Avelumab Induced Severe Disabling Myositis in A Patient with Merkel Cell Carcinoma
Article Information
Arghal Ahmad1*, Hafsa Sheikh2
Ziauddin Medical College, Ziauddin University, Karachi, Pakistan
*Corresponding author: Arghal Ahmad, Ziauddin Medical College, Ziauddin University, Karachi, Pakistan
Received: 02 September 2020; Accepted: 09 September 2020; Published: 22 Septemebr 2020
Georg Lodde1, Lisa Zimmer1, Elisabeth Livingstone1, Christina Drusio1, Sarah Knispel1, Eva Hadaschik1, Axel Wetter2, Jürgen C. Becker3, Dirk Schadendorf1, Selma Ugurel1
1Department of Dermatology, University of Duisburg-Essen, Essen, Germany
2Department of Diagnostic and Interventional Radiology and Neuroradiology, University of Duisburg-Essen, Essen, Germany
3Translational Skin Cancer Research, Deutsches Konsortium für Translationale Krebsforschung (DKTK), Essen, Germany
*Corresponding Author: Georg Lodde, MD, Department of Dermatology, University Hospital Essen, Hufelandstrasse 55, 45122 Essen, Germany
Received: 07 August 2020; Accepted: 01 September 2020; Published: 05 October 2020
Citation: Georg Lodde, Lisa Zimmer, Elisabeth Livingstone, Christina Drusio, Sarah Knispel, Eva Hadaschik, Axel Wetter, Jürgen C. Becker, Dirk Schadendorf, Selma Ugurel. Avelumab Induced Severe Disabling Myositis in a Patient with Merkel Cell Carcinoma. Journal of Cancer Science and Clinical Therapeutics 4 (2020): 434-441.
View / Download Pdf Share at FacebookAbstract
Background: Merkel cell carcinoma (MCC) is a rare, aggressive neuroendocrine skin cancer with a poor survival rate. Nearly 30% of MCC patients develop metastatic disease. Until recently, patients with inoperable metastasised Merkel cell carcinoma had an extremely poor prognosis with a median overall survival of far below 12 months. After the introduction of immune checkpoint blockade it became obvious that the anti-PD-1/PD-L1 inhibitors pembrolizumab, nivolumab and avelumab are highly efficacious in MCC with durable responses in 20-40% of patients leading to a significant prolongation of survival times. PD-1/PD-L1 blockers are generally well tolerated. In the following we report a patient with advanced metastatic MCC who responded to avelumab but developed a severe, disabling therapy-induced myositis which did not resolve after therapy discontinuation. This is the first report on a severe myositis caused by avelumab leaving the patient to be permanently wheelchair-bound.
Case Presentation: An 85 year-old male presented with multiple cutaneous and subcutaneous nodes of the left leg and groin. The patient’s history revealed diagnosis of MCC of the left buttock and synchronous macroscopic lymph node metastasis of the left groin 4 years ago, treated with complete lymphadenectomy followed by adjuvant radiation of all tumor sites. Staging showed an advanced metastatic MCC. Treatment with avevulamb was started. Re-staging 3 months after start of avelumab showed a partial response. The patient developed a therapy-induced myositis despite a corticosteroid support of methylprednisolone, an additional immuno-suppressive treatment with infliximab and discontinuation of treatment. 8 months after start of avelumab the patient is in stable disease. However, the severe weakness of both legs has not resolved and the patient remains immobilized and wheelchair-bound.
Conclusio
Keywords
<p>Merkel cell carcinoma; Avelumab; Myositis; Immune checkpoint inhibition, Toxicity</p> <gdiv></gdiv>
Merkel cell carcinoma articles; Avelumab articles; Myositis articles; Immune checkpoint inhibition articles, Toxicity articles
Merkel cell carcinoma articles Merkel cell carcinoma Research articles Merkel cell carcinoma review articles Merkel cell carcinoma PubMed articles Merkel cell carcinoma PubMed Central articles Merkel cell carcinoma 2023 articles Merkel cell carcinoma 2024 articles Merkel cell carcinoma Scopus articles Merkel cell carcinoma impact factor journals Merkel cell carcinoma Scopus journals Merkel cell carcinoma PubMed journals Merkel cell carcinoma medical journals Merkel cell carcinoma free journals Merkel cell carcinoma best journals Merkel cell carcinoma top journals Merkel cell carcinoma free medical journals Merkel cell carcinoma famous journals Merkel cell carcinoma Google Scholar indexed journals Avelumab articles Avelumab Research articles Avelumab review articles Avelumab PubMed articles Avelumab PubMed Central articles Avelumab 2023 articles Avelumab 2024 articles Avelumab Scopus articles Avelumab impact factor journals Avelumab Scopus journals Avelumab PubMed journals Avelumab medical journals Avelumab free journals Avelumab best journals Avelumab top journals Avelumab free medical journals Avelumab famous journals Avelumab Google Scholar indexed journals Myositis articles Myositis Research articles Myositis review articles Myositis PubMed articles Myositis PubMed Central articles Myositis 2023 articles Myositis 2024 articles Myositis Scopus articles Myositis impact factor journals Myositis Scopus journals Myositis PubMed journals Myositis medical journals Myositis free journals Myositis best journals Myositis top journals Myositis free medical journals Myositis famous journals Myositis Google Scholar indexed journals Immune checkpoint inhibition articles Immune checkpoint inhibition Research articles Immune checkpoint inhibition review articles Immune checkpoint inhibition PubMed articles Immune checkpoint inhibition PubMed Central articles Immune checkpoint inhibition 2023 articles Immune checkpoint inhibition 2024 articles Immune checkpoint inhibition Scopus articles Immune checkpoint inhibition impact factor journals Immune checkpoint inhibition Scopus journals Immune checkpoint inhibition PubMed journals Immune checkpoint inhibition medical journals Immune checkpoint inhibition free journals Immune checkpoint inhibition best journals Immune checkpoint inhibition top journals Immune checkpoint inhibition free medical journals Immune checkpoint inhibition famous journals Immune checkpoint inhibition Google Scholar indexed journals Toxicity articles Toxicity Research articles Toxicity review articles Toxicity PubMed articles Toxicity PubMed Central articles Toxicity 2023 articles Toxicity 2024 articles Toxicity Scopus articles Toxicity impact factor journals Toxicity Scopus journals Toxicity PubMed journals Toxicity medical journals Toxicity free journals Toxicity best journals Toxicity top journals Toxicity free medical journals Toxicity famous journals Toxicity Google Scholar indexed journals neuroendocrine skin cancer articles neuroendocrine skin cancer Research articles neuroendocrine skin cancer review articles neuroendocrine skin cancer PubMed articles neuroendocrine skin cancer PubMed Central articles neuroendocrine skin cancer 2023 articles neuroendocrine skin cancer 2024 articles neuroendocrine skin cancer Scopus articles neuroendocrine skin cancer impact factor journals neuroendocrine skin cancer Scopus journals neuroendocrine skin cancer PubMed journals neuroendocrine skin cancer medical journals neuroendocrine skin cancer free journals neuroendocrine skin cancer best journals neuroendocrine skin cancer top journals neuroendocrine skin cancer free medical journals neuroendocrine skin cancer famous journals neuroendocrine skin cancer Google Scholar indexed journals immunosuppression articles immunosuppression Research articles immunosuppression review articles immunosuppression PubMed articles immunosuppression PubMed Central articles immunosuppression 2023 articles immunosuppression 2024 articles immunosuppression Scopus articles immunosuppression impact factor journals immunosuppression Scopus journals immunosuppression PubMed journals immunosuppression medical journals immunosuppression free journals immunosuppression best journals immunosuppression top journals immunosuppression free medical journals immunosuppression famous journals immunosuppression Google Scholar indexed journals Merkel cell carcinoma articles Merkel cell carcinoma Research articles Merkel cell carcinoma review articles Merkel cell carcinoma PubMed articles Merkel cell carcinoma PubMed Central articles Merkel cell carcinoma 2023 articles Merkel cell carcinoma 2024 articles Merkel cell carcinoma Scopus articles Merkel cell carcinoma impact factor journals Merkel cell carcinoma Scopus journals Merkel cell carcinoma PubMed journals Merkel cell carcinoma medical journals Merkel cell carcinoma free journals Merkel cell carcinoma best journals Merkel cell carcinoma top journals Merkel cell carcinoma free medical journals Merkel cell carcinoma famous journals Merkel cell carcinoma Google Scholar indexed journals pembrolizumab articles pembrolizumab Research articles pembrolizumab review articles pembrolizumab PubMed articles pembrolizumab PubMed Central articles pembrolizumab 2023 articles pembrolizumab 2024 articles pembrolizumab Scopus articles pembrolizumab impact factor journals pembrolizumab Scopus journals pembrolizumab PubMed journals pembrolizumab medical journals pembrolizumab free journals pembrolizumab best journals pembrolizumab top journals pembrolizumab free medical journals pembrolizumab famous journals pembrolizumab Google Scholar indexed journals lymphadenectomy articles lymphadenectomy Research articles lymphadenectomy review articles lymphadenectomy PubMed articles lymphadenectomy PubMed Central articles lymphadenectomy 2023 articles lymphadenectomy 2024 articles lymphadenectomy Scopus articles lymphadenectomy impact factor journals lymphadenectomy Scopus journals lymphadenectomy PubMed journals lymphadenectomy medical journals lymphadenectomy free journals lymphadenectomy best journals lymphadenectomy top journals lymphadenectomy free medical journals lymphadenectomy famous journals lymphadenectomy Google Scholar indexed journals
Article Details
1. Background
Merkel cell carcinoma (MCC) is a rare aggressive neuroendocrine skin cancer with a poor survival rate [1,2]. Incidence is highest in male patients with a fair skin type. Risk factors for developing MCC are immunosuppression, advanced age and high cumulative UV light exposure [3, 4]. Nearly 30% of MCC patients develop metastatic disease [5]. Until recently, patients with inoperable metastasised Merkel cell carcinoma had an extremely poor prognosis with a median overall survival of far below 12 months [6, 7]. After the introduction of immune checkpoint blockade it became obvious that the anti-PD-1/PD-L1 inhibitors pembrolizumab, nivolumab and avelumab are highly efficacious in MCC with durable responses in 20-40% of patients leading to a significant prolongation of survival times [1, 3, 6, 8-11], Thus, anti-PD-1/PD-L1 therapy evolved to the new standard first-line therapy in metastatic MCC. The anti-PD-L1 therapeutic antibody avelumab was the first drug gaining approval for this tumor entity [3, 6, 8, 12]. PD-1/PD-L1 blockers are generally well tolerated. However, severe and long-lasting toxicities have been reported [13-15]. Due to the high efficacy of this class of drugs, the lack of alternative therapeutic strategies and the limited experience of long-term outcome of patients discontinuing treatment, the decision to stop anti-PD-1/PD-L1 treatment in patients developing toxicities is difficult. In the following we report a patient with advanced metastatic MCC who responded to avelumab but developed a severe, disabling therapy-induced myositis which did not resolve after therapy discontinuation. This is the first report on a severe myositis caused by avelumab leaving the patient to be permanently wheelchair-bound.
2. Case Report
An 85 years-old male presented at our skin cancer center in 06/2018 with multiple rapidly growing cutaneous and subcutaneous nodes at the left upper leg and groin (Figure 1A). The patient presented without physical symptoms or disabilities, besides mild abdominal pain; his overall performance status was scored ECOG 0. He had been diagnosed with MCC of the left buttock and synchronous macroscopic lymph node metastasis of the left groin in 09/2014. The patient had received a surgical excision of the primary tumor and complete lymphadenectomy of the groin, followed by an adjuvant radiation of the primary tumor region and the left groin. After the first presentation at our center, the patient underwent CT and MRI staging which revealed an intraabdominal tumor mass of 13 cm diameter, infiltrating the pancreas, liver, and mesenterium (Figure 1C). Blood testing showed an elevated lactate dehydrogenase (LDH) of 875 U/l, all other parameters were within normal range. The multidisciplinary tumor board rated the patient’s advanced metastatic disease as not amenable by surgery. Treatment with the anti-PD-L1 therapeutic antibody avelumab 10 mg/kg Q2W was recommended.
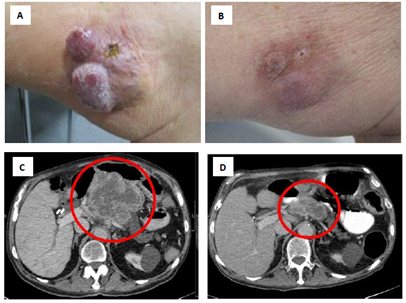
Figure 1: Partial response of MCC skin and intraabdominal metastases upon avelumab therapy. Presentation of the patient at start (A, C) and after 3 months of avelumab therapy (B, D). Representative clinical presentation of skin metastases of the upper leg (A, B) as well as CT scans of the abdomen showing a large intraabdominal metastasis with central necrosis infiltrating the pancreas, liver, and mesenterium (C, D).
At the first application of avelumab the patient developed a CTCAE grade 1 infusion reaction with vertigo and hypotension, which resolved within 20 min after intravenous application of corticosteroids, antihistamines and fluid replacement. All further doses of avelumab were applied without adverse infusion reactions. After the second dose, the skin and subcutaneous metastases started decreasing in size. In parallel, the blood LDH and CRP values decreased rapidly. Adverse events reported by the patient within the first 6 weeks of therapy were fatigue and lack of appetite (both CTCAE grade 1). Before the fourth dose of avelumab the patient noticed an unusual weakness and discomfort of his legs when walking or climbing stairs. Blood tests showed elevated levels of creatine kinase (CK) and myoglobin (Figure 2). Serum anti-smooth muscle (ASMA) and anti-mitochondrial (AMA) antibodies were within normal ranges. The patient had no signs of infection or other physical symptoms. A therapy-induced myositis was diagnosed and confirmed by an MRI scan (Figure 3). Daily laboratory checks revealed a further increase of CK and myoglobin, as well as a slight increase of CK-MB and troponin. The patient refused a muscle biopsy. The patient was examined to exclude myocarditis; electrocardiogram, echocardiography and cardiac stress-MRI gave no pathological findings. The patient’s symptoms from myositis at that time were rather mild and did not impair the patient’s daily activities (CTCAE grade 1). Additionally, a strong tumor response to treatment was already noticed by the patient with a shrinkage of visible and palpable metastases and an improvement of abdominal pain. The patient was carefully informed about the possibility of a further aggravation of the myositis when continuing treatment, including the probability of developing myocarditis or other immune-mediated adverse events. Due to the strong tumor response, he decided to continue avelumab therapy combined with an immuno-suppressive treatment with methylprednisolone 1 mg/kg QD. The first CT/MRI re-staging 3 months after start of avelumab showed a considerable regression of the intraabdominal tumor mass from 13 to 6 cm diameter (Figure 1D). Additionally, the cutaneous and subcutaneous lesions had significantly decreased in size (Figure 1B). Tumor response was classified as partial response (PR).
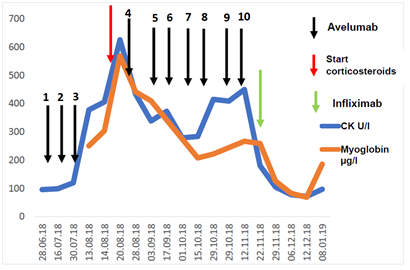
Figure 2: Course of creatine kinase (CK) and myoglobin in the peripheral blood of the patient.
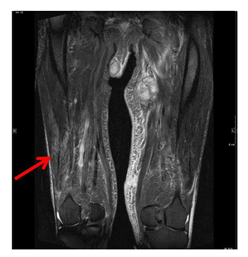
Figure 3: MRI scan of the lower extremities showing T2-hyperintense changes of vastus lateralis and intermedius muscle due to myositis.
The multidisciplinary tumor board recommended to proceed with avelumab treatment for another 3 months, provided the awareness and consent of the patient on the risk of a further deterioration of the myositis. The pathological laboratory values had decreased under corticosteroids but remained elevated (CK 284 U/l; myoglobin 208 µg/l; Figure 2). The methylprednisolone dose was gradually tapered to 0.5 mg/kg QD. After 9 doses of avelumab the patient reported an increasing muscle weakness and restricted mobility of the lower extremities. Blood tests, however, showed stable serum values of CK and myoglobin. Avelumab treatment was continued with an increased corticosteroid support of methylprednisolone 1 mg/kg QD. Nevertheless, the muscle weakness proceeded, so that avelumab therapy was cessated after the 10th avelumab dose. Shortly thereafter, the patient reported a new dyspnoea while attempting to climb stairs. His overall performance status rapidly declined to ECOG 2, he lost the ability to walk, and became wheel chair-bound due to severe muscle weakness. A repeated cardiological examination showed no pathological findings, in particular no evidence of myocarditis. The myositis was now classified as severe with CTCAE grade 4. An immuno-suppressive treatment with infliximab 5 mg/kg was applied in addition to the ongoing corticosteroid therapy. One week after the application of infliximab, the dyspnoea symptoms decreased but the muscle weakness of the lower extremities remained unchanged. The patient was closely followed for the next weeks with fortunately no signs of tumor progression, but also no evidence of improvement of the muscle weakness. Re-staging 6 months after start of avelumab showed a slight progression of the intraabdominal tumor mass and of the tumor lesions of the left groin. However, the tumor board recommended not to re-initiate avelumab due to the risk of further deterioration of the myositis or spread to other muscle regions. Instead, the progressing tumor lesions were irradiated leading to a disease stabilization. Currently, 8 months after start of avelumab and 4 months after treatment discontinuation, the patient is followed for tumor size and side effects showing an ongoing disease stabilization. The methylprednisolone dose was slowly tapered to an actual dose of 16 mg QD. Serum CK and myoglobin values have normalized. However, the severe weakness of both legs has not resolved and did not improve by continuous physical therapy. The patient is still immobilized and remains wheelchair-bound.
3. Discussion
In 2017, MCC was the first cancer entity for which the anti-PD-L1 antibody avelumab gained approval. Thus, data on the toxicity profile of avelumab are not that mature as data on other checkpoint inhibitors. Within the phase-2 trial JAVELIN Merkel 200, 88 patients with chemotherapy-refractory metastatic MCC received avelumab [8]. Immune-related adverse events (thyreoiditis, pneumonitis, diabetes mellitus) were reported in six patients (7%) only, and were of mild to moderate intensity [8]. No severe CTCAE grade 3-4 immune-mediated adverse events were observed. Serum CK values were reported as increased in two patients, however no clinical evidence of myositis was described [8]. Follow-up reports of that trial provided fatigue as the most frequent adverse event followed by musculoskeletal pain and gastrointestinal disorders [17,18]. An interims analysis of an additional first-line treatment cohort (n=39 patients) of the same trial revealed immune-related adverse events (nephritis, cholangitis, hepatitis, thyreoiditis and encephalomyelitis) in 5 patients (13%) [6]. Of those, four adverse events were of grade 3. Again, no cases of myositis were reported. Recently, avelumab was decribed to induce myositis in four of seven patients (57%) with thymoma treated within the JAVELIN Solid Tumor phase-1 trial [19]. However, thymoma is known to be frequently associated with myositis and myasthenia gravis [20]. In the investigated thymoma patients treated with avelumab, pre-existing anti-acetylcholine receptor autoantibodies were shown to be associated with the development of treatment-induced myositis [19]. Also, in thymoma the treatment-induced myositis resolved after discontinuation of avelumab and start of immuno-suppressive therapy. No persistent immobility of patients was reported.
Thus, the present case is the first report of an avelumab-induced myositis in MCC. It must be considered that the clinical course might has been aggravated by the fact that the patient insisted to continue treatment despite the already evident myositis. It is the first report of avelumab to induce a severe myositis which did not resolve upon immuno-suppressive treatment and finally led to an irreversible disabling muscle weakness leaving the patient permanently wheelchair-bound.
Consent for Publication
Consent for publication has been given and can be submitted upon request.
Conflicts of Interest
Georg Lodde declares travel support from Novartis.
Lisa Zimmer declares advisory board and speakers honoraria from Roche, Bristol-Myers-Squibb, Merck Sharp & Dohme, Pierre-Fabre, Sanofi and Novartis, as well as travel support from Bristol-Myers-Squibb, Merck Sharp & Dohme, Amgen, Pierre-Fabre and Novartis.
Elisabeth Livingstone declares advisory board and speakers honoraria from Bristol-Myers-Squibb, Boehringer-Ingelheim, Amgen, Roche, Novartis, and Merck Sharp & Dohme, as well as travel support from Amgen, Boehringer-Ingelheim, Merck Sharp & Dohme and Novartis.
Christina Drusio declares grant and travel support from Roche and Janssen-Cilag.
Sarah Knispel declares travel support from Bristol-Myers-Squibb and Amgen.
Jürgen C. Becker has received speaker honoraria from Amgen, MerckSerono, and Pfizer, advisory board honoraria from 4SC, Amgen, CureVac, eTheRNA, Lytix, MerckSerono, Novartis, Pfizer, Rigontec, and Sanofi as well as research funding from Alcedis, BMS, Boehringer Ingelheim, IQVIA, and MerckSerono; he also received travel support from 4SC and Incyte.
Dirk Schadendorf declares advisory board and speakers honoraria from Roche, Novartis, Bristol-Myers-Squibb, Merck, Amgen, Boehringer Ingelheim and Leo, as well as grant and travel support from Roche, Novartis, Bristol-Myers-Squibb, Merck, Amgen, Boehringer Ingelheim and Leo.
Selma Ugurel declares advisory board and speakers honoraria from Bristol-Myers-Squibb, Merck Sharp & Dohme, Merck Serono and Roche, as well as grant and travel support from Bristol-Myers-Squibb, Merck Sharp & Dohme, Roche.
Eva Hadaschick and Axel Wetter declare no conflicts of interest.
Availability of Data and Materials
All data generated or analysed during this case report are included in this published article.
Authors' Contributions
GL and SU made substantial contributions to the conception, design of the work, the acquisition, analysis, interpretation of data and have drafted the work and substantively revised it. AW made substantial contributions to the acquisition of data. All authors drafted the work and substantively revised it. All authors read and approved the final manuscript.
Funding
There has not been funding for this case report.
Acknowledgements
Not applicable.
References
- Schadendorf D, Lebbé C, Zur Hausen A, et al. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer 71 (2017): 53-69.
- Becker JC, Stang A, DeCaprio JA, et al. Merkel cell carcinoma. Nat Rev Dis Primers 3 (2017): 17077.
- Gallo M, Guarnotta V, Cicco F de, Rubino M, Faggiano A, Colao A. Immune checkpoint blockade for Merkel cell carcinoma: Actual findings and unanswered questions. J Cancer Res Clin Oncol (2019).
- Goh G, Walradt T, Markarov V, et al. Mutational landscape of MCPyV-positive and MCPyV-negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget 7 (2016): 3403-3415.
- Deneve JL, Messina JL, Marzban SS, et al. Merkel cell carcinoma of unknown primary origin. Ann Surg Oncol 19 (2012): 2360-2366.
- D'Angelo SP, Russell J, Lebbé C, et al. Efficacy and Safety of First-line Avelumab Treatment in Patients With Stage IV Metastatic Merkel Cell Carcinoma: A Preplanned Interim Analysis of a Clinical Trial. JAMA Oncol 4 (2018): e180077.
- Becker JC, Stang A, Zur Hausen A, et al. Epidemiology, biology and therapy of Merkel cell carcinoma: Conclusions from the EU project IMMOMEC. Cancer Immunol Immunother 67 (2018): 341-351.
- Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. The Lancet Oncology 17 (2016): 1374-1385.
- Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med 374 (2016): 2542-2552.
- Bichakjian CK, Olencki T, Aasi SZ, et al. Merkel Cell Carcinoma, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 16 (2018): 742-774.
- Chan IS, Bhatia S, Kaufman HL, et al. Immunotherapy for Merkel cell carcinoma: A turning point in patient care. J Immunother Cancer 6 (2018): 23.
- Schadendorf D, Nghiem P, Bhatia S, et al. Immune evasion mechanisms and immune checkpoint inhibition in advanced merkel cell carcinoma. Oncoimmunology 6 (2017): e1338237.
- Zimmer L, Goldinger SM, Hofmann L, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer 60 (2016): 210-225.
- Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer 60 (2016): 190-209.
- Moreira A, Loquai C, Pföhler C, et al. Myositis and neuromuscular side-effects induced by immune checkpoint inhibitors. Eur J Cancer 106 (2019): 12-23.
- S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 Published: November 27, 2017: U.S. Department of Health and Human Services. (2019).
- S. Food and Drug Administration. Avelumab (BAVENCIO). [March 19, 2019, 19.23 Uhr] Available from (): https (): //www.accessdata.fda.gov/drugsatfda_docs/label/2017/761049s000lbl.pdf.
- Kaufman HL, Russell JS, Hamid O, et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer 6 (2018): 7.
- Mammen AL, Rajan A, Pak K, et al. Pre-existing antiacetylcholine receptor autoantibodies and B cell lymphopaenia are associated with the development of myositis in patients with thymoma treated with avelumab, an immune checkpoint inhibitor targeting programmed death-ligand 1. Ann Rheum Dis 78 (2019): 150-152.
- Bernard C, Frih H, Pasquet F, et al. Thymoma associated with autoimmune diseases: 85 cases and literature review. Autoimmun Rev 15 (2016): 82-92.
