Antifogging Properties Brought Upon from Nanopillars
Article Information
Chaselynn Jiannotti, Hai-Feng Ji*
Department of Chemistry, Drexel University, Philadelphia, PA 19104, United States
*Corresponding Author: Dr. Hai-Feng Ji, Department of Chemistry, Drexel University, Philadelphia, United States
Received: 10 September 2020; Accepted: 17 September 2020; Published: 25 September 2020
Citation:
Chaselynn Jiannotti, Hai-Feng Ji. Antifogging Properties Brought Upon from Nanopillars. Journal of Nanotechnology Research 2 (2020): 051-059.
View / Download Pdf Share at FacebookAbstract
Numerous agents such as hydrogels, polymers, and detergents have been used to temporarily subside fogging from surfaces to achieve antifogging effects. To produce a longer lasting solution to fogging, nanopillars were manufactured to resemble the natural surface structure of cicada wings to help produce antifogging effects. Droplet jumping behaviors, wettability, and contact angles are observed through multiple different studies determining the differences between superhydrophobic and superhydrophilic surfaces. Superhydrophobic surfaces displayed droplet jumping behaviors with contact angles > 150°, whereas superhydrophilic surfaces only displayed wettability with contact angles relatively close to 0°. The review summaries the papers on antifogging properties of surface brough upon from nanopillars, which presented guidelines for engineers to consider nanopillar manipulation that will influence antifogging tendencies among many surfaces.
Keywords
Nanopillar; Microstructure; Nanostructure; Antifogging; Wettability; Dewetting; Nucleation; Hydrophobic; Hydrophilic; Topology; Cicada
Nanopillar articles Nanopillar Research articles Nanopillar review articles Nanopillar PubMed articles Nanopillar PubMed Central articles Nanopillar 2023 articles Nanopillar 2024 articles Nanopillar Scopus articles Nanopillar impact factor journals Nanopillar Scopus journals Nanopillar PubMed journals Nanopillar medical journals Nanopillar free journals Nanopillar best journals Nanopillar top journals Nanopillar free medical journals Nanopillar famous journals Nanopillar Google Scholar indexed journals Microstructure articles Microstructure Research articles Microstructure review articles Microstructure PubMed articles Microstructure PubMed Central articles Microstructure 2023 articles Microstructure 2024 articles Microstructure Scopus articles Microstructure impact factor journals Microstructure Scopus journals Microstructure PubMed journals Microstructure medical journals Microstructure free journals Microstructure best journals Microstructure top journals Microstructure free medical journals Microstructure famous journals Microstructure Google Scholar indexed journals Nanostructure articles Nanostructure Research articles Nanostructure review articles Nanostructure PubMed articles Nanostructure PubMed Central articles Nanostructure 2023 articles Nanostructure 2024 articles Nanostructure Scopus articles Nanostructure impact factor journals Nanostructure Scopus journals Nanostructure PubMed journals Nanostructure medical journals Nanostructure free journals Nanostructure best journals Nanostructure top journals Nanostructure free medical journals Nanostructure famous journals Nanostructure Google Scholar indexed journals Antifogging articles Antifogging Research articles Antifogging review articles Antifogging PubMed articles Antifogging PubMed Central articles Antifogging 2023 articles Antifogging 2024 articles Antifogging Scopus articles Antifogging impact factor journals Antifogging Scopus journals Antifogging PubMed journals Antifogging medical journals Antifogging free journals Antifogging best journals Antifogging top journals Antifogging free medical journals Antifogging famous journals Antifogging Google Scholar indexed journals Wettability articles Wettability Research articles Wettability review articles Wettability PubMed articles Wettability PubMed Central articles Wettability 2023 articles Wettability 2024 articles Wettability Scopus articles Wettability impact factor journals Wettability Scopus journals Wettability PubMed journals Wettability medical journals Wettability free journals Wettability best journals Wettability top journals Wettability free medical journals Wettability famous journals Wettability Google Scholar indexed journals Dewetting articles Dewetting Research articles Dewetting review articles Dewetting PubMed articles Dewetting PubMed Central articles Dewetting 2023 articles Dewetting 2024 articles Dewetting Scopus articles Dewetting impact factor journals Dewetting Scopus journals Dewetting PubMed journals Dewetting medical journals Dewetting free journals Dewetting best journals Dewetting top journals Dewetting free medical journals Dewetting famous journals Dewetting Google Scholar indexed journals Nucleation articles Nucleation Research articles Nucleation review articles Nucleation PubMed articles Nucleation PubMed Central articles Nucleation 2023 articles Nucleation 2024 articles Nucleation Scopus articles Nucleation impact factor journals Nucleation Scopus journals Nucleation PubMed journals Nucleation medical journals Nucleation free journals Nucleation best journals Nucleation top journals Nucleation free medical journals Nucleation famous journals Nucleation Google Scholar indexed journals Hydrophobic articles Hydrophobic Research articles Hydrophobic review articles Hydrophobic PubMed articles Hydrophobic PubMed Central articles Hydrophobic 2023 articles Hydrophobic 2024 articles Hydrophobic Scopus articles Hydrophobic impact factor journals Hydrophobic Scopus journals Hydrophobic PubMed journals Hydrophobic medical journals Hydrophobic free journals Hydrophobic best journals Hydrophobic top journals Hydrophobic free medical journals Hydrophobic famous journals Hydrophobic Google Scholar indexed journals Hydrophilic articles Hydrophilic Research articles Hydrophilic review articles Hydrophilic PubMed articles Hydrophilic PubMed Central articles Hydrophilic 2023 articles Hydrophilic 2024 articles Hydrophilic Scopus articles Hydrophilic impact factor journals Hydrophilic Scopus journals Hydrophilic PubMed journals Hydrophilic medical journals Hydrophilic free journals Hydrophilic best journals Hydrophilic top journals Hydrophilic free medical journals Hydrophilic famous journals Hydrophilic Google Scholar indexed journals Topology articles Topology Research articles Topology review articles Topology PubMed articles Topology PubMed Central articles Topology 2023 articles Topology 2024 articles Topology Scopus articles Topology impact factor journals Topology Scopus journals Topology PubMed journals Topology medical journals Topology free journals Topology best journals Topology top journals Topology free medical journals Topology famous journals Topology Google Scholar indexed journals
Article Details
1. Introduction
Many surfaces experience a phenomenon known as fogging when the temperature of a surface is lower than that of the air [1]. Engineers have developed several methods for fogging prevention using antifogging agents. These agents include ploymers [2], hydrogels [3], or detergents [4] which are chemicals that minimize surface tension and prevent water from condensing into small water droplets on a surface. These methods are generally temporary, i.e. once the materials are washed off the surface, the antifogging property of the surface will be gone.
On the other hand, introducing nanopillars on surfaces can alter the structure of a surface to produce a permanent antifogging effect. The antifogging behavior of a surface can be achieved by introducing either hydrophilic or hydrophobic nanopillars [5]. For a surface, its contact angle must be > 90° to be considered hydrophobic, and < 90° for a hydrophilic one. To be antifogging, hydrophobic surfaces also depict a droplet jumping behavior that allows for the water droplets to spontaneously jump from the surface and roll off from the surface. Hydrophilic surfaces do not possess this property, and instead focus more on the wettability of the surface in which water spreads into a thin layer preventing coalescent events. Introducing nanopillars on a surface can change the properties of that surface and further influence the optical, biophysical, and biochemical properties [6]. Although many papers have been published on hydrophobicity and hydrophilicity of nanopillared surfaces, only a few demonstrated their antifogging abilities.
2. Antifogging due to hydrophobic nanostructured surfaces
Studies have suggested that nanostructured hydrophobic surfaces otherwise known as superhydrophobic surfaces have been known to formulate antifogging properties through water repulsion. One such example of this action is caused from spontaneous jumping [7]. This idea of “jumping” occurs upon natural coalescence events in which water droplets collect due to condensation and fogging. Numerous reports have observed that the appropriate water droplet size most suited for jumping averaged at about 10 μm due to its larger jumping contact angle. Hydrophobic surfaces produce the results with a contact angle > 90°, however any angle > 150° is deemed a superhydrophobic surface resulting in the best droplet jumping behavior for antifogging purposes [5].
Tests were constructed to determine if the topology of the nanopillars would affect the critical jumping size. Mulroe et al. concluded that the topological surface is directly correlated to critical jumping size after revealing that tall, narrower nanopillars promoted 2 μm jumping droplets while short stockier nanopillars required over 20 μm before jumping. Slender and tightly condensed nanopillars that promote nanometric jumping are more desirable at enhancing antifogging properties. All of the surfaces reflected an advancing contact angle around ≈ 160° (Figure 1). By revealing the more equipped nanostructured model, proper designs can be made to enhance the water jumping ability and thus increase the antifogging properties on numerous surfaces.
The antifogging phenomenon was also observed in nature. Cicada wings contain thousands of nanostructures that take on hydrophobic behaviors, leading to antifogging and droplet jumping properties [8]. By observing multiple different species of cicada, the dependence upon habitat and life cycle can be determined if it has any correlation to the structural make up of their wings, and thus impact the antifogging properties they possess. One way to test this dependence is by measuring the wettability of the wings surface through contact angles and droplet jumping. Wettability is the ability for a liquid to maintain contact with a solid surface.
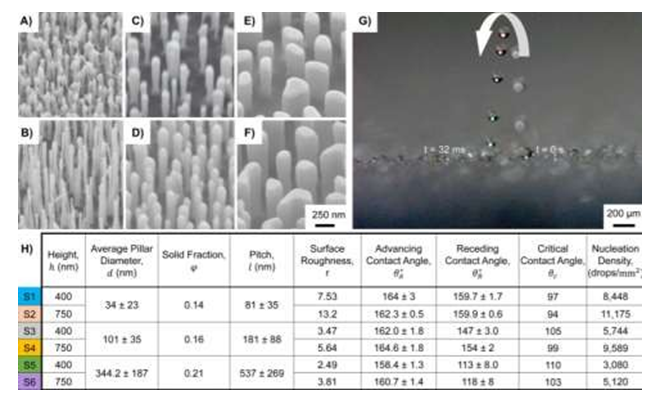
Figure 1: (A-F) Six different SEM images of topological superhydrophobic nanostructured surfaces, S1-S6 ranging from 94 - 110° tilt view are used to determine the droplet jumping size [7]. Multiple pictures were taken to illustrate the droplet jumping behavior (G). The average pillar diameters ranged from A) B) 34 nm to 23nm, C) D) 101nm to 35 nm, and E) F) 344.2 nm to 187nm. Jumping was observed on surfaces S1-S4 but not on S5 and S6. The surface with the highest advancing contact angle was S4 (Figure 1). (Adapted from [7] Figure 1, with permissions from ACS).
The four species, Neotibicen pruinosus, N. tibicen, Megatibicen dorsatus, and Magicicada septendecim were studied and results concluded that out of the four species, three of them were determined as superhydrophobic and experience droplet jumping behaviors, while one of them (Magicicada septendecim) was deemed only hydrophobic and experienced no jumping behaviors (Figure 2). The three species, Megatibicen dorsatus, N. tibicen, and the Neotibicen pruinosus demonstrated minimum droplet sizes of ≈ 7.1 ± 0.5, 4.5 ± 0.8, and 2.3 ± 0.9 μm and contact angles of 155°, 160°, and 163°.
The contact angle for Magicicada septendecim was only 115° which explains why no jumping behavior was observed. Wettability, contact angles, and droplet jumping concluded no correlation to the cicada’s habitat or life cycle; however, the structures of the superhydrophobic wings can be used to further enhance antifogging properties in the industrial field.
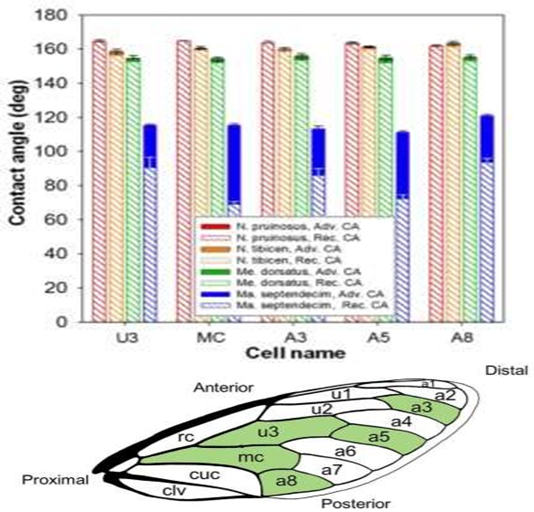
Figure 2: Each of the four cicada species Neotibicen pruinosus, N. tibicen, Megatibicen dorsatus, and Magicicada septendecim and there corresponding advancing and receding contact angles from multiple parts of the wings: U3, MC, A3, A5, A8. Adapted from [8] (Figures 2 and 8), with permissions from ACS.
To understand more about the antifogging multifunctionality and the super hydrophobic nature that cicadas possess, cicada wings for both species Neotibicen pruinosus (superhydrophobic) and Magicicada cassinii (hydrophobic) were examined in a paper publish in 2020 [9]. Cicadas contain numerous nanostructures that coat the surface of their wings which are composed of fatty acids and saturated hydrocarbons. To understand if the leading cause of antifogging behaviors is due to the topological make-up of the cicada wings, microwave-assisted extraction (MAE) was performed. Due to the fatty acids having a considerably low surface energy, droplet jumping occurs and advancing contact angles were measured over multiple periods of extraction. The untreated wings for both N. pruinosus and M. cassinii produced contact angles of about ≈155° and ≈100° which supports the claim that N. pruinosus contains superhydrophobic behavior and M. cassinii contains only hydrophobic behavior (Figure 3).
Since droplet size was not the main focus of the experiment, no measurement was taken. During the first 5 minutes of undergoing MAE contact angles diminished; however, after about 10 minutes the contact angle began to gradually increase its superhydrophobic nature. The data presents the correlation between topology and antifogging properties which could benefit the replication of nanostructures in the industrial world.
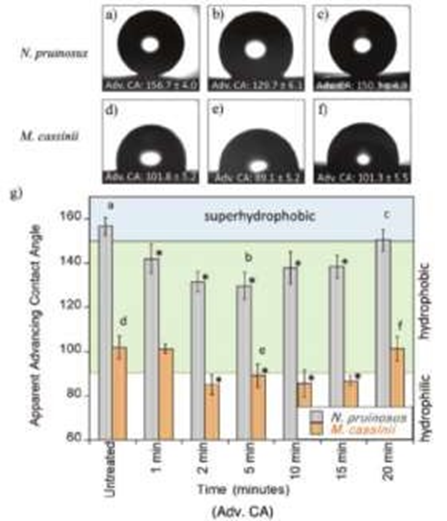
Figure 3: Advancing contact angles for both N. pruinosus (a-c) and M. cassinii (d-f) wing surfaces [9]. Contact angles are listed for untreated wings a,d), MAE treated after 5 min b,c), and MAE treated after 20 min c,f). The letters a-f correspond to the images above the graph (Figure 3).
Multiple different nanostructures have been engineered to replicate those similar to the structure of cicada wings, however, fabrication of a different technique with nanohairs could be more beneficial to producing antifogging effects that could withstand harsh climates [10]. To test antifogging effects at subzero temperatures ZnO nanohairs were planted on micropillars via low- temperature crystal growth. In humid climates these crystals promote superhydrophobicity and coalesced droplets which reduce the amount of droplet adhesion and increase spontaneous jumping (Figure 4). In subzero temperatures (-10°C) the nanohairs delay the icing time for a longer period. Strong water repulsion and delayed freezing allow for water droplets to jump up before they have time to freeze, making antifogging abilities more promising. Seven different nanohair structures were tested, all revealing to be hydrophobic except one which was determined to be superhydrophobic based on its contact angle of ≈152°. These findings determine that micro-nanostructures can produce antifogging applications specific to cold or humid environments.

Figure 4: Microscopic images of droplets on surfaces A-G. Droplets of 7 μL are placed on the surface as reference [10]. The contact angle was measured on a surface of -10°C for seven different structures: A) ≈152°, B) ≈148°, C) ≈145°, D) ≈144°, E) ≈149°, F) ≈138°, and G) ≈135°. There corresponding delay times for freezing are listed in the right hand column (Figure 2).
3. Antifogging by Hydrophilic Nanostructured Surfaces
Tests have concluded that many hydrophobic or superhydrophobic surfaces result in antifogging properties producing contact angles > 90° and droplet jumping sizes of roughly ≈5 μm in diameter. By taking another approach and focusing on the hydrophilic character of a surface, contact angles < 90° the determination of antifogging characteristics can also be achieved [5]. Wettability is a major factor to determine the antifogging property of hydrophilic surfaces. In doing so, a quartz slide is etched by ion reactive etching and induced with a thin dewetting silver mask to form the nanopillar structures [11]. Dewetting is the concept of fluid retraction from a non-wettable surface. The silver was then removed leaving behind a foundation of nanostructures. A 5 mL droplet was placed on the surface to test the wettability factor. A contact angle of 20° resulted from the hydrophilic surface. The smaller the contact angle the more superhydrophilic, and thus better hydrophilic antifogging results. Superhydrophilic surfaces portray antifogging behaviors due to their ability to spread water on the surface instantly avoiding the condensation of water droplets on the surface unlike superhydrophobic surfaces. The main difference between the two surfaces is that hydrophobic surfaces demonstrate droplet jumping behaviors whereas hydrophilic surfaces do not. A test was also conducted to determine how quickly the water droplet (5 mL) could disperse amongst the quartz surface (Figure 5). With the quarts slide cooled to about -5°C and immediately placed in humid conditions the water droplet cleared in about ≈0.5 seconds to completely spread amongst the surface. By manipulating a surface to create nanostructures, this hydrophilic method could be utilized to help improve antifogging performances in the optical field.
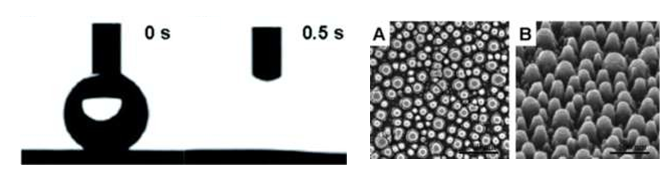
Figure 5: Digital image of 5 mL droplet producing hydrophilic effects after being placed in -5°C refrigerator and immediately placed in a humid setting [11]. Droplet took ≈ 5 seconds to completely disperse on the glass surface. SEM images of silver mask before (A) and after (B) removal at a 45° angle (Figure 5 and Figure 3).
Methods such as lithography, chemical etching, and plasma-based etching have been used to replicate many nanostructures similar to those found in nature to induce antifogging properties in the optical and industrial field. To enhance the antifogging capacity of a glass surface, a non- lithographic method is fabricated without requiring any metallic dewetting to create a hollowed nanopillar design [12]. With the use of plasma etching, the removal of surface material via plasma, a sacrificial SiO2 layer is introduced to the surface to display hydrophilic behaviors. Through tests such as exhalation and water spraying the wettability and contact angles were measured (Figure 6).
The contact angle was measured on both untreated glass as well as the etch glass. Results concluded that the etched glass dropped the contact angle significantly from 35° to below 10° deeming the glass superhydrophilic. Breath test displayed droplets of ranging sizes from 2–7.3 μm in diameter that were dispersed into a thin film over the glass resulting in strong antifogging character. The method of fabrication for this glass resulted in a simpler, more effective enhancement in antifogging production and contact angle decline.
Figure 6: (a) Top view of an SEM image of the hollowed nanopillars [12]. (b) Optical images of bare untreated glass (left), SiO2 layer with 250 nm thickness (middle), and SiO2 with 500 nm thickness (right) after water spray test. (c) Observation of dental mirror after exhalation test with half bare glass (left) and the other treated with 250 nm SiO2 (right). (d) Optical image of the red rectangle box from the same mirror in (c) displaying the water droplets (Figure 3 and Figure 7).
4. Conclusion
Nanopillars have been manufactured to enhance the antifogging capability of a surface. Surfaces that displayed a ultrahydrophobic manner revealed a droplet jumping characteristic which caused the water droplets on the surface to spontaneously “jump” leaving behind a fogless surface. Those surfaces that contained hydrophilic properties however did not depict jumping behavior, instead these surfaces experience a water nucleation behavior known as wettability. Wettability allowed for the droplets to not jump but coalesce and disperse amongst the surface into a thin film reflecting antifogging behavior. With the knowledge to create long lasting antifogging effects from nanopillar manipulation, these surfaces can be beneficial for the optical, biochemical, and biophysical fields. However, although thousands of papers have been published on superhydrophobic and superhydrophilic properties of nanopillared surfaces, only a few papers in this review has shown their antifogging properties. For this reason, this short review provides only a brief introduction, other than a comprehensive review on this concept. Although it appears both superhydrophobic and superhydrophilic nanopillared surfaces are naturally associated with their antifogging property, more studies are needed in order to prove it and to understand how nanopillars affect the antifogging properties of a surface and how to optimize the effect.
References
- Canet, Silvia, Name *. Anti-Fog Coating: The Mechanism and Applications. Adv. Nanotech. S.L., (2019).
- Sason E, Kolitz-Domb M, Chill JH, et al. Engineering of Durable Antifog Thin Coatings on Plastic Films by UV-Curing of Proteinoid Prepolymers with PEG-Diacrylate Monomers. ACS Omega 4 (2019): 9352-9360.
- Xu B, Liu W, Yuan J, et al. Synthesis, Characterization, and Antifogging Application of Polymer/Al2O2 Nanocomposite Hydrogels with High Strength and Self-Healing Capacity. Polymers 10 (2018):1362.
- Piromchai P, Kasemsiri P, Thanaviratananich S. Alternative Agents to Prevent Fogging in Head and Neck Endoscopy. Clin. Med. Insights Ear Nose Throat 4 (2011): 1-4.
- Law K.-Y. Definitions for Hydrophilicity, Hydrophobicity, and Superhydrophobicity: Getting the Basics Right. J. Phys. Chem. Lett 5 (2014): 686-688.
- Zahir T, Pesek J, Franke S, et al. Model-Driven Controlled Alteration of Nanopillar Cap Architecture Reveals Its Effects on Bactericidal Activity. Microorganisms 8 (2020): 186.
- Mulroe MD, Srijanto BR, Ahmadi SF, et al. Tuning Superhydrophobic Nanostructures To Enhance Jumping-Droplet Condensation. ACS Nano 11 (2017): 8499-8510.
- Oh J, Dana CE, Hong S, et al. Exploring the Role of Habitat on the Wettability of Cicada Wings, ACS Appl. Mater. Interfaces 9 (2017): 27173-27184.
- Roman-Kustas J, Hoffman JB, Reed JH, et al. Molecular and Topographical Organization: Influence on Cicada Wing Wettability and Bactericidal Properties. Adv. Mater. Interfaces 7 (2020): 2000112.
- Shi W, Wang L, Guo Z, et al. Excellent Anti-Icing Abilities of Optimal Micropillar Arrays with Nanohairs. Adv. Mater. Interf 2 (2015): 1500352.
- Xu H, Liu L, Wu F, et al. Fabrication of biomimetic patterns for high transmission and antifogging property, RSC Adv 5 (2015): 28014-28018.
- Yoon SM, Lee YA, Park KC, et al. Subwavelength Hollow- Nanopillared Glass with Gradient Refractive Index for Ultralow Diffuse Reflectance and Antifogging. ACS Appl. Mater. Interfaces 12 (2020): 6234-6242.
