Vonoprazan-Based versus Esomeprazole-Based Triple Therapy for Helicobacter Pylori: A Randomized Trial
Article Information
Yasser A. Abdelghani*, 1, Mahmoud M. Moussa2
1MD, Dept. of Tropical Medicine and Gastroenterology, Minia University, Egypt
2MD, Department of clinical pathology, Minia University, Egypt
*Corresponding author: Yasser A. Abdelghani. MD, Dept. of Tropical Medicine and Gastroenterology, Minia University, Egypt
Received: 21 August 2023; Accepted: 29 August 2023; Published: 04 September 2023
Citation: Yasser A. Abdelghani, Mahmoud M. Moussa. Vonoprazan-Based versus Esomeprazole- Based Triple Therapy for Helicobacter Pylori: A Randomized Trial. Journal of Pharmacy and Pharmacology Research. 7 (2023): 157-162
View / Download Pdf Share at FacebookAbstract
Background: The anti-acid effects of vonoprazan, a potassium-competitive acid blocker, are stronger and more long-lasting than those of proton pump inhibitors.
Objective: This study aimed to evaluate the efficacy of vonoprazan against esomeprazole for eradicating Helicobacter Pylori (H. pylori) for 14 days.
Patients and Methods: This randomized clinical experiment was conducted in a hospital affiliated with Minia University. Those who tested positive for having an active H. pylori infection, whether or not they had previously been treated, were split into two groups and given either VAL (vonoprazan 20 mg bid, amoxicillin 1000 mg bid, plus levofloxacin 500 mg once a day) or EAL (Esomeprazole 20 mg bid., amoxicillin 1000 mg bid, and levofloxacin 500 mg once a day). An H. pylori antigen test was performed 4–6 weeks after treatment ended to assess clearance.
Results: 122 people (61 in VAL and 61 in EAL) were split evenly between the two groups. H. pylori eradication rates were significantly higher (P = 0.031) in the VAL group compared to the EAL group. Adverse treatment-related occurrences were minor and not significantly different between the two groups.
Conclusions: The VAL regimen was effective in increasing eradication rates and was well tolerated; hence, it may be considered a potent regimen for treating H. pylori, especially in countries with high levels of antibiotic resistance.
Keywords
Vonoprazon, Esomeprazole, Helicobacter pylori, Randomized
Article Details
1. Introduction
Helicobacter pylori (H. pylori) is a gram-negative, microaerophilic bacterium on the gastrointestinal mucosa, including well over half of the world's population (1). Infection with H. pylori often begins in infancy and lasts a lifetime if medication is not used to treat it (2). Most affected individuals go long without exhibiting symptoms (2). Infection with H. pylori over an extended period may cause damage to the stomach's mucosa, and it is the primary form of persistent gastritis, duodenal ulcers, gastric ulcers, gastric adenocarcinoma, and gastric mucosa-associated lymphoid tissue lymphoma (2, 3).
The most crucial element in eliminating H. pylori is medication adherence. It is affected by several variables, including the complexity and length of the therapy, any adverse effects, the patient's willingness, educational level and socioeconomic status, and the physician's motivation (4, 5, 6). According to a previous study on H. pylori treatment, the typical triple therapy lasting seven days has been used most often in Asia-Pacific. Most medical professionals in Taiwan, Japan, and Korea have opted to start treatment for H. pylori infections with either a 7-day or 14-day course of standard triple therapy (62 % and 21 %, 100 % and 0 %, and 81 % and 7 %, respectively). Antibiotic resistance, on the other hand, is growing increasingly widespread around the globe, and the majority of nations now have triple therapy eradication rates lower than 80 % (7, 8). Even though a large number of newly invented first-line treatments, including such bismuth quadruple therapy as well as a non-bismuth quadruple (sequential, concurrent, as well as hybrid treatment), can increase the rate of eradication of H. pylori infection, eradication failure still occurs in anywhere from 3 % to 24 % of infected patients (9, 10).
Potassium-competitive acid blockers (P-CABs), such as vonoprazan, due to their greater efficacy, the rapid start of the action, dose-dependent effects, and prolonged duration of action in suppressing acid production are good alternatives to proton pump inhibitors (PPIs) (11). Vonoprazan is a P-CAB used to prevent acid from doing its damage (12). Vonoprazan binds reversibly to K+ ions and inhibits the H+, K+ ATPase enzyme (13, 14), resulting in a higher intragastric pH maintained for 24 hours after a single daily dose. It is not affected by the CYP2C19 polymorphism and is metabolized mostly by cytochrome P450 (CYP) 3A4, in contrast to PPIs (12). Since H. pylori eradication requires sustained acid suppression throughout therapy, vonoprazan is expected to improve eradication rates compared to standard PPI-based therapies. Furthermore, it has been suggested that shorter treatment periods for H. pylori may be possible with vonoprazan due to its more efficient acid control (15, 16). Levofloxacin has excellent safety data, making it easier for doctors to utilize in clinical settings (17). Additionally, it is often well tolerated, and most adverse reactions are mild to moderate in degree and brief (18). Numerous studies in Egypt revealed that levofloxacin-based triple treatment resulted in more H. pylori eradication (84.5 % vs. 69.00 %) in patients than in clarithromycin-based triple therapy (18, 19). This difference suggests that the levofloxacin-based triple treatment for H. pylori is more effective than the clarithromycin-based therapy. We have substantially greater levels of clarithromycin resistance in our region, as seen by the decreased eradication rate of clarithromycin-based triple treatment. Standard levofloxacin-based triple treatment has a less-than-ideal eradication rate for our H. pylori-infected individuals.
The effectiveness of the VAL regimen (vonoprazan 20 mg bid, amoxicillin 1000 mg bid, and levofloxacin 500 mg once a day for 14 days) has not been compared to that of the EAL regimen (Esomeprazole 20 mg bid, amoxicillin 1000 mg bid, and levofloxacin 500 once daily for 14 days) in a randomized study. Since the vonoprazan-based regimen can boost or maintain the eradication rate and is growing in popularity among patients, it may be the best option for treating H. pylori. This randomized clinical research was thus designed to compare the efficacy and safety of the VAL and EAL treatments.
2. Patients and Methods
a. Study population
The Minia Gastroenterology Clinic in Egypt conducted a series of prospective, open-label, randomized controlled studies on patients with digestive issues. The regional ethics board approved the study's procedure. For eligibility criteria, participants must be above 18 and have had an H. pylori infection without having therapy or medicines to remove the illness. Antigen (Ag) testing of stool samples and histopathologic examination of stomach samples obtained during upper gastrointestinal tract (GI) endoscopy confirmed the presence of H. pylori infection. All participants offer to have biopsies taken from at least four places (2 antrums and 2 bodies).
To narrow the pool of candidates for participation in the research, the following criteria were used as exclusions: (i) history of an allergy to esomeprazole, vonoprazan, penicillin, or levofloxacin; (ii) history of substance abuse or existing alcohol abuse; (iii) use of an antibiotic that affects H. pylori within the past 4 weeks; (iv) pregnancy as well as lactation; (v) significant cardiovascular, pulmonary, or renal, as well as active malignancy; (vi) surgery for stomach or duodenum; (vii) cirrhotic patients were excluded, since in patients with severe hepatic impairment, the metabolism of esomeprazole is decreased leading to a doubling of the AUC.
b. Randomization and procedures
Eligible patients were assigned to receive either the VAL group's treatment (vonoprazan 20 mg bid, amoxicillin 1000 mg bid, and levofloxacin 500 mg once a day for 14 days) or the EAL group's treatment (esomeprazole 20 mg bid, amoxicillin 1000 mg bid, and levofloxacin 500 once daily. for 14 days). After obtaining their signed informed permission, patients were instructed to follow prescribed regimens and advised on potential adverse effects. Patients filled out a standard questionnaire for demographic data and medical history. They disclosed their age, sex, smoking and drinking records, consumption of alcoholic beverages, caffeine-containing beverages, non-steroidal anti-inflammatory drug (NSAID) use, and any coexisting conditions. Patients were monitored during therapy to assess their compliance with treatment; full compliance was defined as taking at least 80% of the prescribed dose.
After two weeks, medication adherence, adverse events, and side effects were assessed upon their visit to the clinic. Some of the side effects mentioned in the surveys were feeling sick, throwing up, tasting bitter, having a rash, feeling bloated, having a headache, feeling dizzy, or having stomach pain. In this context, "treatment-related side effects" refers to any symptoms that emerged or intensified as a direct result of the therapy. Serious adverse events were classified as those that required hospitalization of the patient and significantly hindered their ability to go on with their everyday lives. Six weeks after commencing treatment, patients needed a negative H. pylori stool Ag test to be symptom-free and clinically cured. A treatment failure was defined as the inability to attain any of these goals.
c. Endpoints
To define the primary objective, which would have been the complete eradication of H. pylori infection, the occurrence of any adverse events and patient compliance with the medication, have been the secondary objectives.
2.1 Statistical analysis
The predicted sample size was found to be in line with previous studies. Vonoprazan-based triple treatment (VAL) had a 97% eradication rate, whereas esomeprazole-based combination therapy (EAL) only managed a 68.5 % success rate. Our planned sample size for each group was 50, assuming 80% power, 95% confidence, and a 20% follow-up loss rate. The sample size estimation indicated that more than 120 patients would be required. The results were analyzed using a protocol-mandated "intention to treat" criterion. In all regiments, statistical differences in eradication rates between treatments were evaluated by using 2 test. As appropriate, the 2 test or Fisher's exact test was used to compare demographic data and adverse reaction rates, and a P-value of 0.05 or below was deemed statistically significant. The statistical analysis was carried out using SPSS.
3. Results
3.1 Study groups characteristics
122 participants with active H. pylori infections were enrolled and were given a random assignment to undergo either the VAL or EAL regimen. All 122 patients enrolled in the study finished the treatment; therefore, both intention-to-treat and per-protocol analysis results were similar. The starting characteristics of the two groups did not vary significantly from one another in any way (Table 1). In both patient groups, every patient followed their drug regimens to a higher degree than 80 %.
Table 1: The individuals' baseline characteristics for the two therapy groups.
|
Characteristics |
VAL group |
EAL group |
P-value |
|
(n = 61) |
(n = 61) |
||
|
Male gender, n (%) |
27 (44.2%) |
30 (49.1%) |
0.384 |
|
Age (years), mean ± SD |
55.31 ± 12.4 |
54.89 ± 14.25 |
0.367 |
|
BMI (kg/m2), mean ± SD |
25.23 ± 3.76 |
27.35 ± 5.13 |
0.251 |
|
Smoking > 1 pack/week, n (%) |
9 (14.7%) |
8 (13.1%) |
0.291 |
|
Alcohol > 1 day/week, n (%) |
0 (0%) |
0 (0%) |
1 |
|
Underlying disease |
|||
|
Cardiovascular disease, n (%) |
2 (3.2%) |
7 (11.4%) |
0.062 |
|
Diabetes mellitus, n (%) |
13 (21.3%) |
15(24.5%) |
0.352 |
|
Hypertension, n (%) |
17 (27.8%) |
20 (33.3%) |
0.457 |
|
Dyslipidemia, n (%) |
7 (11.4%) |
9 (15%) |
0.621 |
|
Cerebrovascular disease, n (%) |
0 (4.9%) |
1 (1.6%%) |
0.987 |
|
Connective tissue disease, n (%) |
3 (4.9%) |
5 (8.2%) |
0.621 |
|
Other, n (%) |
7 (11.4%) |
5 (8.2%) |
0.356 |
|
Endoscopic findings |
|||
|
Non-erosive gastritis, n (%) |
21 (34.4%) |
17 (27.8%) |
0.323 |
|
Erosive gastritis, n (%) |
26 (42.6%) |
27(44.2%) |
0.785 |
|
Atrophic gastritis, n (%) |
7 (11.4%) |
5 (8.2%) |
0.296 |
|
Gastric or duodenal ulcer, n (%) |
11 (18.03%) |
17 (27.8%) |
0.076 |
*EAL group received therapy with esomeprazole, amoxicillin, and levofloxacin for 14 days; the VAL group received treatment with vonoprazan, amoxicillin, and levofloxacin. Body mass index = BMI
3.2 H. pylori eradication
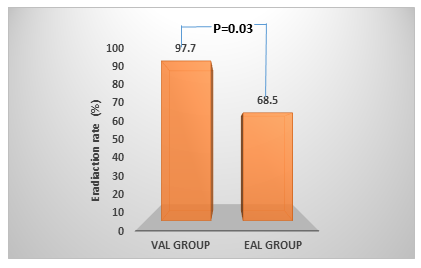
The research found that patients who received VAL had an elimination rate of 97.7 %, and the EAL group achieved an elimination rate of just 68.5 %. Figure 1 shows a statistically significant difference in eradication rates between the VAL and EAL groups.
3.3 Side effects of treatment
Both groups often complained of nausea and dizziness without any statistically significant differences. The reported adverse effects are shown in Table 2. There were no significant variations in the side effects between the two groups. No significant side effects or negative occurrences led individuals to stop getting medication.
Table 2: Treatment side effects
|
Side effects of therapy |
VAL group |
EAL group |
P-value |
|
(n = 61) |
(n = 61) |
||
|
Nausea, n (%) |
8 (13.1%) |
7 (11.4%) |
0.417 |
|
Vomiting, n (%) |
1 (1.6%) |
0 (0%) |
0.315 |
|
Bitter taste, n (%) |
1 (1.6%) |
0 (0%) |
0.315 |
|
Skin rash, n (%) |
2 (3.2%) |
1 (1.6%) |
0.418 |
|
Bloating, n (%) |
5 (8.2%) |
2 (3.3%) |
0.243 |
|
Dizziness, n (%) |
7 (11.5%) |
9 (14.8%) |
0.592 |
|
Headache, n (%) |
1 (1.6%) |
0 (0%) |
0.315 |
|
Diarrhea, n (%) |
7 (11.5%) |
3 (4.9%) |
0.187 |
|
Constipation, n (%) |
3 (4.9%) |
3 (4.9%) |
1 |
|
Abdominal pain, n (%) |
4 (6.55%) |
7 (11.5%) |
0.267 |
|
Dry mouth or throat, n (%) |
2 (3.2%) |
1 (1.6%) |
0.418 |
|
Others, n (%) |
3 (4.9%) |
2 (3.3%) |
0.648 |
*EAL group received therapy with esomeprazole, amoxicillin, and levofloxacin for 14 days; the VAL group received treatment with vonoprazan, amoxicillin, and levofloxacin. Not relevant = N/A
4. Discussion
According to the study's results, the efficacy of treatments to eradicate H. pylori has steadily declined worldwide. The key factors contributing to the failure of these treatments are noncompliance with medication regimens and the development of antibiotic resistance in H. pylori. The eradication rates of H. pylori have been studied with many different approaches. As a secondary treatment for H. pylori, the Maastricht V/Florence Consensus Report suggests amoxicillin-fluoroquinolone triple or quadruple therapy (20). Since H. pylori have a low degree of in vivo antibiotic sensitivity, changing or adding antibiotics is also one of the fundamental treatments; nevertheless, doing so may increase treatment toxicity, antibiotic resistance, and dysbiosis of the gut microbiota, making the regimen more complicated. Numerous studies revealed the ineffectiveness of fluoroquinolone-containing triple therapy (21, 22). Compared to standard triple therapy involving clarithromycin, the PPI levofloxacin-amoxicillin triple therapy had a cure rate of 76%, according to a meta-analysis of randomized controlled trials (23).
The presented study hypothesized that replacing esomeprazole with the more potent acid inhibitor vonoprazan for 14 days would increase patient compliance because of its more convenient application, because all drugs in the VAL group are given after meals. On the other hand, according to the literature, esomeprazole is most effective when given 30 minutes before eating or drinking (24). As clarithromycin is avoided in our regimen, the treatment-related side effects are also reduced. Accordingly, patient compliance is raised, and thus the success rate of H. pylori eradication is increased.
Moreover, our VAL treatment plan was extremely successful, and the research outcomes demonstrate a statistically significant tendency towards greater eradication rates inside the VAL group than the EAL group. Our result of a greater eradication rate with vonoprazan (VAL) treatment as opposed to an esomeprazole therapy has a variety of pharmacological reasons. First, vonoprazan suppresses acid secretion more strongly and consistently than PPI (25). In contrast to esomeprazole, it is superior because it can maintain the stomach's pH at a level > 5 for nine out of every ten hours (13). In contrast, studies on esomeprazole revealed that it could maintain an intragastric pH of > 4 hours (26), which is lower than the intragastric pH obtained by vonoprazane. Therefore, the transport of antibiotics into the gastrointestinal mucosa highly depends on the effectiveness and duration of acid suppression. Treatment with vonoprazan is significantly more likely to increase the concentrations and stability of amoxicillin and levofloxacin in the gastrointestinal mucosa than treatment with PPIs (27).
In addition, previous research has demonstrated that a pH range of 6 to 8 may be optimal for eradicating H. pylori because this range increases the bacteria's reproductive capacity and the effectiveness of antibiotics dependent on bacterial growth (28). To get the same level of acid-inhibiting impact as other proton pump inhibitors (PPI), esomeprazole must first be taken for a minimum of five to six doses, which must be maintained throughout H. pylori therapy. Vonoprazan, on the other hand, has a substantially faster onset to attain its peak acid-inhibitory activity, probably after the first dosage (29). Thirdly, unlike the drug esomeprazole, the CYP2C19 polymorphism of vonoprazan does not affect its pharmacokinetic characteristics (23, 25). Accordingly, the CYP2C19 polymorphism status does not impact vonoprazan's ability to inhibit acids (25, 30). Compared to PPIs, vonoprazan had a higher proportion of H. pylori eradication, according to a study by Kiyotoki S et al. (2020). When triple treatment (vonoprazan, amoxicillin, and clarithromycin) was used to treat H. pylori, over 90 % of cases were cured (31). It was shown that vonoprazan-containing antibiotic therapy is a successful method of H. pylori eradication in a single-center, exploratory clinical study of patients 18 years of age or older who tested positive for the infection. It can achieve 100 % success in patients receiving its first treatment and even 91 % efficacy in those who have previously had eradication failure (32).
The study concluded that nausea was the most common side effect, and all other treatment-related adverse effects were minor and underreported for both groups. The incidence and severity of problems were similar between the two groups, with medication compliance rates in both groups over 80%. An interesting finding in our study is the combination of a high eradication rate for H. pylori (97.7%), associated with a compliance rate of over 80%, and minor treatment-related adverse effects. This association may be explained by using levofloxacin instead of clarithromycin, where the clarithromycin is associated with more adverse effects. Also, the extensive instructions, encouragement, and caution regarding adverse effects that patients got from their physicians may contribute to the higher compliance rate in our study.
Still, this study has some limitations; firstly, the urea breath test was not used because of unavailability. Secondly, the exact cause of the therapeutic failure of the VAL group, including whether it was caused by inadequate acid suppression, antibiotic resistance, or other factors, was not determined. The prevalence of CYP2C19 polymorphism and antimicrobial resistance within the sample population was not assessed, and the exact cause of treatment failure was not resolved. Even though this study was conducted in a single clinic in Egypt, there may be regional variations in the antibiotic resistance patterns of H. pylori or other relevant characteristics. These regional differences may affect how successfully the treatment strategy works. Larger multicenter trials, including susceptibility testing, are required to compare the eradication rates. Last but not least, we tried to minimize bias in open-label trial designs by employing randomization and precise measurement of the major results.
5. Conclusion
This study indicated that triple therapy based on vonoprzan achieved considerably higher eradication rates for H. pylori infection than that of esomeprazole. Additionally, the vonoprzan-based triple treatment was well tolerated. As a consequence of this, VAL has the potential to be regarded as an effective treatment plan for H. pylori, especially in countries with high rates of antibiotic resistance.
Acknowledgements:
All authors have contributed to writing, designing, compiling and editing the final manuscript
Data availability statement:
Data is available within the manuscript and will be provided at the editor's request.
Funding:
No funding sources are reported
Conflict of interest: None
References
- Hooi JK, Lai WY, Ng WK, Suen MM, Underwood FE, Tanyingoh D, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 153 (2017): 420-9.
- Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology 136 (2009): 1863-73.
- Muhammad JS, Sugiyama T, Zaidi SF. Gastric pathophysiological ins and outs of helicobacter pylori: a review. J Pak Med Assoc 63 (2013): 1528-33.
- Li H, Liang X, Chen Q, Zhang W, Lu H. Inappropriate treatment in Helicobacter pylori eradication failure: a retrospective study. Scandinavian Journal of Gastroenterology 53 (2018): 130-3.
- O'Connor JPA, Taneike I, O'Morain C. Improving compliance with Helicobacter pylori eradication therapy: when and how? Therapeutic advances in gastroenterology 2 (2009): 273-9.
- Sabaté E, Sabaté E. Adherence to long-term therapies: evidence for action: World Health Organization (2003).
- Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. Bmj 348 (2014).
- Sung JJ, Chung SS, Ling TK, Yung MY, Leung VK, Ng EK, et al. Antibacterial treatment of gastric ulcers associated with Helicobacter pylori. New England Journal of Medicine 332 (1995): 139-42.
- Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 59 (2010): 1143-53.
- Wu DC, Hsu PI, Wu JY, Opekun AR, Kuo CH, Wu IC, et al. Sequential and concomitant therapy with four drugs is equally effective for eradication of H pylori infection. Clinical Gastroenterology and Hepatology 8 (2010): 36-41. e1.
- Scott DR, Marcus EA, Sachs G. Vonoprazan: MarKed Competition for PPIs? Digestive diseases and sciences 61 (2016): 1783-4.
- Garnock-Jones KP. Vonoprazan: first global approval. Drugs 75 (2015): 439-43.
- Kagami T, Sahara S, Ichikawa H, Uotani T, Yamade M, Sugimoto M, et al. Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to CYP 2C19 genotype. Alimentary Pharmacology & Therapeutics 43 (2016): 1048-59.
- Ohkuma K, Iida H, Inoh Y, Kanoshima K, Ohkubo H, Nonaka T, et al. Comparison of the early effects of vonoprazan, lansoprazole and famotidine on intragastric pH: a three-way crossover study. Journal of Clinical Biochemistry and Nutrition 63 (2018): 80-3.
- Abdel-Aziz Y, Metz DC, Howden CW. potassium-competitive acid blockers for the treatment of acid-related disorders. Alimentary Pharmacology & Therapeutics 53 (2021): 794-809.
- Sugimoto M, Yamaoka Y. Role of vonoprazan in Helicobacter pylori eradication therapy in Japan. Frontiers in Pharmacology 9 (2019): 1560.
- Cuadrado-Lavín A, Salcines-Caviedes JR, Carrascosa MF, Dierssen-Sotos T, Cobo M, Campos MR, et al. Levofloxacin versus clarithromycin in a 10 day triple therapy regimen for first-line Helicobacter pylori eradication: a single-blind randomized clinical trial. Journal of Antimicrobial Chemotherapy 67 (2012): 2254-9.
- Elantouny NG, Abo Bakr AA, EL-Sokkary RH, Elshahat YE. Levofloxacin versus clarithromycin-based therapy for eradication of Helicobacter pylori infection: a comparative study. Zagazig University Medical Journal 25 (2019): 500-7.
- Zaki M, Othman W, Ali MA, Shehta A. Fluoroquinolone-resistant Helicobacter pylori strains isolated from one Egyptian University Hospital: molecular aspects. J Microbiol Antimicrobial Agents 2 (2016): 26-31.
- Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, et al. Management of Helicobacter pylori infection—the Maastricht IV/Florence consensus report. Gut 61 (2012): 646-64.
- Hsu PI, Chen WC, Tsay FW, Shih CA, Kao SS, Wang HM, et al. Ten-Day Quadruple Therapy Comprising Proton-Pump Inhibitor, Bismuth, Tetracycline, and Levofloxacin Achieves a High Eradication Rate for H elicobacter pylori Infection after Failure of Sequential Therapy. Helicobacter 19 (2014): 74-9.
- Kuo C-H, Hu H-M, Kuo F-C, Hsu P-I, Chen A, Yu F-J, et al. Efficacy of levofloxacin-based rescue therapy for Helicobacter pylori infection after standard triple therapy: a randomized controlled trial. Journal of Antimicrobial Chemotherapy 63 (2009): 1017-24.
- Marin AC, McNicholl AG, Gisbert JP. A review of rescue regimens after clarithromycin-containing triple therapy failure (for Helicobacter pylori eradication). Expert opinion on pharmacotherapy 14 (2013): 843-61.
- Shah SC, Iyer PG, Moss SF. AGA clinical practice update on the management of refractory Helicobacter pylori infection: expert review. Gastroenterology 160 (2021): 1831-41.
- Sakurai Y, Nishimura A, Kennedy G, Hibberd M, Jenkins R, Okamoto H, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK-438 (vonoprazan) doses in healthy male Japanese/non-Japanese subjects. Clinical and translational gastroenterology 6 (2015): e94.
- Hatlebakk J. gastric acidity− comparison of esomeprazole with other proton pump inhibitors. Alimentary Pharmacology & Therapeutics 17 (2003): 10-5.
- Vakil N, Megraud F. Eradication therapy for Helicobacter pylori. Gastroenterology 133 (2007): 985-1001.
- Sachs G, Scott DR, Wen Y. Gastric infection by Helicobacter pylori. Current gastroenterology reports 13 (2011): 540-6.
- Hunt RH, Scarpignato C. Potent acid suppression with PPIs and P-CABs: what's new? Current treatment options in gastroenterology 16 (2018): 570-90.
- Jenkins H, Sakurai Y, Nishimura A, Okamoto H, Hibberd M, Jenkins R, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Alimentary pharmacology & therapeutics 41 (2015): 636-48.
- Kiyotoki S, Nishikawa J, Sakaida I. Efficacy of vonoprazan for Helicobacter pylori eradication. Internal Medicine 59 (2020): 153-61.
- Gunaratne AW, Hamblin H, Clancy A, Magat AJMC, Dawson MVM, Tu J, et al. Combinations of antibiotics and vonoprazan for the treatment of Helicobacter pylori infections—Exploratory study. Helicobacter 26 (2021): e12830.