What’s in a Name? The Tricky Issue of Diagnosing Juvenile Temporal Arteritis When Histological Findings are not Consistent
Article Information
Chiara Castellani1*, Giulia d’Amati2, Bruna Cerbelli3, Marco Biffoni4, Carlo Perricone5, Cristiano Alessandri1, Fabrizio Conti1, Rossana Scrivo1
1Department of Clinical Internal, Anesthesiological and Cardiovascular Sciences, Sapienza University of Rome, Italy
2Department of Radiological Sciences, Oncology and Anatomical Pathology, Sapienza Univesrity of Rome, Italy
3Department of Medico-Surgical Sciences and Biotechnologies", Sapienza University of Rome, Italy
4Department of Surgical Sciences, Sapienza University of Rome, Italy
5Reumatology, Department of Medicine and Surgery, University of Perugia, Italy
*Corresponding Author: Chiara Castellani, Department of Clinical Internal, Anesthesiological and Cardiovascular Sciences, Sapienza University of Rome, Italy.
Received: 22 May 2022; Accepted: 14 June 2022; Published: 12 July 2022
Citation: Chiara Castellani, Giulia d’Amati, Bruna Cerbelli, Marco Biffoni, Carlo Perricone, Cristiano Alessandri, Fabrizio Conti, Rossana Scrivo. What’s in a Name? The Tricky Issue of Diagnosing Juvenile Temporal Arteritis When Histological Findings are not Consistent. Archives of Clinical and Medical Case Reports 6 (2022): 528-531
View / Download Pdf Share at FacebookAbstract
Juvenile Temporal Arteritis (JTA) is a rare vasculitis characterized by a nodule occurring without previous trauma. We report the case of a young woman with a lesion clinically suggesting JTA, but histologically consistent with Angiolymphoid Hyperplasia with Eosinophilia (ALHE). A 25-year-old woman casually noticed a pulsatile, not erythematous, 2 x 0.5 cm lump in her right temporal area, without previous trauma. She denied visual impairment, difficulties in mastication, and headache. Erythrocyte sedimentation rate and C-reactive protein were normal and no peripheral eosinophilia was recorded. An ultrasound scan of the temporal artery showed the “halo sign”. The biopsy revealed proliferating vessels with plump eosinophilic endothelial cells surrounding the temporal artery, severe luminal stenosis, and lymphocyte and eosinophil infiltrate. Although the clinical features were consistent with JTA, a histological diagnosis of ALHE was established. ALHE usually presents with multiple, itchy, erythematous nodules or plaques of the scalp and the neck. Unlike ALHE, the overlying skin of the lesion in JTA is usually normal, while the “halo sign” may be present in both diseases. Furthermore, both JTA and ALHE show lymphocyte and eosinophil infiltrate and vascular proliferation. Although overlap conditions may occur, we observed a rare case with incongruous clinical and histological aspects, in which the clinical pattern of JTA matched the histological features of ALHE. JTA and ALHE might be considered as part of the spectrum of a single vascular disorder.
Keywords
Angiolymphoid hyperplasia with eosinophilia; Differential diagnosis; Histology; Juvenile temporal arteritis; Vasculitis
Angiolymphoid hyperplasia with eosinophilia articles; Differential diagnosis articles; Histology articles; Juvenile temporal arteritis articles; Vasculitis articles
Article Details
1. Introduction
We report the case of a young woman with a lesion clinically suggesting a diagnosis of Juvenile Temporal Arteritis (JTA), while the histological findings were consistent with Angiolymphoid Hyperplasia with Eosinophilia (ALHE). Although the overlap between JTA and ALHE may occur [1, 2], in our patient we observed a challenging condition, characterized by the clinical pattern of the former and the histological features of the latter. To the best of our knowledge, two other similar cases have been described in the medical literature; hence, we question the possibility that JTA and ALHE might be considered part of the spectrum of a single vascular disorder. We searched PUBMED up to the 1st of May 2022, on the main differential diagnosis of JTA, using the following keywords: juvenile temporal arteritis, differential diagnosis, angiolymphoid hyperplasia with eosinophilia, Kimura disease, juvenile temporal arteritis AND angiolymphoid hyperplasia with eosinophilia, juvenile temporal arteritis, angiolymphoid hyperplasia with eosinophilia, Kimura disease overlap. All articles were reviewed in detail. A total of 31 articles were included in the final literature review.
2. Case presentation
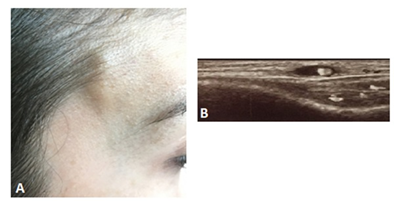
A 25-year-old woman casually noticed a pulsatile, not erythematous lump measuring 2 x 0.5 cm in her right temporal area when combing her hair (Figure 1A). No previous trauma had occurred. Since the lesion was slightly painful at palpation, she sought help from her general practitioner, who did not consider further investigation nor medical therapy. Within a few days, the patient experienced an enlargement of the lesion, still mildly painful at palpation. She presented to the local Emergency Room but was rapidly discharged with a diagnosis of headache, although vasculitis was suspected. On this basis, the general practitioner prescribed a 10-day course of prednisone 25 mg daily. Before the end of the treatment, ultrasonography demonstrated a 3 cm-long wall thickening of the superficial temporal artery with a hypoechoic halo. She was admitted to the Rheumatology Unit of our Hospital on the 10th day after prednisone withdrawal. The enlargement was still evident, with no changes on the skin overlying the nodule. She denied visual impairment, difficulties in mastication, headache while reporting sporadic pain of the scalp in the left parietal and right occipital area. She was afebrile and the laboratory testing showed an erythrocyte sedimentation rate of 5 mm/h and a C-reactive protein of 1600 mg/dL (normal, 0–6000 mg/dL). Furthermore, no peripheral eosinophilia was recorded (316/μl). An ultrasound scan of the temporal artery confirmed the hypoechoic halo around the arterial lumen (“halo sign”), indicative of blood vessel wall edema (Figure 1B).
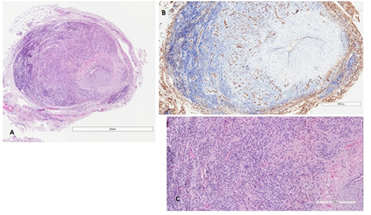
A biopsy of the right temporal artery was performed, after removing 1.5 cm in size of the macroscopic ectatic artery. The histological exam revealed proliferating small capillary vessels with plump eosinophilic endothelial cells, arranged in lobular structures surrounding the temporal artery; a prominent inflammatory infiltrate, with small lymphocytes, plasma cells, and abundant eosinophils was also seen, together with severe luminal stenosis (Figure 2A-C). An immunohistochemical stain for CD31 was performed to highlight the proliferating capillary vessels (Figure 2B).
Figure 2: Histological features. (A) The temporal artery is surrounded by proliferating small capillary admixed with a marked inflammatory infiltrate. There is intimal hyperplasia with severe lumen stenosis; (B) Immunohistochemical stain for CD31 highlights the proliferating capillary vessels; (C) The inflammatory infiltrate is made up of small lymphocytes, plasma cells, and abundant eosinophils.
3. Discus
To the best of our knowledge, only two cases with clinical manifestations highly suggestive of JTA or Giant Cell Arteritis (GCA) but histologically consistent with ALHE have been described [3, 4]. JTA is a rare, localized vasculitis of unknown etiology affecting more frequently male subjects and is characterized by the presence of a nodule in the temporal region. The swelling can be unilateral or bilateral and occurs in the absence of previous trauma [1, 5]. Less than 50 cases have been described so far [5, 6]. The typical features of this clinical entity may be summarised as follows: 1) people affected are younger than 40 years; 2) patients experience a palpable, painless or tender nodule; 3) inflammation indexes are normal by laboratory work-up; 4) neither ophthalmic symptoms nor ischemic cranial manifestations are reported; 5) histologic examination shows intimal proliferation, disruption of the media with an inflammatory infiltrate of lymphocytes, eosinophils, and plasma cells which may extend to the perivascular area; possible sparse giant cells and thrombosis; 6) surgical excision is often curative, with no need for glucocorticoids or any other systemic treatment [1, 7]. Even though eosinophilia is not typical of JTA, it was reported in up to one-third of the patients, and also sporadic cases of elevated serum Immunoglobulin E (IgE) have been documented [5]. No visceral involvement has been described in JTA. The condition usually occurs just once, with rare cases of relapse [5]. The differential diagnosis of JTA must take into account Kimura Disease (KD), systemic vasculitides affecting the temporal arteries, the very rare vasculitis resembling the elderly GCA, and ALHE. In heavy smokers, including young people, also thromboangiitis obliterans involving the temporal artery should be considered, with the histological evidence of thrombi occluding the vessel lumen [1, 8]. ALHE is a vasoproliferative cutaneous disease of uncertain etiology [9], although a possible etiopathogenetic link with the use of contraceptives in women has been described [10]. It is an uncommon and benign condition, usually presenting with multiple, itchy, erythematous nodules or plaques of the skin mainly affecting the scalp and the neck of middle-aged and young women [9]. It may sometimes occur after local trauma [11] and affect different areas of the body, such as the upper limbs [12] and the tongue [13]. Spontaneous bleeding of the lesion has been reported [9, 14]. Differently from ALHE, the overlying skin of the lump in JTA usually shows no changes, while the ultrasonographic “halo sign” may be present in both diseases [2, 15]. As far as histopathology is concerned, both JTA and ALHE show eosinophilic and lymphocytic infiltrates, and some degree of vascular proliferation and perivascular infiltrates.
However, in JTA those inflammatory infiltrates are mostly confined to the vessel wall, while in ALHE the inflammatory infiltrates are mainly perivascular, and vascular proliferation is often conspicuous with capillaries surrounding blood vessels that may also appear ectatic [5, 16]. Although rarely, ALHE nodules have been found in the lungs [11, 17]. Eosinophilia may be present in up to 20% of ALHE patients, without serum IgE elevation [9]. Treatment of ALHE is usually surgical, including Mohs micrographic surgery [18], but many other therapeutic strategies have been performed, such as electrocoagulation and photodynamic therapy [19], 32P brachytherapy [20], LASER therapy (pulsed dye and CO2 lasers) [21], cryotherapy [9], intralesional and topical glucocorticoids [14], intralesional bleomycin, vinblastine or interferon [22]. After surgical excision, ALHE may recur in 30% [23] to 40.8% of the cases [24]. Another condition to be considered for the differential diagnosis of JTA and ALHE is the aforementioned KD, which is a rare, benign, chronic immune-mediated disorder [24] mostly observed in Asian populations. The peak age for onset is the third decade, with a male:female ratio of 6.7:1 [25]. KD is characterized by subcutaneous, painless nodules, predominantly localized in the head and neck, frequently associated with mostly locoregional lymphadenopathy or salivary gland enlargement [25]. The overlying skin of the nodules may be normal or hyperpigmented, and itchy [25]. Apart from the head and neck, other areas of the body can be affected, such as the upper limbs, especially the forearms, and the inguinal area [25], together with other unusual localizations, such as the epiglottis [26] and breasts [27]. Similar to ALHE and JTA, KD’s etiology is unknown, but many pathogenetic hypotheses have been formulated, including the probable link with certain infectious agents (such as Candida, human polyomavirus 6, and arthropod bites) [28, 29].
As for the laboratory work-up, eosinophilia is a typical feature of KD, with a median percentage of eosinophils of 21.1% and a median absolute value of 1850/μl [25]. In a cohort of 45 patients with KD, 44 had increased IgE levels, with a median absolute value of 1591 U/L [25]. Renal involvement has been described in 10 to 60% of Kimura’s patients, mainly consisting of nephrotic syndrome (10-12% of cases) with findings of 24-hour proteinuria up to 20.4 g and histological evidence of membranous glomerulonephritis [29].
At the histological examination, lymphoid and massive eosinophil infiltrate, often forming perivascular lymphoid follicles with reactive germinal centers (which are found nor in JTA neither ALHE), and occasional eosinophil microabscesses may be found [1]. Germinal center necrosis, deposits of proteinaceous material, vascularisation of germinal centers, sclerotic areas, and vascular proliferation with normal-appearing endothelial cells were also reported [1, 25]. Surgical excision alone or combined with local radiotherapy, oral glucocorticoids (0.5–1 mg/kg/day), cyclophosphamide (100 mg/day) in addition to glucocorticoids, especially in patients with renal involvement, were the most common options used to treat KD [25, 30]. About 46% of patients were reported to relapse in a median follow-up time of 8.5 years [25]. Overlap conditions between JTA, ALHE, and KD have been documented [2, 3, 4, 15, 31], with one report of overlap amongst the three conditions [2]. To the best of our knowledge, this is the third case described so far in the medical literature about a patient with clinical manifestations highly suggestive for JTA but histological features consistent with ALHE. We might consider the possibility that JTA and ALHE are part of the spectrum of a single disorder, whose clinical and histological characteristics mix in the context of benign, heterogeneous manifestations.
Author contributions
C.C., C.A., F.C., and R.S. cared for the patient; C.C. and R.S. wrote the original draft; G.d’A. and B.C. performed the histological examination; M.B. performed the surgical excision of the lesion; C.P. performed the ultrasound of the temporal artery. All authors critically revised and approved the manuscript.
Funding
No external funding for this manuscript.
Compliance with ethical standards
Conflict of Interest
The authors have indicated that they do not have any financial and non-financial potential conflicts of interest to disclose.
Informed Consent
Written informed consent was obtained from the presented patient.
References
- Nesher G, Oren S, Lijovetzky G, et al. Vasculitis of the temporal arteries in the young Semin Arthritis Rheum 39 (2009): 96-107.
- Grum F, Hufendiek K, Franz S, et al. High-resolution color-coded sonography in angiolymphoid hyperplasia with eosinophilia presenting as temporal arteritis. Circulation121 (2010): 1045-1046.
- Li E, Sinard J, Distefano A, et al. Angiolymphoid hyperplasia with eosinophilia with clinical presentation concerning for juvenile temporal arteritis. Ocul Oncol Pathol 6 (2020): 25-30.
- Kitamura H, Kitamura H, Ito S, et al. Epithelioid hemangioma of the temporal artery clinically mimicking temporal arteritis. Pathol Int 49 (1999): 831-835.
- Journeau L, Pistorius MA, Michon-Pasturel U, et al. Juvenile temporal arteritis: a clinicopathological multicentric experience. Autoimmun Rev 18 (2019): 476-483.
- Blanco Alonso S, Mellor-Pita S, Alfageme F, et al. Temporal arteritis in a young patient. A clinical case. Reumatol Clin (Engl Ed) 17 (2021): 297-299.
- Tomlinson FH, Lie JT, Nienhuis BJ, et al. Juvenile temporal arteritis revisited. Mayo Clin Proc 69 (1994): 445-447.
- Prokesch B, Law K, Conn DL. Thromboangiitis obliterans involving the temporal artery. Arthritis Care Res (Hoboken) 63 (2011): 918-920.
- Caca-Biljanovska N, Arsovska-Bezhoska I. Angiolymphoid hyperplasia with eosinophilia successfully treated with cryotherapy. Open Access Maced J Med Sci 7 (2019): 794-796.
- Theofilou NE, Scolozzi P, Lombardi T. Angiolymphoid hyperplasia with eosinophilia located on the forehead: a possible association with oral contraceptive use? Dermatopathology (Basel) 6 (2019): 225-230.
- Marka A, Cowdrey MCE, Carter JB, et al. Angiolymphoid hyperplasia with eosinophilia and Kimura disease overlap, with evidence of diffuse visceral involvement. J Cutan Pathol 46 (2019): 138-142.
- Mak CW, Tzeng WS, Chen Y, et al. Sonographic appearance of angiolymphoid hyperplasia with eosinophilia in the upper arm. J Clin Ultrasound 36 (2008): 448-450.
- Lin SF, Wei YM, Fan Y, et al. Angiolymphoid hyperplasia with eosinophilia of the tongue with a Sézary syndrome history: Evidence for immune mediation of the disease. Australas J Dermatol 13 (2020).
- Adler BL, Krausz AE, Minuti A, et al. Epidemiology and treatment of angiolymphoid hyperplasia with eosinophilia (ALHE): A systematic review. J Am Acad Dermatol 3 (2016): 506-512.
- Paparo F, Fulcheri E, Garlaschi G, et al. Vasculitis of the temporal artery in a young woman. Rheumatology 50 (2011): 1968.
- Bastos JT, Rocha CRMD, Silva PMCE, et al. Angiolymphoid hyperplasia with eosinophilia versus Kimura’s disease: a case report and a clinical and histopathological comparison. An Bras Dermatol 92 (2017): 392-394.
- Ribeiro L, Souto M, Loureiro A. Angiolymphoid hyperplasia with eosinophilia of the lung. Arch Broncopneumol 54 (2018): 337-350.
- Trivedi A, Norris I, De Witt CM, et al. Angiolymphoid hyperplasia with eosinophilia treated with Mohs micrographic surgery. Dermatol Online J 12 (2019): 9.
- Wu L, Li F, Shi W, et al. Combination of electrocoagulation and photodynamic therapy for angiolymphoid hyperplasia with eosinophilia in the external ear. Photodiagnosis Photodyn Ther 27 (2019): 449-451.
- Zhang J, Li Y, Wen G, et al. Novel application of 32P brachytherapy: treatment of angiolymphoid hyperplasia with eosinophilia in the right auricle with 8-year follow-up. Cancer Biother Radio 33 (2018): 282-284.
- Sagi L, Halachmi S, Levi A, et al. Combined pulsed dye and CO2 lasers in the treatment of angiolymphoid hyperplasia with eosinophilia. Lasers Med Sci 31 (2016): 1093-1096.
- Labib A, Estawrow M. Angiolymphoid hyperplasia with eosinophilia: new concept to lower recurrence. J Craniofac Surg 30 (2019): e386-e388.
- Nakandakari MD, de la Rosa DN, Arias J. Hiperplasia angiolinfoide con eosinofilia. Actas Dermosifiliogr 108 (2017): 586.
- Zhou P, Zhang W, Shi W, et al. Kimura disease. Dermatol Online J 23 (2017): 16.
- Zhang X, Jiao Y. The clinicopathological characteristics of Kimura disease in Chinese patients. Clin Rheum 38 (2019): 3661-3667.
- Dezoteux F, Dubois R, Lefèvre G, et al. An unexpected asymptomatic epiglottal site of Kimura Disease. Eur Ann Otorhinolaryngol Head Neck Dis 136 (2019): 47-49.
- Kakkar A, Gupta RK, Khanna P, et al. Kimura disease of the breast – a previously undescribed entity. Breast J 4 (2016): 456-459.
- Rascovan N, Monteil-Bouchard S, Grob JJ, et al. Human polyomavirus-6 infecting lymph nodes of a patient with an angiolymphoid hyperplasia with eosinophilia or Kimura disease. Clin Infect Dis 62 (2016): 1419-1421.
- Fouda MA, Gheith O, Refaie A, et al Kimura disease: a case report and review of the literature with a new management protocol. Int J Nephrol 2010 (2011): 673908.
- Lee CC, Feng IJ, Chen YT, et al. Treatment algorithm for Kimura’s disease: a systematic review and meta-analysis of treatment modalities and prognostic predictors. Int J Surg 100 (2022): 106591.
- Tomizuka T, Kikuchi H, Asako K, et al. Is Kimura's disease associated with juvenile temporal arteritis? A case report and literature review of all juvenile temporal arteritis cases. Mod Rheumatol Case Rep 5 (2021): 123-129.