of the species; thereby, illustrating that growth inhibitory compounds were not generated during agar preparation.
For assessing whether the formulated colourless agar was able to support the growth of various types of microbes present in the environment as well as the presence of growth inhibitory compounds generated during agar preparation, spread plate culture of deionized water inoculum was performed on both the gold standard R2A agar and the formulated colourless agar medium. Many different types of microbial colonies of different morphologies and pigmentation were recovered on both types of agar, which confirmed the presence of a large variety of microorganisms in deionized water (Figure 7). Specifically, the ability of formulated colourless agar medium in recovering a large variety of different types of microbial colonies attested to the fact that the formulated colourless agar medium was able to support the growth of many microbial species. More importantly, the result also showed that growth inhibitory compounds were not generated during agar preparation. Overall, the high optical transparency of the formulated colourless agar medium enabled greater ease in identifying small colonies recovered from the deionized water inoculum. Great diversity in microbes present in deionized water pointed to the important role that monochloramine played in suppressing microbial growth in drinking water distribution system and the detrimental impact to microbiological quality of water upon its removal by the ion exchange system that produced deionized water.
Conclusions
By separately sterilizing sugars and ammonium compounds during agar preparation and reconstituting the different solutions at ~48oc, a method for preparing colourless agar was developed. The colourless agar improved colour contrast between microbial colonies and agar background. More importantly, the formulated colourless agar was amenable to supplementation with yeast extract (up to a concentration of 1 g/L) without introduction of colour. Growth experiments with Escherichia coli DH5α, Bacillus subtilis NRS-762 and Pseudomonas protegens Pf-5 in liquid versions of the colourless agar (without agar powder) confirmed that the medium could support the growth of common bacteria to an optical density of ~1.0 in an otherwise low nutrient content medium. On the other hand, streak plate culture of the same bacteria on solid formulated colourless agar medium recovered growth of the microbes in morphologies similar to those obtained on LB Lennox agar. Loss of pigmentation was an issue with growth of E. coli DH5α and B. subtilis NRS-762 on colourless agar but not P. protegens Pf-5. Specifically, whitish, round colonies were obtained for E. coli DH5α and B. subtilis NRS-762 instead of beige colonies. Culture experiments aimed at assessing the ability of the colourless agar medium in supporting the growth of a variety of microbes confirmed that the medium could recover many types of microbial species from deionized water, which highlighted that the medium was suitable for use in microbial ecology studies aimed at assessing the microbial flora of a habitat. More importantly, the result indicated that no growth inhibitory compounds were generated during agar preparation. Collectively, the formulated colourless agar medium could find use in a wide variety of applications in microbiology ranging from microbial ecology studies to cell counting experiments.
Supplementary materials
Additional information on the growth of different bacteria on formulated colourless agar can be found in the appended supplementary materials.
Conflicts of interest
The author declares no conflicts of interest.
Funding
The author thank the National University of Singapore for financial support.
References
- Choudhry P. High-Throughput method for automated colony and cell counting by digital image analysis based on edge detection. Plos one 11 (2016): e0148469.
- Clarke, M. L. et al. Low-cost, high-throughput, automated counting of bacterial colonies. Cytometry A 77 (2010): 790-797.
- Croxatto A. Towards automated detection, semi-quantification and identification of microbial growth in clinical bacteriology: A proof of concept. Biomed J 40 (2017): 317-328.
