Why Only Manage POTS?!? POTS MAY BE CURED!! A Review
Article Information
DePace NL2, Weintraub MI2, Munoz, R2, Kaczmarski K2, Manno R2, Ahmed G2, Colombo J1,2*
1Chief Technology Officer & Senior Medical Director, Physio PS, Inc., Atlanta, GA 30339, USA
2 Franklin Cardiovascular, Sicklerville, NJ 08081, USA
*Corresponding author: Colombo J, Chief Technology Officer & Senior Medical Director at Physio Ps.
Received: 25 April 2024; Accepted: 06 May 2024; Published: 26 June 2024
Citation: DePace NL, Weintraub MI, Munoz, R, Kaczmarski K, Manno R, Ahmed G, Colombo J. Why Only Manage POTS?!? POTS MAY BE CURED!! A Review. Cardiology and Cardiovascular Medicine. 8 (2024): 247-266.
View / Download Pdf Share at FacebookAbstract
Postural Orthostatic Tachycardia Syndrome (POTS) is but a type of Dysautonomia. The Dysautonomia describes the other symptoms that are usually associated with POTS, but not due to POTS. Through Dysautonomia, POTS is thereby also a symptom of many other disorders, including Long- COVID, PTSD, Anxiety, EDS/Hypermobility, and many more. The ‘O’ in POTS is the autonomic dysfunction. The ‘T’ in POTS is perceived as a symptom; however, it is truly a compensatory mechanism. A primary problem with POTS and its diagnosis is that in ⅓ to ½ of the POTS patient population, depending on history, POTS is comorbid with Vasovagal Syncope (VVS). Patients do not faint from POTS. POTS is compensated. Patients do faint from VVS. The problem is the fact that the Orthostatic dysfunction in POTS is a Sympathetic dysfunction and the Vagal component in VVS is a Parasympathetic dysfunction. Most current autonomic monitoring techniques are not able to differentiate Sympathetic from Parasympathetic function without assumption or approximation. Unfortunately, the accepted assumptions and approximations are not valid in younger patients which characterize most POTS patients.
Herein, we introduce an autonomic monitoring technique, P&S Monitoring, that is able to mathematically independently and simultaneously measure and differentiate Parasympathetic from Sympathetic activity, as well as alpha Adrenergic (α-Sympathetic) from beta-Adrenergic (β-sympathetic) activity. With P&S Monitoring POTS and VVS are able to be differentiated, identified even if comorbid, and treated in a classical or standard manner and both dysfunctions relieved concurrently; thereby helping to restore patient’s quality of life and productivity.
Keywords
Postural Orthostatic Tachycardia Syndrome (POTS); Dysautonomia; Vasovagal Syncope
Article Details
Introduction:
Postural Orthostatic Tachycardia Syndrome (POTS) is but a type of Dysautonomia. The Dysautonomia describes the other symptoms that are usually associated with POTS, but not due to POTS. Through Dysautonomia, POTS is thereby also a symptom of many other disorders, including Long- COVID, PTSD, Anxiety, EDS/Hypermobility, and many more [1,2,3,4,5,6]. The ‘O’ in POTS is the autonomic dysfunction. The ‘T’ in POTS is perceived as a symptom; however, it is truly a compensatory mechanism. In POTS patients, Tachycardia is the heart’s attempt to properly profuse the brain when the Orthostatic dysfunction causes blood to pool in the lower extremities. Underlying the Orthostatic dysfunction is a Dysautonomia known as Sympathetic Withdrawal (SW). SW is an abnormal alpha-Adrenergic response characterized by a decrease in α-Sympathetic activity with upright-posture or Stand. This leads to improper vasoconstriction and thereby blood pooling in the lower extremities [7]. In the insert, below, the red portion of the curve is the average Stand Sympathetic response as compared with a supine or seated state (see Appendix 1). SW, as displayed in the insert, below, top graph, is represented by the red portion of the curve which should extend into the gray (normal) area. LFa is the engineering term used for the Sympathetic measurement.
The (blue) Parasympathetic response in the insert below, top graph, (a decrease from seated to standing) is normal.
An example of a Sympathetic Withdrawal response (SW, an abnormal alpha-Adrenergic response) underlying Orthostatic Dysfunction. LFa is the engineering term used for the Sympathetic measurement. The Sympathetic, red portion of the curve, would normally extend into the gray (normal) area. The (blue) Parasympathetic response, a decrease from seated to standing, is normal.

An example of a Vasovagal Syncope (VVS) response, which is a combination of two abnormal responses: (1) an excessive Parasympathetic response (PE, an abnormal Vagal or Cholinergic response), see the (abnormal) increase in the blue portion of the curve, and (2) a β-Sympathetic dysfunction, known as Sympathetic Excess (SE, a beta-Adrenergic or Hyperadrenergic response) see the (abnormal) increase in the red portion of the curve which upon Stand presents as (pre-clinical) Syncope.
A primary problem with POTS and its diagnosis is that in ? to ½ of the POTS patient population, depending on history, POTS is comorbid with Vasovagal Syncope (VVS, see insert, above, bottom graph) . Patients do not faint from POTS. POTS is compensated. Patients do faint from VVS. Specifically, this problem is the fact that the Orthostatic dysfunction is an α-Sympathetic dysfunction and VVS is a combination of a Vagal dysfunction, which is an excessive Parasympathetic response (PE, a Vagal or Cholinergic response) [8] (see the increase in the blue portion of the curve in the insert above, bottom graph), and a β-Sympathetic dysfunction, known as Sympathetic Excess (SE, a beta-Adrenergic or Hyperadrenergic response) which upon Stand presents as (pre-clinical) Syncope [9]. SE, when co-morbid with PE, is always secondary to PE and is a compensatory mechanism, not a symptom. PE with SE is displayed in the insert above, bottom graph. PE is represented by the blue, Parasympathetic, portion of the curve which should decrease normally, instead it increases, opposite of normal.
SE, when co-morbid with PE, is always secondary to PE and is a compensatory mechanism, not a symptom. SE, as displayed in the insert above, bottom graph, is represented by the red, Sympathetic, portion of the curve which should end in the gray (normal) area. Often PE and SE (VVS) masks SW, but decreases in BP, or excessive increases in HR, from sitting to standing identify the presents if SW (underlying the Orthostatic dysfunction, POTS, Orthostatic Intolerance or Orthostatic Hypotension). Note, POTS and Orthostatic Intolerance may also be comorbid. [9]
Most current autonomic monitoring techniques are not able to differentiate Sympathetic from Parasympathetic function without assumption or approximation (see Appendix 2). Unfortunately, the accepted assumptions and approximations are not valid in younger patients which characterize most POTS patients. Note: one clue to this dilemma is the fact that POTS alone does not cause patients to faint. That's the purpose of the Tachycardia, to prevent fainting. Of course, if the Tachycardia is heavily medicated it's possible for POTS patients to faint, but usually the body finds a way to defeat the therapy so that the brain may be properly perfused. In general, fainting is due to VVS. Herein, we introduce an autonomic monitoring technique, P&S Monitoring, that is able to mathematically independently and simultaneously measure and differentiate Parasympathetic from Sympathetic activity, as well as alpha Adrenergic (α- Sympathetic) from beta-Adrenergic (β-Sympathetic) activity. With P&S Monitoring POTS and VVS are able to be differentiated, identified (even if comorbid), and treated in a classical or standard manner and both dysfunctions relieved concurrently; thereby helping to restore patient’s quality of life and productivity.
Methods
A database of 8128 consecutive, serial Dysautonomia patients (57.3% Female; average age of 56.9 years; age range: 12 to 100 years), including 4488 (55.2%) POTS or pre-clinical POTS were followed over a six year period, in three large suburban practices (two cardiology practices in the Philadelphia, PA and Memphis, TN area and one Neurology practice in the New York City area) drawing from both urban and suburban populations.
Parasympathetic and Sympathetic (P&S) function was assessed noninvasively using the Physio PS, Inc. (Atlanta, GA) software (ANX 4.0 P&S Monitor, see insert below). The ANX 4.0 is a FDA cleared (1995) Software as a Medical Device which computes simultaneous, independent measures of P&S activity based on continuous, time-frequency analysis of Heart Rate Variability(HRV) with concurrent, continuous, time-frequency analysis of respiratory activity (RA) [9] (See Appendix 2).

A photo of the ANX-4.o P&S Monitoring System.
The test is a 15.5 minute clinical study which collects EKG and Respiratory Activity (RA) and BP from a patient while they perform breathing challenges and a quick postural change followed by a period of quiet upright posture. The patient wears three EKG electrodes and a BP cuff (see insert, below).

This is an observational study. Patient testing and clinical outcomes measures were collected as an authorized part of the subjects’ care and treatment given their clinical history. All data were handled according to HIPPA regulations. All patients were notified of their involvement in a large population study as part of their clinical routine. No patient requested to be excluded. Data were analyzed, statistically, with SPSS v 22.0, with the null hypothesis indicating significance at p ≤ 0.05.
P&S Monitoring
The clinical study employed to determine P&S activity includes four well-known autonomic challenges, the Ewing Challenges [10,11,12], separated by resting baseline periods. These six periods are labeled as: A) Resting Baseline, B) Deep Breathing, C) Valsalva baseline, D) Valsalva maneuvers, E) Stand baseline, and F) Stand (postural change)* (see Appendix 1 for more details). BPs were taken during each phase of the clinical study. The method includes five minutes of rest, in a relaxed seated position with sturdy back support, and then a quick postural change to standing, followed by 5 minutes of quiet standing. As documented in Bloomfield, et al. [13], this active standing maneuver results in P&S responses that are “nearly identical to the changes in autonomic balance that occurs during the first five minutes of passive tilt.” Their results suggest “nearly identical” is approximately 96.3%. These results were repeated, using hemodynamic measures as biomarkers [13]
P&S Monitoring also facilitates reading P&S responses in the presence of arrhythmia [9,14]. Given that patients with Depression-like symptoms may present with palpitations and possible arrhythmia (typically benign, e.g., sinus arrhythmia), arrhythmia patients were included in this analysis.
*Note: Resting Baseline ('A') is differentiated from the other baselines ("C" & "E"), since the other baselines are (purposely) not long enough to establish a stable resting baseline. They are just long enough to permit a return to baseline before the next challenge, without the total test being longer than necessary. This removes the redundancy of several Resting Baselines.
HR Variability (HRV) analyses alone are mixed measures of P&S activity (see Appendix 2 for more details). For example, spectral HRV analyses result in a Low Frequency (LF) and a High Frequency (HF) terms [15,16]. LF is a mix of both P&S activity (see Figure 1), unless the subject’s breathing rate is greater than about 15 breaths per minute [9,15,16]. HF is also a broad- band term [9,15,16] (see Appendix 2, Figure 1), more than twice as broad as the known Parasympathetic frequency range [17,18,19,20,21,22]. Therefore, even if the subject’s breathing rate is greater than approximately 15 breathes/min, the HF term is mixed with noise (other information that is not related to Parasympathetic activity), thereby inflating the resulting Parasympathetic measurement. Because HRV terms are based on a measure of cardiac function only and the heart is controlled by both P&S branches, HR terms are mixed measured of both P&S activity and more. As a result, HRV terms, including LF & HF, require assumption and approximation to specify P&S activity [9] (see Appendix 2).
In order to eliminate the need for assumption and approximation required by LF & HF, mathematically independent spectral analyses (i.e., analyses of a totally different measurement involving the autonomic nervous system), in this case Respiratory Activity (RA), is added to spectral analyses of HRV [9] (see Appendix 2). This second independent P&S measure of RA satisfies the algebraic requirement for a system with two independent components, fully characterizing the system, eliminating the need for assumption and approximation when differentiating Parasympathetic from Sympathetic activity [17,18,19,20,21,22] (see Appendix 2). There is still another major assumption that remains that centers on the analysis protocol itself.
The standard for autonomic spectral analysis is a Fourier Transform [15,16]. The assumptions and approximations forced by this method are eliminated with the application of Wavelet Transforms [9] (see Appendix 2). Eliminating these additional assumptions and approximations in the analysis method also enables a significantly shorter data collection time to compute P&S activity. (See Appendix 2). This enables autonomic transients and the dynamic activity of P&S interactions to be captured and analyzed. The resulting P&S terms are Respiratory Frequency area (RFa) and Low Frequency area (LFa), respectively, and Sympathovagal Balance (SB = LFa/RFa) is computed as a true ratio of independent parameters [9]. See the differences between LF & HF and LFa & RFa in Figure 1 [9] (also see Appendix 2).

Therapy Options
There are three therapy options offered to patients: Pharmaceutical, non-Pharmaceutical, or a combination of the two. Many patients present after many years of many other physicians and providers prescribing more and many medications. More is not better in P&S treatment, for it typically leads to more symptoms and therefore more P&S dysfunction. For example, antidepressants may include anti-cholinergic effects, but only at the lowest doses do they do so without additional, and typically unwanted, autonomic effects. At the standard, higher doses, antidepressants are known to cause other symptoms, such as Tardive Dyskinesia [23,24]. Further, since many antidepressants are not offered at low enough doses, the options are limited. When used off-label, at the lowest possible doses only, they may then be helpful without causing additional symptoms. The pharmaceutical options are homeostatic doses and have been found to be interchangeable with the supplement and lifestyle modifications that are also recommended. A major difficulty in treatment is the sensitivities that patients have developed, not necessarily to the pharmaceutical agents themselves, but to the fillers and capsules/coatings used to convey the agent. This is another reason for providing non-pharmaceutical options.
Pharmaceutical Therapy Options
First-line pharmaceutical therapy options are prescribed based on patient history [25]. In general, they include the following. For Sympathetic Withdrawal (αSW) low-dose, oral vasoactive therapy is prescribed; specifically, 2.5 mg Midodrine titrated as needed from qd in the morning to tid. Midodrine is an α-1 Sympathetic agonist or stimulant. Midodrine is contraindicated for patients with supine hypertension (diagnosed as an abnormal increase in BP from upright posture to supine). Midodrine typically relieves the β-Sympathetic symptoms organically, after relieving any Orthostatic dysfunction caused by the SW. In other words, vasoactive therapy helps to relieve blood pooling in the lower extremities, moving the blood to the abdomen, helping the heart pump blood to the brain, thereby restoring proper brain perfusion. This in turn prevents the need for adrenaline storms, thereby relieving the β-Sympathetic symptoms, like tachycardia, stress, and inflammation. In terms of the car model (see Appendix 3) Checking to “see if gas is getting to the engine” (αSW) and treating to ensure it does (e.g., unclogging the fuel filter or fuel line), relieves βSE organically (reducing the amount of acceleration needed), ultimately relieves the β-Sympathetic symptoms

For Parasympathetic Excess (PE), Nortriptyline 10.0 mg, qd, 12 hours before waking. Nortriptyline, at first will help to pattern the patient’s sleep. Since it has a 12 hours window of action, it must be dosed 12 hours before waking, but before 8 pm to prevent interfering with the normal Melatonin to Serotonin transition that helps to induce sleep in the evening between 8 pm and 10 pm. This helps the patient begin to feel better as the anti-cholinergic effect relieves the PE and its constellation of symptoms. For (stand) Sympathetic Excess (βSE), therapy depends on the differential. If SE is demonstrated with PE, indicating (pre-clinical) Vasovagal Syncope [26], then PE therapy is followed and SE is considered secondary with a beta-blocker (e.g., Propranolol, 10 mg prn up to tid) prescribed only if the Tachycardia from POTS or pre-clinical POTS presents frequently. If SE is demonstrated with a drop in HR from resting to stand indicating (pre- clinical) Neurogenic Syncope, volume building and often low-dose Midodrine helps to treat the stand SE. For autonomically mediated arrhythmia, the rest of the P&S test helps to determine whether the arrhythmia is due to P or S abnormality or not autonomic (e.g., cardiogenic) [27] to be treated accordingly.
Non-Pharmaceutical Therapy Options
See the Autonomic Dysfunction Therapy Algorithm (Appendix 4) [adapted from 28]. In general, psychosocial stress reduction is recommended with history-specific antioxidant and supplement recommendations to reduce oxidative stress and improve cerebral and coronary blood flow. Non-Pharmaceutical therapy options were offered if patients were intolerant or unresponsive to the pharmaceutical options. For Sympathetic Withdrawal (αSW), 600 mg tid, Alpha-Lipoic Acid (a potent antioxidant selective for nerves, including brain cells), titrated as needed and tolerated, [29] was recommended. Alpha-Lipoic Acid was a precursor to Midodrine to relieve Orthostatic dysfunction which is caused by αSW, as well as to slow and possibly reverse neuralgia and neuropathy [28]. Alpha-Lipoic Acid does this by not only entering the cell, but by entering the Mitochondria as well and helping to heal them from within; thereby “energizing” nerve cells, including brain cells. In addition, compression garments are recommended when a patient is to be standing for periods of time during the day.
Similarly, checking to see if the brakes are “on” (PE) and treating to “release the brakes” (relieve PE) may resolve all of the above beta-Sympathetic (βSE) abnormalities. Relieving PE, relieves βSE organically (releasing the brakes reducing the amount of over-acceleration needed without the need for any other action), which ultimately relieves the β-Sympathetic symptoms. Low- and-slow exercise is the primary non-pharmaceutical remedy for PE and is recommended for six months or more depending on history [adapted from 30]. The maximum exercise is walking at no more than two miles per hour for up to 40 minutes contiguous as tolerated per day [28]. The minimum exercise on days of extreme fatigue is a supine exercise as depicted in the insert, below, left, with kicking of the legs at two miles per hour for up to 40 minutes contiguous, per day as tolerated, without inducing more fatigue.

If the patient reports significant sleep difficulties, head-down posture (see insert, right) is recommended. As depicted a 15° head-down posture, supine (e.g., by rearranging couch cushions), for no more than 20 minutes at around two hours before bed-time for sleep, and up to three times per day, as needed to relieve brain-fog, anxiety, tension headache, migraine, etc.. Head-down posture may also help to relieve resting or stand Sympathetic Excess (βSE), as long as there is no (additional) head pressure.
With POTS, to address the Tachycardia and help maintain heart health, especially if patients are prescribed β-blockers, Co-Enzyme Q10 (a potent antioxidant selective for the heart), 200 mg qd, is recommended to “energize” the heart by healing the Mitochondria in the heart muscle cells. Autonomically induced arrhythmia (Sinus Arrhythmia) was treated based on underlying etiology (e.g., PE or βSE).
In either case (SW or PE or both), proper daily hydration is required. This includes a minimum of 64 oz of water taken throughout the day with an additional 5.5 g of electrolytes dosed every two hours throughout the day. The electrolytes may be sodium from salt (1 tablespoon, including flavored salts) if the patient’s BP is normal to low, less than approximately 160/90 mmHg, history dependent. If BP is high, then the electrolytes may be potassium from (bananas or prunes, for example). For days with more activity (loosing water and electrolytes through sweat for example) or in women’s days of their period, more water is required, up to 96 oz with 8.25 g of electrolytes (e.g., 1½ tablespoons of salt).
Of course, the process of normalizing and balancing the P&S nervous systems (both at rest and dynamically) takes time. It is not a quick fix. In fact, it may take up to 24 months, depending on the patient’s history, including daily stress levels . To use another analogy: the P&S Nervous systems are like a swinging pendulum, if to correct it you hit it hard or fast, you knock the pendulum off its hinge and create more problems. Therapy must be slow, gentle, easy nudges over time, or for another analogy, it is like stopping a bad habit and establishing and maintaining a good habit. This also may take several months.
Typically, patients may not feel significantly different during the first two to three months after the introduction of treatment. This is because the P&S systems control the organs, and the organs control the symptoms. As it takes time for the P&S systems to adapt, a change in symptoms is typically delayed. A follow-up P&S Monitoring test at this time is thought of as an “encouragement visit” to demonstrate that the numbers are moving in the right direction and offers a chance to titrate more to the individual patient. Then six to nine months after introduction of treatment, a P&S Monitoring test offers the opportunity to consider weaning patients from therapy if there is not a chronic condition (e.g., Diabetes or Ehlers-Danlos Syndrome/Hypermobility) that continues to work to imbalance the nervous systems. The goal is to have patients weaned from pharmaceutical treatments by the 15 to 24 month depending on history and carry forward on lower dose supplements and lifestyle therapies, as needed. It also helps to encourage the patient by explainingthat the reason why the duration of the visits are long is because numerous body systems are being assessed all in one visit, in some cases as many 11; (1) Central and (2) Peripheral nervous systems, (3) GI system, the (4) Heart and (5) Vascular systems, (6) Women’s Health, (7) Urinary system, (8) Pulmonary system, (9) Endocrine system (hormones, diabetes), (10) Immune system (auto-immunity), and (11) Mental and Behavioral Health.
Diagnosing POTS
Many “experts” will state that POTS is not real. That may be because they cannot measure it effectively to diagnose it consistently. POTS is often co-morbid with Vasovagal Syncope (VVS). Many of the same experts claim that POTS and VVS cannot be co-morbid. This is because, for the most part, they have never been able to measure both together; therefore, it must not exist. Yet the two dysautonomias are mediated by two different branches of the autonomic nervous system; three, in fact: 1) Sympathetic Withdrawal (αSW, an alpha-adrenergic response) underlies Orthostatic dysfunction (the “O” in POTS), 2) Parasympathetic Excess (PE; aka., Vagal, referring to Vagus Nerve, is another name for Parasympathetic, a cholinergic response); and 3) Sympathetic Excess (βSE, a beta-adrenergic response) underlies Syncope, it is the result of the “adrenaline storm” released by the brain to increase cerebral perfusion, compensating for either or both Orthostatic dysfunction (i.e., POTS) or VVS. The good news is that any or ALL three of these autonomic dysfunction mechanisms leading to dysautonomias are treatable, simultaneously. Furthermore, for many patients they may be relieved and the patient returned to a normal lifestyle, quality of life and productivity.

Abnormal Trends Plot of a POTS Patient
Here is data (see the Trends plot, above) from a sample POTS patient with VVS; a female in her early twenties. The P&S responses (blue & red curves, respectively) to the standard P&S test are within normal limits for Resting Baseline (‘A’), the Parasympathetic challenge of Deep Breathing (‘B’), a return to baseline (‘C’), the Sympathetic challenge of short Valsalva maneuvers (‘D’), and a return to baseline (‘E’). However, the Stand, or postural change, challenge (‘F’) is not normal. There is too much activity after the initial (red) Sympathetic peak that is the Gravitational Reflex (compare with a normal Trends Plots, insert, below). The stand phase (‘F’) from the patient starts normally with a proper Sympathetic response to the gravitational challenge during the first 15 seconds or so of Stand. However, the rest of the Stand response is filled with all sorts of P&S activity which is abnormal indicating the struggle of the P&S to maintain an upright posture (including proper cerebral perfusion). Normally as shown in the Normal Trends Plot, the quiet-Stand portion of the study (in ‘F’) resembles the initial resting baseline portion of the study (‘A’). In fact, in this patient, it takes a relatively similar amount of P&S energy to remain standing as it does to support the stress simulated by the Valsalva challenge. Physiologically, that makes no sense.

Normal Trends Plot
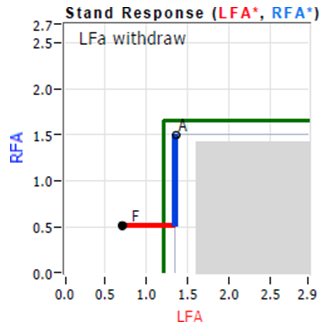
The excessive beta-Sympathetic (red) activity during the Stand phase (after the first 15 seconds, as seen in the abnormal Trends plot) indicates a (β)SE. The excess Parasympathetic (blue) activity in the abnormal Trends plot is confirmed by the abnormal Stand Response graph, below, left). Normally during Stand (see “Normal Stand Response Plot,” insert, below, right), first the Parasympathetics (the blue portion of the Stand response curve) decreases from point ‘A’ (the resting Baseline response) and then the alpha- Sympathetics should increase to the point ‘F’ (the Stand response), ending in the middle of the gray (normal) area. Since the beta-Sympathetics normally remain quiet during the quiet-Stand portion of the Stand challenge, the alpha-Sympathetic response dominates. This is much like the brakes and accelerator of a car (see Appendix 3). To go, first you take your foot off the brake pedal, then you apply your foot to the accelerator. In cases of PE (VVS, see “Abnormal Stand Response Plot,” insert, left), it is like the “car” is driven with the foot still on the brakes, forcing excess acceleration and undue wear and stress on the car and the brakes, just like the human body.

This “over-acceleration” is exacerbated by an “insufficient amount of gas being delivered to the engine.” This is like the αSW causing blood to pool in the lower extremities as opposed to being move to the abdomen by the vasculature being vasoconstricted by an α-Adrenergic, or α-Sympathetic, surge. The combined, averaged, αSW and βSE results in a normal looking Sympathetic (red) response. This is a problem with averaging, some data are lost. That is why the Trends Plot, HR and BP are also recorded to ensure all data are considered. In this case the αSW is masked by the βSE which is apparent from the Abnormal Trends Plot and excessive increases in HR and decreases in BP from Resting Baseline to Stand (shown in a portion of the Summary Table and HR graph below), indicating the presents of αSW which underlies Orthostatic dysfunction. Combined, the PE indicated in the Abnormal Stand Response plot and the βSE indicated in section ‘F’ of the Trends plot documents VVS, and in this case αSW is indicated by both an excessive rise in HR (see HR graph below) and a decrease in BP (see Summary Table, below) indicating Orthostatic Intolerance and pre-clinical POTS.

As mentioned, the masked αSW, in this case POTS and Orthostatic Intolerance or pre-clinical Orthostatic Hypotension, are documented elsewhere in the HR & BP responses to Stand. The ‘O’, the Orthostatic dysfunction, is the clue.
The average or mean HR increases upon standing from 74 to 121 bpm, documenting the Tachycardia associated with POTS (the ‘T’ in POTS). This is also graphically indicated in the HR plot depicting the cardiogram in response to the clinical study showing the jump in HR upon standing (section ‘F’). In this case, a sharp rise in HR after an initial (normal) return to baseline before the onset of Tachycardia. The BP responses drop from Resting Baseline (‘A’) 120/87 to 111/86 mmHg upon standing (‘F’), documenting Orthostatic dysfunction (the ‘O’ in POTS). Note, there may not be as much of a drop as indicated here, but there is typically an abnormal BP response to Stand, even if it is only a weak rise in BP (< a 10% increase over the resting BP). Some or all of these three indications help to diagnose masked SW and add therapy for Orthostatic dysfunction to the plan.
With POTS, marked abnormalities are more often seen early in the morning and these abnormalities may normalize later in the day, especially when patients acclimate with compensatory mechanisms and properly hydrate throughout the day. Therefore, POTS may be intermittent and not consistently documented due to the compensatory mechanisms and proper hydration. As a result, later in the day, POTS patients may not demonstrate significant HR and BP changes. Also, in warm ambient temperatures POTS signs and symptoms may be significant, whereas in a more temperate climate the symptoms may be masked or controlled.
Another reason why so many doctors miss POTS is because the patient seems normal. Of course they do! When does the typical doctor assess a patient? When the patient is resting (sitting or supine). The vast majority of POTS patients are indeed normal at rest, like the one whose Resting Baseline Response Plot is below, where the patient’s resting response is point ‘A’ is right in the middle of the normal gray shaded region. They better be normal at rest. As our typical patient reports, they have had dozens of doctors over decades of time working very hard to make them normal … at rest! The problem is not while the patient is resting. In fact, the P&S nervous systems are rarely at rest, and when the patient is resting the P&S systems are typically most active. The problem is when the patient is active, as in the cases of POTS, while upright, standing, and the heart must fight gravity.

Restore And Improve Quality Of Life
No, it is not acceptable to make the poor quality of life with POTS the new normal; even if the patient cannot remain standing while s/he brushes their teeth in the morning or washes their hair in the shower while standing. For most, POTS came on suddenly, apparently from nowhere.
But you have to go back three to six months before the onset to determine a potential cause (e.g., COVID-19 or trauma) or consider a chronic disease or disorder (e.g., Diabetes or Hypermobility). Often the P&S nervous systems will struggle to maintain normal function even when they are abnormal before they may fail to maintain. There are many possible causes: concussion, psycho-social stress, all sorts of abuses (physical as well as mental), serious viral infections (including COVID-19) and other types of infections (molds, mildews and bacterial), in other words, trauma of some sort (mental or physical), as well as puberty. However, those caused by puberty often do grow out of it.
POTS typically affects young women because they typically have physically (by volume) smaller hearts than men and older women. Sorry, that is just the way it is. (Notice we say physically, this has nothing to do with the fact that most women are bigger hearted than men; emotionally and nurturing. ?). Due to the smaller cardiac stroke volume, when the brain calls for more blood (e.g., with an “Adrenalin Storm”), the smaller heart does not have the mass to leverage more pressure, so it must leverage more rate to increase blood flow to the brain; attempting to restore proper cerebral perfusion. POTS is also, typically, more symptomatic earlier in the day, and may often be missed later in the day.
To help healthcare providers, more information is needed. P&S monitoring provides more information. As shown in the graphs and tables above, P&S monitoring may document if VVS is involve with POTS requiring additional therapy to relieve PE (to get the foot off the brakes, see Appendix 3) so the βSE may normalize organically. Relieving PE will relieve SE, which in turn will relieve tachycardia and other beta-Sympathetically mediated symptoms (e.g., inflammation, pain, anxiety, etc.). In other words, by taking the foot off the brakes, the need to accelerate harder is relieved, which in turn, relieves stresses on the engine and the brakes. P&S monitoring is able to document both at the same time in one single test. POTS may involve (pre-clinical) Orthostatic Intolerance or Orthostatic Hypotension and αSW (possibly masked, if VVS). Therapy may unmask αSW and it may be documented upon follow-up testing to help properly titrate therapy to the individual patient.
Diagnostic Summary
The separate parts of the ANS may be involved and all at the same time (especially in more serious and complex cases):
- ‘O’ = SW, an α-adrenergic dysfunction,
- ‘V’ = PE, a Vagal dysfunction,
- ‘S’ = SE, an β-adrenergic dysfunction.
αSW & PE are associated with the following: lightheadedness, cognitive dysfunction or “brain fog”, and frequent headache or migraine, sleep difficulties, fatigue, exercise intolerance, difficult to control BP, blood glucose, hormone level, or weight, difficult to describe pain syndromes (including CRPS), unexplained arrhythmia (palpitations) or seizure, GI disturbance, temperature dysregulation (both response to heat or cold and sweat responses), and symptoms of depression or anxiety, ADD/ADHD, and sex dysfunction.
The results of amplified β-Sympathetic activity (amplified acceleration, see Appendix 3) may be Anxiety, Inflammation, allergic or histaminergic over-reactions, amplified pain, hypertension or difficult to control BP, tachy-arrhythmia, amplified stress responses, ADD/ADHD, OCD and even high-functioning Autism, and more. All of these are Sympathetic symptoms, including over-activity, and some may be critical in nature. As indicated above, there are two Sympathetic branches in the body: one branch, the α-Sympathetic branch, which primarily controls and coordinates the vasculature outside the thorax (the rib cage); the other branch, the β-Sympathetic branch, which primarily controls and coordinates the heart and lungs (inside the rib cage). The β-Sympathetic symptoms may be secondary to either or both SW (an α-Sympathetic response) or PE. Regardless of the mechanism (SW or PE or both), not enough blood gets to the brain. This results in the β-Sympathetic symptoms due to the β-Sympathetic response attempting to stimulate the heart to pump more, compensating for poor cerebral perfusion (see the extra Sympathetic peaks in the Trends Plot above, section ‘F’, what are called “Syncope Spikes”). If the physician does not think to consider the β-Sympathetic responses as secondary, attempting to treat only the β-Sympathetics or treating them as the primary, may exacerbate the conditions and cause the potentially critical nature of βSE (heart attack or stroke) to become a self-fulfilling prophecy.
Summary
Unfortunately, most autonomic tests only test total autonomic activity and are not able to measure the two autonomic branches independently and simultaneously without assumption and approximation. Even tilt table is often unrevealing because of complications, its inability to differentiate P from S independently and simultaneously without assumptions, approximations, chemicals, or from ignoring symptoms until they are so bad that the assumptions are valid. However, even then as soon as therapy is implemented the assumptions are no longer valid and therefore Tilt-testing is no longer revealing. As an aside, for VVS, the stress and anxiety (Sympathetic stimuli) caused by the tilt table itself temporarily “cures” VVS (balances the Parasympathetics), leaving the physician with no diagnosis to treat and believing that the patient is normal. Further, in many suspected POTS cases, often the real issue is that there is no POTS to begin with. It is VVS and the stress of the tilt-table “cures” the VVS by balancing P&S.
The good news with P&S monitoring is that all three dysautonomias may be documented, thereby are treatable, and all treated simultaneously. Again, however, it is not “quick fix” it may take up to 24 months. Therapy must be low-and-slow. Again, like correcting a pendulum, or essentially breaking one or more “bad habits” and establishing good habits, it is never fast. Therapy may be both pharmaceutical, and for those that have been put on high doses and are desensitized, non- pharmaceutical. Therapies have been developed and published (see summary diagram in Appendix 4: “Autonomic Dysfunction Algorithm”). The main issue is that the heart is made to act as if it is deconditioned by the P&S nervous systems and must be reconditioned. At the same time the lower vasculature must be re-integrated with the heart. Once all of this is done, symptoms are relieved and Quality of Life is restored or improved, until some other clinical event. The only reason for maintenance dosing of any kind, let alone life-long therapy, is if there is some remaining end-organ dysfunction that will continue to pull the P&S systems out of balance. A summary therapy plan for POTS is included below.
- First, suppress any β-1 Sympathetic stimulation with low-dose β- blockers, such as Propranolol or Nadolol. This is typically short-term, to reduce and prevent palpitations and rapid HR, until the nervous system is re-trained to respond normally upon standing. Typically, the patient will self-wean.
- Second, stimulate α-1 Sympathetics with oral vasoactives, fluids, electrolytes, compression garments, and Alpha-Lipoic Acid. The oral vasoactives are also often short- term. All of this is to relieve the brain-fog, cognitive and memory difficulties, lightheadedness, fatigue, etc., associated with POTS.
- Typically, these first two steps are administered simultaneously.
- Third, if blood volume remains an issue (e.g., water and electrolytes are not sufficient), then low-dose volume expanders are recommended (i.e., Florinef or Desmopressin).
- Last is low and slow exercise, if the POTS is not relieved by the second step or VVS is documented. Low-dose anti-cholinergic treatment may also be included to treat PE underlying VVS.
Treating β-Sympathetic issues as primary typically exacerbates the patient’s condition, including making them more difficult to manage, or patients do not tolerate the therapy. Granted mortality risk(s) need to be addressed, such as high resting BP or high Standing HR, Mast Cell over-activation, Pain, etc., but recognize that these may be secondary and typically are relieved with the SW underlying POTS or PE underlying VVS. SW or PE therapy must be “low and slow” with SW often the first and most difficult dysautonomia to fully correct.
Ultimately, POTS is (and all other neurogenic forms of Orthostatic dysfunction are) blood flow issues that are caused by “bad nerves,” P or S dysfunction(s). Without testing for both P&S activity, independently and simultaneously, at rest and in response to challenge, the best that may be offered the patient is a guess and a bad guess at that. This is the reason why the general sentiment towards POTS has moved to a life-long management: 1) to keep it a research project and 2) to sell more drugs. Without a clear, objective, quantitative measure of the Parasympathetic nervous system, and therefore a clear, objective, quantitative measure of the Sympathetic nervous system, patients are being done a dis-service. This is evidenced by the millions of patients taking matters into their own hands and still diligently seeking more information.
Figure 1: A spectral domain comparison of the LFa & RFa method [9] and the LF & HF method [15,16], see the Methods section for abbreviations. The vertical broken line represents the Respiratory frequency over the four second measurement period. The Respiratory frequency is independently computed in the Respiratory Activity spectrum (not shown) and then transferred here, to the HRV spectrum, to locate the RFa (Parasympathetic) spectrum. In this way, the RFa spectrum is based on the breathing rate of the subject. In this example, the Respiratory frequency is 0.125Hz (equivalent to 7.5 breathes per minute). The LF spectrum is represented in dark grey, from 0.04Hz to 0.15Hz [15,16]. The HF spectrum is represented in light grey, from 0.15Hz to 0.40Hz [15,16]. The RFa spectrum, in this example, is from 0.065Hz to 0.185Hz [9]. The RFa is computed from a frequency range centered on the Respiratory frequency (0.125Hz, see above) and moves as the Respiratory frequency moves [17]. The LFa spectrum, in this example, is from 0.04Hz to 0.065Hz. The LFa is computed as the (fixed) LF frequency range (0.04Hz to 0.15Hz) minus the portion of the RFa frequency range that overlaps the LF frequency range (in this example 0.065Hz to 0.15Hz) [17]. LFa, in (beats per minute)2 or bpm2, represents Sympathetic activity and RFa, in bpm2, represents Parasympathetic activity [9,15,17,18,19,20].
APPENDIX 1: P&S Monitoring – More Information to Differentiate Orthostatic Dysfunction
HR and Respiratory Activity (RA) traces for sections (Figure 2) and the complete (Figure 3) study are displayed below.
Figure 2: Sample HR (red) and Respiratory Activity (RA, gray) traces from a young normal subject in response to the four main challenges of the clinical study. The (A) Resting Baseline and (F) quiet Stand phases of the clinical study (top, left graph) often look similar (in both the patients are quiet) with prominent Respiratory Sinus Arrhythmia (RSA) in the HR traces similar to the Respiratory traces, as is normal. The (B) Deep Breathing phase of the clinical study (upper, middle left graph). Notice the exaggeration of RSA due to the paced breathing. The (D) Valsalva Challenge phase of the study (lower, middle left graph), is a 15 second Valsalva maneuver followed by 20 seconds of rest followed by four ten second Valsalva maneuvers with a five second rests in between (three of which are displayed).
The time requirement, as well as the safety factor, is further improved with the implementation of a spectral analysis technique that eliminates the time-frequency compromise. In this case, the Wavelet Transform [9,21,22,30,31,32,33,34,35,36,37,38,39,40]. P&S Monitoring employs the Wavelet Transform, along with the appropriate time and safety considerations (see Appendix).
Figure 3: The HR & RA traces and P&S traces for the complete study from a young adult patient without POTS. As is normal there is little P&S activity at rest (A). The significant blue, Parasympathetic response to (B) Deep Breathing is normal. Followed by a return to baseline (C). The significant red, Sympathetic response to (D) Valsalva challenge is normal. Followed by a return to baseline (E). The first red peak after Stand (F) is the normal Gravitational response; however, the elevated P&S activity during quiet standing is elevated but within normal limits and is associated with the more HR activity during Stand (F) as compared with Resing Baseline (A). The increase in depth of respiration in Stand is normal. It is a result of more freedom of motion in the diaphragm.
Figure 4: The averages of the four clinical study challenge results as depicted in the P&S graph in Figure 3 are plotted against normal regions (gray areas) for those challenges [9]. The P&S averages for all phases are listed in the yellow highlighted portion of the table, flanked by HR (mean HR) and BP. While this patient’ s BP did increase with Stand, it was a weak increase and accounts for the additional P&S activity in the P&S plot of Figure 3 with Stand.
Figure 5: Comparison of two subjects, one without (left) and one with (right) POTS. Normally, immediately upon standing, there is an initial normal peak in HR, known as the Gravitational Reflex (see HR plot on left, section F). Then the standing HR returns to Resting Baseline HR (see HR plot on left, section ‘A’). Stand BP increases about 10% higher than the Resting Baseline BP (in this case from 108/62 to 118/66) without reports of lightheadedness or other symptoms while standing. In this example, the average HR for the subject without POTS changed from 68 bpm at rest (section A) to 79 bpm while standing (section F). From the P&S Trends plot (middle graph, left) there is a peak in Sympathetic (red) activity at the very beginning of the Stand challenge (section F). This peak causes the peak in HR known as the Gravitational reflex and drives the increase in BP. In the subject without POTS case this is a normal average increase as indicated in the bottom, left graph showing a normal increase in Sympathetic activity with Stand (as indicated by the red portion of the curve increasing into the gray area indicating a normal response). The POTS patient demonstrates an initial normal peak in HR (the Gravitational Reflex, see HR plot on right, section F). However, as is common in POTS patients, the standing HR increases, in this case from 97 bpm to 121 bpm. Notice the Sympathetic peak at the very beginning of Stand in this case (see P&S plot, red curve, middle, right) is diminished as compared with that of a subject without POTS. In this POTS case Stand BP decreases from 99/47 to 86/52 exacerbating the Orthostatic dysfunction. She reports significant lightheadedness upon standing and all the while standing. In the subject with POTS case the average Sympathetic response (the red portion of the curve in the bottom, right graph) decreases, indicating Sympathetic Withdrawal (SW, an alpha-Adrenergic response).
Differentiating Orthostatic Dysfunction
Alpha-Sympathetic Withdrawal (αSW, an alpha-Adrenergic reaction, see insert below, the Sympathetic (red) portion of the curve decreases away from the normal (gray) area; LFa is the engineering term for the Sympathetic measure) is the more information that helps to differentiate POTS from Orthostatic Intolerance from Orthostatic
Hypotension, from Parasympathetic Excess (PE, an excessive Cholinergic response) underlying Vasovagal Syncope (VVS, the combination of PE, the Vagal component, and beta-Sympathetic Excess (βSE, a beta-Adrenergic reaction). This additional information when combined with the hemodynamic data collected (HR & BP from the report Table as in Figure 4) provides more information to diagnose and treat these conditions earlier and as needed concurrently.

Alpha-Sympathetic Withdrawal
(Pre-Clinical) POTS αSW as seen in the Stand response plot above with a increase in HR of more than 20 bpm but less than 30 bpm. POTS is an increase of more than 30 bpm, and confirmed by an αSW response. In the case presented to the right it is pre-clinical POTS (or pre-POTS) with an increase in HR from Resting Baseline of 21 bpm.

Mean HR change in an example of pre-clinical POTS.
Orthostatic Intolerance is αSW as seen in the Stand response plot above with any decrease in BP of less than 20 mmHg Systolic or less than 10 mmHg Diastolic from Resting Baseline (A) or an increase in either or both Systolic or Diastolic BP of less than 10% as compared with Resting Baseline. In the case presented to the right, it is a decrease in Systolic BP that presents as Orthostatic Intolerance.

An example of an abnormal BP change in Orthostatic Intolerance.
Orthostatic Hypotension is a 20/10 mmHg drop in BP or more (a three-fold abnormality or more). It does not require αSW to define it, but it may help to confirm it. Also, if Orthostatic Hypotension presents without αSW, it may not be neurogenic, but rather vascular and an venous ultrasound of the lower extremities may be indicated to image the valves.

An example of (pre-clinical) Vasovagal Syncope.
Vasovagal Syncope as discussed above does not involve αSW. It may be the sole reason for lightheadedness and indicated by the Stand response plot as shown in the insert, left, or it may be in combination with Orthostatic dysfunction as presented in the example in the “Diagnosing POTS” section of the manuscript. From the insert, left, the Vagal or PE component is indicated by the increase in Parasympathetic activity with Stand as shown by the vertical, blue, portion of the curve from ‘A’ (Resting Baseline) to ‘F’ (Stand). The Syncope or βSE component is indicated by the excessive increase in Sympathetic activity with Stand as shown by the horizontal, red, portion of the curve from ‘A’ to ‘F’ extending beyond the gray (normal) area.
APPENDIX 2: P&S Monitoring v. ANS Monitoring
We define ANS monitoring as those based on measurements of only cardiac intervals (HR Variability or beat-to-beat BP) [15,16]. The main difference between P&S Monitoring [9] and ANS monitoring is that the latter is based on only one mathematically independent parameter (measurement, the heart), whereas the ANS has two independent components: P&S. With only one mathematically independent measurement, only dependent measures of P&S are possible, forcing assumptions and approximations are required to theorize the measurements of the P&S. P&S Monitoring resolves the algebraic requirements of a system with two independent components by adding a second independent measurement: Respiratory Activity (RA), a measurement of the lungs, an independent system separate from the heart. Here we focus on the spectral analysis method of measurements, but similar arguments are valid for the other methods of ANS monitoring. Spectral and temporal analyses are mathematically commutative.
First, we’ll review spectral analysis, then we’ll discuss the assumptions and approximations. For spectral or frequency analysis (they are the same), consider a piano. Listening to the music from the sound board at the back of the piano is time domain analysis of the music you hear. You hear the rhythms, colors, and beats. However, it is difficult to determine how the music was made – what frequencies were included in the music, and the fingers from which hand struck the keys. This requires spectral domain or frequency domain analysis. For the purposes of analyzing the Autonomic nervous system in general, the left hand would represent the Sympathetic nervous system and the right hand, the Parasympathetic nervous system. Watching the keyboard is like frequency domain analysis. Each note in the music is a different frequency.
Assume that each piano key generates a single frequency (in actuality it does not). The place on the keyboard indicates the specific frequency stimulated and the force with which the key was struck is related to the power or strength of the frequency in the music created. Striking more than one key at a time causes the sound of more than one frequency with varying amplitudes (power). The amplitudes are dependent on how hard the keys were struck. These multiple frequencies may be plotted against their amplitude (see insert, below), and a spectral plot (a spectrum) would be generated representing the music from the chord which was stuck. If, instead of discrete frequencies, the spectrum were continuous, then a curve, as depicted in Figure 1, would result.

An example of a spectrum with discrete frequencies sampled.
This is Fourier Transform analysis. As you may have noticed there is no mention of time. This is the spectrum for a single instant in time, or if a continuous signal from another source that does not change with time (a “stationary” signal) this is the spectral characteristic of that source. However, autonomic signals change with time as well as frequency. This is the basis for the time-frequency “trade-off” and the set of assumptions and approximations that are required to make Fourier Analysis work for P&S Monitoring. Therefore, another type of spectral analysis is needed that is able to analyze spectral and temporal changes simultaneously. One such analysis is Wavelet Transforms [35,36,37].
In fact, there are two sources of assumption and approximation in standard ANS Monitoring with (Fourier) spectral analyses as implicated above and further discussed below. One is a single independent measure of P&S activity (resulting in mixed, mathematically dependent measures) versus two independent measures (resulting in two, mathematically independent, unmixed measures). The other is based on the spectral analysis method: Wavelets v Fourier Transforms.
The differences between spectral analysis of cardiac-interval monitoring (ANS Monitoring) and P&S Monitoring are presented in Figure 1. It is a fundamental principle of mathematics and physics that the area under a spectral curve is the definition of power or tone or activity. In this case, since the HR was measured in beats per minute (bpm) the area under the cure is measured in bpm2. The area under the entire curve is known as total spectral power (TSP, bpm2), both the activity in the Parasympathetics plus the activity in the Sympathetics.
Consider a piano: listening to the music from the sound board at the back of the piano is time domain analysis of the music you hear. You hear the rhythms, colors, and beats. However, it is difficult to determine how the music was made. This requires spectral domain or frequency domain analysis. Watching the keyboard is like frequency domain analysis. Assume that each key generated a single frequency (in actuality it does not). The place on the keyboard indicates the specific frequency stimulated and the force with which the key was struck is related to the power or strength of the frequency in the music created. Striking more than one key at a time causes the sound of more than one frequency with varying amplitudes. The amplitudes are dependent on how hard the keys were struck. These multiple frequencies may be plotted against their amplitude, and a spectral plot (a spectrum) would be generated representing the music from the chords which were stuck, resulting in spectral curve as depicted in Figure 1.
The spectral curve presented in Figure 1 is from a short recording of EKG, from which the cardiogram (HBI signal) was determined and processed through a spectral analyzer. This is the extent of spectral analysis for HRV-alone (the combination of the gray areas in Figure 1). For the purposes of the P&S Monitoring method, the concurrent respiratory activity signal (e.g., from an impedance plethysmography circuit within the EKG recorder) was also captured and processed through a copy of the same spectral analyzer, separate from, but simultaneous with the cardiogram. The method of P&S Monitoring is depicted as the blue and red shaded areas in Figure 1.
The darker grey area delineates the low frequency (LF) region (0.04Hz to 0.15Hz [[15] (p1047, Table 2),[16]). The area under the HR spectral curve within the LF region is known to be a mixed measure of Sympathetic power or activity as modulated by Parasympathetic activity [[15] (p1052- 53), [16]]. Unfortunately, this area is often assumed to be a pure Sympathetic measure. The lighter gray area of Figure 1 is a broadband region (0.15Hz to 0.40Hz [[15] (p 1047, Table 2), [16]], known as the high frequency (HF) region. The area under the spectral HR curve within the lighter grey area is thought to be indicative of PSNS activity alone. It is a relatively broad, fixed frequency band designed to ensure that Parasympathetic activity is captured, assuming the patient is breathing fast enough (> 0.21 Hz, which is equal to 12.5 breaths per minute) [[15] (p1052-53), [16]]. Unfortunately, the broadband nature of the HF also permits noise to be captured (not depicted for clarity).
Noise is anything that is not of Parasympathetic activity. Noise artificially inflates the HF measure of Parasympathetic activity. The noise includes harmonics form the ULF, VLF, and LF spectra, resulting in an HF measure that reflects Sympathetic activity as well. This has caused controversy. For example, when a subject performs the deep breathing exercise, at 6 breaths per minute, the entirety of the Parasympathetic activity region is located within the LF region, and yet the HF measure in these cases is not zero. So, what is being measured? Further, since the noise inflates the HF measure, it facilitates the appearance of health in patients, and fosters misdiagnoses, including Cardiovascular Autonomic Neuropathy (CAN).
The P&S Monitoring method first captures the respiratory activity waveform. Then the Respiratory Activity (RA) spectrum is determined and the frequency of Respiratory Sinus Arrhythmia (RSA, or the FRF, or Fundamental Respiratory Frequency as MIT called it) [[17] (p220-222)] is located. The FRF is then transferred to the HR spectrum, identifying the center of the Parasympathetic (RFa, Respiratory Frequency area) spectrum for that analysis period for that patient, breathing at 0.125 Hz, or 8 breaths per minute, in this case. Since RSA solely represents cardio-vagal interaction (i.e., Vagal Nerve activity at the heart), the FRF indicates the center frequency of Vagal or Parasympathetic activity. The width of the frequency region that represents Parasympathetic activity was first documented by Akselrod et al. [[17] (p220-222)] and subsequently validated [18,19,21,22,38,40].
In this representation at the given FRF (0.125 Hz, or 8 breaths/min), the Parasympathetic activity region is depicted in blue in Figure 1. In general, the patient’s respiratory frequency is variable over the time of the recording. As a result, the FRF is variable over the time of the recording.
As a result, the Parasympathetic activity region moves left or right along the frequency axis, depending on where along the RA spectrum the FRF is discovered. Notice the blue region (RFa) is not as wide as the lighter grey region (HF, see Figure 1). Also, notice that the blue region is not totally confined within the lighter grey region. This is due to the fact that the patient’s respiratory frequency is only 8 breaths per minute (0.125 Hz), and not at least 12.5 breaths per minute (0.21 Hz); the minimum required for HRV-alone analysis (typically close to hyperventilation for many patients – a Sympathetic stimulus).
In this example, even though the Parasympathetic activity window (blue) is smaller than the HF (light grey) window, the Parasympathetic measure (RFa) is greater than the HF, due to the different areas under the spectral curve within the blue and grey windows. Given the breathing rate of 8 breaths per minute (close to 6 breathes per minute known to be a very powerful Parasympathetic stimulus [41]), it is reasonable, therefore, that the Parasympathetic activity should be more significant. This is not what is indicated by the HF measure. The HF measure, including the noise that it includes) indicates very little Parasympathetic activity in this case.
Further, notice that the blue region also overlaps the darker grey region (see Figure 1). On the one hand, this confirms the standards article’s claim that the LF region is a mixed measure of Sympathetic activity as modulated by Parasympathetic activity [[15] (p1052-53), [16]], and not simply a pure Sympathetic measure. On the other hand, this demonstrates how P&S Monitoring method extracts the Parasympathetic activity from the LF region, leaving the rest of the LF region to independently represent the (pure) Sympathetic activity for the patient over this time period of recording. The area under the HR spectral curve within the LF region not within the (blue) Parasympathetic activity window is defined as the (unambiguous) Sympathetic activity measure and is depicted in red (see Figure 1).
n the case of HRV-alone, under the assumption that the LF represents Sympathetic activity alone, this patient would seem to be hyperventilating and anxious or stressed, rather than breathing normally and relaxed. Clinically, this is a reason for the failure of HRV-alone. HRV- alone misrepresents the underlying P and S activity which leads to false positives and false negatives. P&S Monitoring resolves the failure of HRV-alone.
Again, HRV-alone merely informs you that the patient’s ANS is functioning. Assuming that the patient is breathing, this fact is already known! What is not known are P&S activity levels in response to disease, lifestyle, therapy, and cumulative history. Is the patient truly responding as expected, and assumed from symptoms, or is there a hidden (asymptomatic) autonomic dysfunction perpetuating an unstable condition that may be responsible for difficult to manage or unresponsive patients, PTSD, Long-COVID, or worse: heart attack, stroke, or sudden death?
P&S Monitoring specifies the activity in the P and S branches, providing more information to reduce morbidity and mortality and improve outcomes. To guide therapy, document patient responses, and answer these questions, you need to document the patients P&S activity periodically.
CONTINUOUS WAVELET TRANSFORM (CWT) v FAST FOURIER TRANSFORM (FFT)
Wavelet analysis was declared by the International Autonomic Society, during their joint American and International conference in 2007, as the proper means of non-invasively, assessing autonomic activity [42,43] (later published in 2009 [44]). Wavelet analysis eliminates the signal stationarity requirement and treats frequency and time criteria simultaneously [21,22,45,46]. Only Wavelets enable specific P&S measurements from the administration of the standard autonomic study in about 15.5 minutes without further assumption and approximation (see below). The standard autonomic study is (in order): 1) a five minute rest baseline, 2) a one minute deep (paced) breathing challenge, 3) one minute rest, 4) a series of five short Valsalva maneuvers over an approximately one and a half minute period, 5) two minutes rest, and 6) a quick head-up PC (stand) followed by 5 minutes of quiet head-up posture (stand).
Wavelets analyze the patient’s steady state and transient responses, revealing more information to improve outcomes. FFTs cannot analyze the transient responses. For example, Orthostatic dysfunction is known as an inappropriate response to the gravitational challenge that occurs immediately upon standing. Only with Wavelet analysis is the gravitational response captured and therefore, only with Wavelets is Orthostatic dysfunction properly assessed [46]. Diagnoses based on BP or HR require extreme changes before diagnosing and treatment, for several reasons, including the fact that they are controlled by both P&S systems which adds ambiguity due to the ANS Monitoring methods. Wavelets instantaneously assess the variable breathing rate of the patient being tested. Wavelets enable identification and isolation of Parasympathetic activity from Sympathetic activity in the LF region [45]. No more assumptions or approximations are needed; simply measure patients as they are, including with disease, therapy, lifestyle, genetics, and cumulative history.
What does this mean? The FFT, which continues to be the declared standard, ubiquitous spectral analysis technique throughout ANS Monitoring technology, has two very well known, short- comings [47]. For one, the FFT requires a compromise between accuracy in the time domain and accuracy in the frequency domain (see Figure 6). This compromise inherently reduces the sensitivity and specificity of the results. As demonstrated in Figure 6 (left panel), the shorter window length (WL) of 32 samples captures the frequency details with acceptable fidelity. The frequency results, represented on the ordinate, include the f3 = 0.3 Hz component. However, the temporal fidelity is compromised. The longer WL (64 samples, Figure 6, left pane, right) loses the frequency details (e.g., the 0.3 Hz component is missing in this example) but preserves temporal fidelity.
The second short coming of the FFT is the requirement known as signal stationarity [47]. This requires the signal to not change significantly over the analysis period. The problem is that biological signals always change, especially P and S signals, which are designed to cause changes in the body. (They change the changes!) So, this makes it difficult to measure anything but quiescent (relatively “steady-state” or stable, not changing) conditions. The minimum, accepted, valid, FFT-based HRV-alone analysis time period is five minutes [15,16]. To measure challenge conditions properly and accurately, the challenge (e.g., including Valsalva, DB, and postural change or stand) would need to be at least six to seven minutes long so that the middle five minutes can be properly analyzed, assuming no changes in the middle five minutes. For example, these five minutes must also be arrhythmia-, or artifact-, free to satisfy stationarity.
Unfortunately, all transitions are lost with this requirement from the FFT (including short-term FFTs). For example, the transition during the quick PC would be lost and with it the information needed to simply and directly differentiate a patient’s possible Orthostatic response from a possible Syncope response.
Wavelet transforms (e.g., CWT or Wavelets) “fixes” the short-comings of FFTs [45]. Wavelets address both the time and the frequency domain issues simultaneously (see Figure 6, right panel), therefore, no compromise or artificial conditions are required. As shown in Figure 6 (right panel), all frequency components are represented and there is no loss in temporal fidelity (compare the three spectrograms along the bottom of the two panels in Figure 6). This inherently improves the sensitivity and specificity of the measure; and (as a result) clinical sensitivity and specificity. Furthermore, Wavelets “thrive” on “non-stationary” signals, including ones that change significantly and often [45]. In this way, the patient breathes naturally, changes posture and performs short breathing challenges (especially Valsalva maneuvers). Together, this enables the P&S responses to challenges to be properly and accurately measured, including the P&S responses during the transitions and active phases of the challenges. As in the example above, P and S activity is now measured during the quick PC, so that the underlying P and S physiology contributing to Orthostatic dysfunction (e.g., POTS) or Syncope is documented.
In summary, HRV analysis with Respiratory Activity analysis with Wavelets as the analysis technique truly provides non-invasive (due to Wavelet spectral analysis of HRV), independent (due to Wavelet spectral analysis of Respiratory Activity), simultaneous (due to Wavelets), quantitative (also due to Wavelets), real-time (also due to Wavelets) assessment of both branches of the ANS: the P and S nervous systems.
Figure 6: An example demonstrating the difference between FFT (left panel) and CWT analyses. The left and right panels, respectively, presents the FFT and the CWT time-frequency representation of a signal with three spectral components: 0.1 Hz, 0.05 Hz, and 0.3 Hz. With the FFT a coice has to be made and either way, information is lost. Not so with CWT. See text for details.
APPENDIX 3: The “Brakes and Accelerator” or “Car” Model of P&S Activity
The “brakes and accelerator” model is the better way to consider the P&S (or autonomic) nervous systems. The brakes represent the Parasympathetics and the accelerator represent the beta-Sympathetics. The “see-saw” model taught in Medical School is still included and addresses resting conditions. Typically, at rest or under normal conditions, a foot is either on the brakes and off the accelerator or vice versa; there is the “see-saw.” For normal as well as abnormal responses to challenge, the “see-saw” model often fails. For example, as mentioned above, upon standing, first the Parasympathetics should decrease and then the alpha- Sympathetics should increase. This is much like the brakes and accelerator on a car. To go, first the foot taken off the brake pedal, then the foot is applied to the accelerator. Upon releasing the brakes, the car already begins to roll (accelerate) minimizing and potentiating the amount of acceleration, stress, and wear, required to normally reach speed. If the brakes are not released, and the accelerator is depressed, the car will still go, but it takes more acceleration, including stress to reach speed. In effect, the acceleration is amplified (even though the speeds reached are not as fast because the brakes are engaged). This also amplifies the results of acceleration. This is the effect of PE. PE also often causes secondary β-SE that is the “adrenaline storm” or “Syncope Spikes” or β-Sympathetic stimuli of the heart meant to increase blood flow to the brain and everywhere above the heart.
To extend the brake and accelerator analogy, SW (an abnormal α-Sympathetic response) is like hitting the accelerator and abnormally low amounts of gas gets to the engine, so either the engine barely goes (fatigue, brain-fog, sleep difficulties, memory or cognitive dysfunction, vision effects, ringing in the ears, lightheadedness, taste or smell disorders, etc.) or the engine stalls (fainting or you feel like fainting and you have to sit or lay down). In the case of αSW, blood pools in the feet, ankles and lower legs, causing poor coronary (heart) and cerebral (brain) perfusion. These symptoms are also caused by PE.
APPENDIX 4: The Autonomic Dysfunction Therapy Algorithm
This completes the Autonomic Dysfunction Therapy Algorithm [adapted from 28] discussed throughout the text.
To complete the daily Low-and-Slow exercise recommendation, it is recommended that before rising in the morning, Dysautonomia patients that become lightheaded upon rising, including POTS patients should perform a couple of dozen leg lifts to help circulate the blood and stimulate the nervous system.
To complete the proper daily hydration recommendation, it is recommended to drink water to replace what was breathed out throughout the night†. If the water is not tolerated, then it may be its temperature. The stomach is approximately 100°F. Any drink cooler will cause the stomach to constrict, causing a full or bloated feeling or causing the gastric juices to be pushed up into the Esophagus causing nausea.
†If you have been tent camping and wake up to some water condensed on the roof of the tent on a cool morning, that is an sample of what is breathed out overnight. All that water is from the occupant(s) of the tent.
For some patients elevating the head of the bed at night helps relieve any head pressure that may occur in a supine position. This head pressure is from the fact that upon assuming a supine position the heart is no longer working against gravity and the brain becomes properly perfused and the patient is not yet acclimated to the extra (normalized) pressure that results. Night-time head elevation is also thought to keep the α-Sympathetic nerves working throughout the night as well, help to relieve the Orthostatic dysfunction. However, elevating the head may also promote anxiety-like symptoms or nightmares at night if the α-Sympathetic nerves fail to help maintain proper cerebral perfusion.
Due to βSE, histaminergic and inflammatory responses are amplified. In fact, many of the newer inflammatory auto-immune disorders that are being reported may actually be a result of PE induced βSE causing excess or prolonged immune activity (due to PE) with amplified inflammation (due to secondary βSE). Much of the pain that is reported by Dysautonomia patients is due to prolonged or excessive inflammation, possibly exaggerated by prolonged or excessive histaminergic responses. Zyrtec, 10 mg qd, and Pepcid, 20 mg qd, are recommended to relieve the excessive and prolonged histaminergic reactions. This is also the recommended therapy for those diagnosed with Mast Cell Activation Syndrome (MCAS). Thes two supplements are H1 and H2 blockers, respectively, and both are required to prevent the body from overloading the histamine receptor system that is not blocked.
Note, MCAS may also be a cause of women’s health issues. Upon menstruation, PE releases too many Mast Cells. The secondary βSE releases too much Histamine from each Mast Cell, causing an order of magnitude increase in Histamine release drawing more blood into the area perhaps for prolonged periods. The Histamine causes inflammation, which is amplified by the βSE, drawing more blood. Then the progesterone half of the cycle occurs causing vasodilation drawing even more blood into the area and perhaps for prolonged periods. This excess bleeding may cause menstrual cramps and Dysmenorrhea. The prolonged presents of the excess blood may cause it to leak into the abdomen leading to Endometriosis which may cause adhesion to sequester the blood and possibly lead to PCOS. Again, the Histamine blockers may helps to relieve these Women’s Health issues by reducing the bleed. To complete this thought, the Parasympathetics also control and coordinate hormone activity. PE also causes too much Estrogen to be released during the period also causing more bleeding. The Parasympathetics communicate with the reproductive organs through the reproductive hormones. PE should be considered a type of over-communication.
To address the excessive or prolonged inflammation, there are two very powerful anti- inflammatories that do not require prescription. They are Turmeric, 1000 mg tid titrated as needed and Omega-3 Fatty Acid, 4000 mg qd, titrated as needed. A prescription of Vascepa may be easier to swallow, requiring fewer pills for the recommended dosage. For pain due to inflammation that is not controlled by these two supplements, then consider Low-dose Naltrexone, up to 8 mg bid, as needed; often only 2 mg to 4 mg.
Another supplement used to address inflammation is Methylfolate, 7.5 mg qd, titrated up to 30 mg qd as needed. Methylfolate relieves inflammation of the small C-nerve fibers (known as Small Fiber Disorder) that transmit pain information from the periphery and Sympathetic information to the periphery controlling sweating (Sudomotor function) and peripheral circulation, thereby controlling temperature regulation and wound healing. Relieving Small Fiber Disorder helps to normalize these functions. If the C-fibers are not only inflamed but also dysfunctional, Alpha-Lipoic Acid will help to heal the dysfunction.
Nitric Oxide boosts are recommended to help improve circulation as well as it provides other benefits. Nitric Oxide promote Endothelial health. Inflamed Endothelium in the GI tract may be a cause of the acid burn feeling by permitting the food and stomach acid to leak into the stomach lining causing the burn; a type of “Leaky-Gut Syndrome.” Endothelial dysfunction in the intestines together with the abnormal motility due to Dysautonomia may be ultimately the cause(s) of inflammatory bowel diseases such as Irritable Bowel Syndrome, Ulcerative Colitis and perhaps Crohn's disease. In the arteries and veins, Endothelial health promotes proper blood flow. Inflamed Endothelium in the vasculature causes turbulence which may lead to blood clots. Also inflamed Endothelium provides anchor points in the vasculature for cholesterol to adhere leading to Atherosclerosis. The Endothelium is part of the Blood-Brain Barrier helping to maintain brain health. In the brain Nitric Oxide is also a Neurotransmitter in certain Parasympathetic circuits. It also signals the need for repair and healing. Any where the body interfaces with the outside environment, there is Endothelium working to ensure inflow and outflow of substances from the body. Inflamed Endothelium exposes the body to more than it needs.
Nitric Oxide production in the body may be increased through two pathways. The primary pathway is essential Amino Acids, primarily L-Arginine. Unfortunately, in supplement form, L- Arginine does not survive the stomach well. It is also rate limited. L-Carnitine and L-Citrulline are precursors to L-Arginine and in supplement form they due survive the stomach well. Of course these are also available through a proper diet. The other Nitric Oxide pathway is from the nitrates in Beet Root Extract. There are bacteria in the mouth and stomach that will produce as much Nitric Oxide as they are fed Nitrates such as from Beet Root extract.
Typically, those with Orthostatic dysfunction, including POTS, instinctively perform physical counter-maneuvers to prevent lightheadedness and dizziness upon assuming upright posture.
While proper diet as well exercise is always important, the best diets to consider are the Mediterranean Diet or the Japo-Mediterranean Diet. These will provide all of the supplements recommended, but in natural form so they are more bio-available, reducing the need for higher doses of the supplements. Still, it is important to avoid heavy meals for they shunt more blood to the stomach for longer periods of time taking the blood from the brain, promoting lightheadedness. Also avoid alcohol as well as sugar, sugar substitutes and caffeine as all of these will promote dehydration and alcohol will also vasodilate making it harder to pump blood to the brain. Heat exposure, as well as cold exposure may cause undue stress on the body causing a flare of the Dysautonomia, including exacerbating POTS.
References
- Colombo J, Weintraub MI, Munoz R, et al. Long-COVID and the Autonomic Nervous System: The Journey From Dysautonomia to Therapeutic Neuro-Modulation, Analysis of 152 Patient Retrospectives. NeuroSci. 3 (2002): 300-310.
- DePace NL, Colombo J. Long-Covid Syndrome: A Multi-Organ Disorder. Cardio Open 7 (2022): 213-224.
- DePace, N.L., Colombo, J. Long-COVID Syndrome and the Cardiovascular System: A Review of Neurocardiologic Effects on Multiple Systems. Curr Cardiol Rep (2022).
- Bloom HL and Colombo J. Coronavirus Induces Oxidative Stress Which Leads to Parasympathetic and Sympathetic Dysfunction and Delayed Symptoms Often Not Related to COVID. J Individ Med Ther 1 (2022).
- DePace NL, Santos, L; Goldis M, et al. Hypermobility/Ehlers-Danlos Syndrome and the Parasympathetic and Sympathetic Nervous Systems. J Individ Med Ther 1 (2022).
- DePace N, Murray G (posthumously), Plotnikoff G, et al. Long-COVID, PTSD and EDS/Hypermobility Have Common Parasympathetic and Sympathetic Dysfunctions Causing Poor Cerebral and Coronary Perfusion: A Review. J Individ Med Ther 2 (2023).
- Arora RR, Bulgarelli RJ, Ghosh-Dastidar S, et al. Autonomic Mechanisms and Therapeutic Implications of Postural Diabetic Cardiovascular Abnormalities. J Diabetes Science and Technology 2 (2008): 568-571.
- Tobias H, Vinitsky A, Bulgarelli RJ, et al. Autonomic nervous system monitoring of patients with excess parasympathetic responses to sympathetic challenges – clinical observations. US Neurology 5 (2010): 62-66.
- Colombo J, Arora RR, DePace NL, et al. Clinical Autonomic Dysfunction: Measurement, Indications, Therapies, and Outcomes. Springer Science + Business Media, New York, NY (2014).
- Ewing DJ. Cardiovascular reflexes and autonomic neuropathy. Clin Sci Mol Med 55 (1978): 321-327.
- Ewing DJ, Clarke BF: Diagnosis and management of diabetic autonomic neuropathy. British Medical Journal 285 (1982): 916-918.
- Ewing DJ, Martyn CN, Young RJ, et al. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care 8 (1985): 491-498.
- Bloomfield DM, Kaufman ES, Bigger JT Jr, et al. Passive head-up tilt and actively standing up produce similar overall changes in autonomic balance. Am Heart J 134 (1997): 316-320.
- Nanavati SH, Bulgarelli RJ, Vazquez-Tanus J, et al. Altered autonomic activity with atrial fibrillation as demonstrated by non-invasive autonomic monitoring. US Cardiology 7 (2010): 47-50.
- Malik, M. The Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability, standards of measurement, physiological interpretation, and clinical use. Circulation 93 (1996): 1043-1065.
- Malik, M. and the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability, standards of measurement, physiological interpretation, and clinical use. European Heart Journal 17 (1996): 354-381.
- Akselrod S, Gordon S, Ubel FA, et al. “Power spectrum analysis of heart rate fluctuations: a quantitative probe of beat-to- beat cardiovascular control.” Science 213 (1981): 213-220.
- Akselrod S, Gordon D, Madwed JB, et al. Hemodynamic regulation: investigation by spectra analysis. Am J Physiol 249 (1985): H867-875.
- Akselrod S, Eliash S, Oz O, et al. Hemodynamic regulation in SHR: investigation by spectral analysis. Am J Physiol 253 (1987): H176-183.
- Akselrod S: Spectral analysis of fluctuations in cardiovascular parameters: a quantitative tool for the investigation of autonomic control. Trends Pharmacol Sci 9 (1988): 6-9.
- Aysin B, Aysin E. Effect of respiration in heart rate variability (HRV) analysis. [PubMed: 17946068]. Conf Proc IEEEEng Med Biol Soc 1 (2006): 1776-1779.
- Aysin B, Aysin E, Colombo J Comparison of HRV analysis methods during orthostatic challenge: HRV with respiration or without? IEEE Engineering in Medicine and Biology Conference, Lyons, France (2007).
- Aia PG, Revuelta GJ, Cloud LJ, Factor SA. Tardive dyskinesia. Curr Treat Options Neurol. 2011 Jun;13(3):231-41. doi: 10.1007/s11940-011-0117-x. PMID: 21365202.
- Revet A, Montastruc F, Roussin A, Raynaud JP, Lapeyre-Mestre M, Nguyen TTH. Antidepressants and movement disorders: a postmarketing study in the world pharmacovigilance database. BMC Psychiatry. 2020 Jun 16;20(1):308. doi: 10.1186/s12888-020-02711-z. PMID: 32546134; PMCID: PMC7298955.
- Piña IL, Di Palo KE, Ventura HO. Psychopharmacology and Cardiovascular Disease. JACC 71 (2018): 2346-2359.
- DePace NL, Bateman JA, Yayac M, et al. Improved Patient Outcomes by Normalizing Sympathovagal Balance: Differentiating Syncope-Precise Subtype Differentiation Leads to Improved Outcomes. Cardiol Res Pract (2018): 9532141.
- Nanavati SH, Bulgarelli RJ, Vazquez-Tanus J, et al. Altered autonomic activity with atrial fibrillation as demonstrated by non-invasive autonomic monitoring. US Cardiology 7 (2010): 47-50.
- DePace NL, Colombo J. Clinical Autonomic and Mitochondrial Disorders: Diagnosis, Prevention and Treatment for Mind-Body Wellness. Springer Nature Switzerland, AG (2019).
- Ziegler D, Schatz H, Conrad F, et al. Effects of treatment with the antioxidant alpha-lipoic acid on cardiac autonomic neuropathy in NIDDM patients. A 4-month randomized controlled multicenter trial (DEKAN Study). Deutsche Kardiale Autonome Neuropathie. Diabetes Car 20 (1997): 369-373.
- Fu Q, Vangundy TB, Shibata S, Auchus RJ, Williams GH, Levine BD. Exercise training versus propranolol in the treatment of the postural orthostatic tachycardia syndrome. Hypertension. 58 (2011): 167-75. Epub 2011 Jun 20. PMID: 21690484; PMCID: PMC3142863 (2011).
- Keissar K, Davrath LR, Akselrod S. Coherence analysis between respiration and heart rate variability using continuous wavelet transform. Philos Transact A Math Phys Eng Sci 367 (2009): 1393-1406.
- Arora RR, Bulgarelli RJ, Ghosh-Dastidar S, et al. Autonomic mechanisms and therapeutic implications of postural diabetic cardiovascular abnormalities. J Diabetes Science and Technology 2 (2008): 568-571.
- Olivera MM, Santos-Bento M, Xavier R, et al. Wavelet analysis for the evaluation of cardiovascular autonomic nervous response to postural change in healthy subjects in relation to age. Second Joint Meeting of the European Federation of Autonomic Societies and the American Autonomic Society, Vienna, Austria. Clinical Autonom Res 17 (2007): 301.
- Olivera MM, Feliciano J, da-Silva N, et al. Wavelet Analysis for the evaluation of autonomic nervous system during orthostatic stress in paroxysmal atrial fibrillation. Second Joint Meeting of the European Federation of Autonomic Societies and the American Autonomic Society, Vienna, Austria. Clinical Autonom Res 17 (2007): 301.
- Olivera MM, da-Silva N, Timoteo AT, et al. Alterations in autonomic response during head-up tilt testing in paroxysmal atrial fibrillation patients: a wavelet analysis. Rev Port Cardiol 28 (2009): 243-257.
- Ducla-Soares JL, Santos-Bento M, Laranjo S, et al. Wavelet analysis of autonomic outflow of normal subjects on head-up tilt, cold pressor test, Valsalva manoeuvre and deep breathing. Exp Physiol 92 (2009): 677-686.
- Xavier R, Laranjo S, Ducla-Soares E, et al. The Valsalva Maneuver Revisited by Wavelets. Rev Port Cardiol 27 (2008): 435-441.
- DePace, Bateman, Yayac, et al. Improved patient outcomes by normalizing sympathovagal balance as measured by parasympathetic and sympathetic monitoring: 0. The technology. Submitted, IEEE EMBS; (2018).
- Components of Heart Rate Variability: Basic Studies, in Heart Rate Variability, Malik M. (ed), Futura Publishing Co., Armonk, NY (1995): 147-163.
- Arora RR, Bulgarelli RJ, Ghosh-Dastidar S, et al. Autonomic mechanisms and therapeutic implications of postural diabetic cardiovascular abnormalities. J Diabetes Science and Technology 2 (2008): 568-571.
- Ewing DJ. Cardiovascular reflexes and autonomic neuropathy. Clin Sci Mol Med 55 (1978): 321-327.
- Olivera MM, Santos-Bento M, Xavier R, et al. Wavelet analysis for the evaluation of cardiovascular autonomic nervous response to postural change in healthy subjects in relation to age. Second Joint Meeting of the European Federation of Autonomic Societies and the American Autonomic Society, Vienna, Austria. Clinical Autonom Res 17 (2007): 301.
- Olivera MM, Feliciano J, da-Silva N, et al. Wavelet Analysis for the evaluation of autonomic nervous system during orthostatic stress in paroxysmal atrial fibrillation. Second Joint Meeting of the European Federation of Autonomic Societies and the American Autonomic Society, Vienna, Austria. Clinical Autonom Res 17 (2007): 301.
- Olivera MM, da-Silva N, Timoteo AT, et al. Alterations in autonomic response during head-up tilt testing in paroxysmal atrial fibrillation patients: a wavelet analysis. Rev Port Cardiol 28 (2009): 243-257.
- Benedetto JJ and Frazier MW (eds.) Wavelets: Mathematics and applications. CRC Press, Inc., Boca Raton, FL (1994).
- Arora RR, Bulgarelli RJ, Ghosh-Dastidar S, et al. Autonomic mechanisms and therapeutic implications of postural diabetic cardiovascular abnormalities. J Diabetes Science and Technology 2 (2008): 568-571.
- Oppenheim AV, Willsky AS, with Young IT. Signals and Systems. Prentice-Hall, Englewood Cliffs, NJ, (1983).







