Von Hippel-Lindau Syndrome: Clinical Features, Genetics and Surveillance of a Family
Article Information
Roxana Dumitriu1*, Iulia Burcea1, 2, Roxana Dusceac1, 2, Catalina Poiana1, 2
Department of Pituitary and Neuroendocrine Disorders, C. I. Parhon National Institute of Endocrinology, Bucharest, Romania
Department of Endocrinology, University of Medicine and Pharmacy Carol Davila, Bucharest, Romania
*Corresponding author: Roxana Dumitriu, Department of Pituitary and Neuroendocrine Disorders, C. I. Parhon National Institute of Endocrinology, Bd. Aviatorilor no. 34-38, Bucharest, Romania
Received: 09 June 2020; Accepted: 18 June 2020; Published: 22 June 2020
Citation:
Roxana Dumitriu, Iulia Burcea, Roxana Dușceac, Cătălina Poiană. Von Hippel-Lindau Syndrome: Clinical Features, Genetics and Surveillance of a Family. Archives of Internal Medicine Research 3 (2020): 146-155.
View / Download Pdf Share at FacebookAbstract
Background: Von Hippel-Lindau (VHL) syndrome represents a rare familial condition caused by germline mutations of the tumorsuppressor gene VHL, which is located on the short arm of chromosome 3p25. Only 20% of the cases arise from de novo mutations, most patients having a positive family history with an autosomal dominant transmission. VHL is characterized by the presence of vascular tumors including hemangioblastomas of the cerebellum, spinal cord, brain stem and retina and the appearance of multiple cysts in different organs. Frequently, patients can associate clear cell renal carcinoma (RCC) and pheochromocytomas, or endolymphatic sac tumors.
Case reports: We present the cases of two sisters, diagnosed with VHL, with a positive family history (their father was diagnosed with VHL as well). The first patient, a 40 year old female, non-smoker, presented with multiple hemangioblastomas (right cerebellar, brainstem, right optic nerve, spinal cord), bilateral pheochromocytoma and multiple cysts (hepatic, renal and ovary) had a positive genetic analysis (VHL heterozygote mutation p.Arg167Gln) and underwent multiple surgical interventions. The second patient, her sister, 38 year old, presented with multiple emangioblastomas (cerebellar hemangioblastomas, right retinal hemangioblastoma), ovarian and breast cysts.
Conclusions: Patients with rare tumors characteristic of VHL (e.g. retinal or cerebellar hemangioblastomas) should be evaluated clinically and molecularly for VHL. This includes reviewing a detailed family history focused on characteristic tumors.
Keywords
Von Hippel-Lindau, Genetic Sequencing, Genetic Disorders, Familial Inheritance
Von Hippel-Lindau articles, Genetic Sequencing articles, Genetic Disorders articles, Familial Inheritance articles
Article Details
Abbreviations:
VHL-Von Hippel Lindau; RCC-Renal Cell Cancer; MRI-Magnetic Resonance Imaging; CT-Computed Tomography; HIF-Hypoxia-Inducible Factors
1. Introduction
Von Hippel-Lindau disease is an autosomal dominant syndrome which is associated with a variety of tumors. The VHL gene coding sequence contains three exons, two isoforms of mRNA exist, reflecting the presence or absence of exon 2. Tumors appear following the loss or inactivation of the wild-type allele in a cell. Data shows that 20% of patients have germline mutations detected by Southern blot analysis, 27% missense mutations and 27% nonsense or frameshift mutations [1, 2]. Clinical heterogeneity is a hallmark of VHL, with four phenotypes that exhibit widely differing frequencies of pheochromocytoma and renal cancer [3, 4]. The VHL gene is a tumor suppressor gene that encodes two different proteins (pVHL) of 213 and 160 amino acids, respectively. Both gene products have a role in the degradation of the hypoxia-inducible factors (HIF-1α and HIF-2α): the pVHL proteins have the α domain that binds with the protein elongin, and the β domain of pVHL is open to bind HIF that has been hydroxylated. The complex binds ubiquitin that targets HIF for intracellular destruction by proteases and well oxygenated cells destroy HIF. In this way, cells are oxygen-deprived. Also, lack of functional pVHL can cause accumulation of HIF (that is a transcription factor that induces the production of VEGF- vascular endoth-elial growth factor, erythropoietin, erythropoietin receptor, glucose transporter-1 and platelet-derived growth factor-B that allow adaptation to hypoxia, but in excess probably enhance tumorigenesis).
Estimates of VHL prevalence and incidence vary widely. Prevalence is estimated at 1/53.000 and annual birth incidence at 1/36.000. Men and women are equally affected [3]. Mean age of onset (the age of first sign or symptom) is 25, 25 years old with a mean age at diagnosis of 30, 87 years. The most common appearance is represented by cerebellar hemangio-blastoma (34,9%) and the most common cause of death are the complications of cerebellar hemangioblastomas (47,7%). The life expectancy is under 50 years with a mean age at death of 40, 9 years [1, 2]. Signs and symptoms in patients with cerebellar hemangio-blastomas can include: headache, nystagmus, positional vertigo, hypertension, slurred speech, vomiting, wide-based gait and dysmetria. Data from the literature show that the cerebellar lesions are the most frequent tumors (44-72%) and the mean age of the appearance is 30 years old (age range 11-78), followed by renal cell carcinoma (40-70 %) with a mean onset age of 37 years old (age range 16-67) [5].
Typically, genetic testing is the standard method to diagnose VHL, but usually the diagnosis is established on clinical findings [6]. In a patient with family history of VHL, the presence of a single retinal or cerebellar hemangioblastoma, pheochromocytoma or RCC is considered sufficient to justify the diagnosis. Multiple pancreatic cysts are enough for the diagnosis, but renal or epididymal cysts alone are not enough, due to their frequent occurrence in the general population. Spinal hemangioblastomas are more specific, 80% of them are due to VHL and can be found in up to 59% of patients with VHL [7, 8]. VHL syndrome was found in 30% of patients with cerebellar hemangioblastomas [7]. The cumulative occurrence of cerebellar hemangioblastoma is 60,2%, retinal hemangioblastoma 41%, RCC 25, 3 %, spinal hemangioblastoma 14, 5% and pheochromo-cytoma 14, 5% [7, 9]. If the patient does not have a familial history of VHL, positive diagnosis requires the presence of two or more retinal hemangioblastomas or one hemangioblastoma and one visceral tumor. Pheochromocytoma can be the initial presentation. The prognostic of lifetime risk of pheochromocytoma can be determined by the underlying mutation, even in patients with no family history of VHL. Pheochromocytoma occurs in association with specific alleles (usually mutations opposed to deletions), therefore a family history of pheochromocytoma in association with VHL is an indication for surveillance in affected family members [10].
Specific VHL subtypes display genotype–phenotype correlations but, unlike other familial syndromes such as MEN-2, the phenotype in VHL has not yet been stratified at the codon level [11]. Clinical heterogeneity exhibits widely differing frequencies of pheochromocytoma and RCC [4]. In type 1 VHL, affected members of these families do not develop pheochromocytoma, they tend to have loss-of-function mutations (gene deletions, frameshifts, truncations). In type 2 VHL, patients carry missense mutations and are prone to develop pheochromocytomas. Type 2 can be subdivided into other 3 subtypes: type 2A – patients develop pheochromocytomas, hemangioblastomas, and low risk renal cell carcinoma; type 2B – patients develop pheochromocytomas, hemangioblastomas, and high risk renal cell carcinoma; type 2C - patients develop pheochromocytomas, but no hemangio-blastomas, and no renal cell carcinomas. Types 2A and 2B are distinguished by rare versus high rates (70%) of RCC, and type 2C carries the risk of pheochromocytoma alone [12]. In VHL type 2 patients with pheochromocytoma, 96% of germline mutations are of the missense type [12]. Von Hippel–Lindau (VHL) disease type 2A is an inherited tumor syndrome characterized by predisposition to pheochromocytoma, retinal hemangioma (RA), and central nervous system hemangioblastoma (HB). Family studies show a reduced penetrance of the clinical phenotype. A retrospective study that included 63 VHL patients from two large VHL kindreds (family 1: Y112H mutation and family 2: Y98H mutation) with pheochromo-cytoma/paraganglioma found that pheochromocytoma expressivity differed by genotype [11].
Studies on genotype–phenotype correlations confirmed that pheochromocytoma is linked to VHL missense mutations. The age at onset for VHL syndrome is significantly earlier and the age-related risks of RA and RCC are higher for individuals with nonsense or frameshift mutations compared to those with deletions. Importantly, the results of these studies provided valuable strategies for genetic counseling and clinical prophylactic surveillance for VHL family members [13]. In VHL, pheochromocytomas exclusively produce norepinephrine. Plasma free normetanephrine levels are usually elevated. When an abdominal CT scan shows a solid renal lesion, this must be surgically removed, because even simple cystic renal lesions are considered premalignant. If renal cysts are observed, they must be followed with dynamic thin-section CT scan every 6 months.
A suggested lifetime surveillance protocol for patients with VHL2 germline mutations is proposed: patient must self-monitor blood pressure, and report hypertension or any suspicious symptoms; age 10-15 years is recommended for yearly retinal examination, blood pressure measurement and plasma free normetanephrines level; after the age of 15, it is recommended a twice yearly physical examination with blood pressure measurements and plasma free normetanephrines; every two years MRI scanning (with intravenous contrast) of the brain and spinal cord and abdomen; for abnormal biochemical screening, MRI or CT scan of the abdomen with thin-sections adrenal cuts, and 123I-MIBG SPECT or 18F-FDA PET scan to confirm the identity of the small masses.
2. Case Reports
2.1 Case number 1
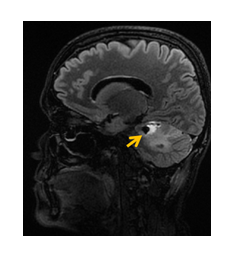
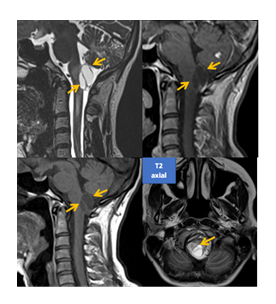
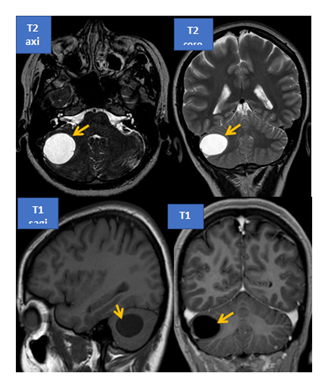
A 40 years old woman, non-smoker, with positive family history for VHL (her father and one sister were diagnosed with VHL) presented at the age of 18 years old with blurred vision, headache and unilateral visual loss (left eye). After the ophthalmological exam, the diagnosis of left eye retinal angiomatosis was established. The patient underwent multiple laser argon photocoagulation interventions and cryotherapy with poor outcome and with the development of proliferative retinopathy at the age of 23 years old. She developed glaucoma and cataract, with crystalline replacement. Also, she presented a hemangioblastoma which affected the right optic nerve. The cerebral MRI exam showed a right cerebellar hemangioblastoma (of the brainstem) (Figure 1). The patient underwent neurosurgical resection (posterior fossa craniotomy – total laminectomy of the first cervical vertebra and partial laminectomy of the second cervical vertrebra). The histopathological exam established the diagnosis – cerebellar hemangioblastoma (grade I WHO) and the genetic and molecular testing confirmed the presence of the germline heterozygote mutation VHL c.500G>A; p.Arg167Gln (at 32 years old). Further follow-up and MRI exams revealed the presence of spinal hemangioblastomas, (T12-L2) (Figure 2). Also, she had multiple cerebellar hemangioblastoma and one affecting the right optic nerve.
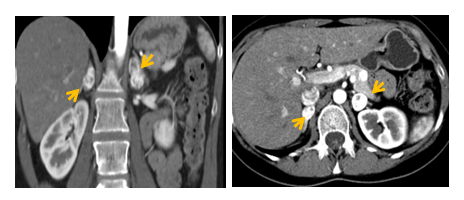
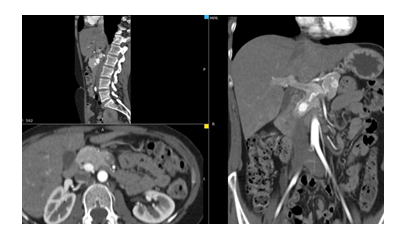
Initially, at presentation in our clinic (at the age of 33 years old), the full hormonal panel was normal, excepting a slightly elevated level of the plasmatic normetanephrines of 228 pg/ml (15-180), with normal plasmatic levels of metanephrines, 38 pg/ml (normal range: 10-90), neuroendocrine markers in normal range - plasmatic chromogranin A 59 ng/ml (20-125), specific neuronal enolase 6.94 ng/ml (0-12), plasmatic serotonin 152 ng/ml (80-450), urinary 5-hydroxy indole-acetic acid 1.84 mg/24 hours (1-10), ACTH 22.81 pg/ml (3-66), 8 am cortisol 24.23 micrograms/dl and normal thyroid function. An abdominal CT scan showed multiple cysts (pancreatic, hepatic, renal) and bilateral adrenal tumors (right – 18/16/25 mm, left – 20/18/29), and multiple nodular pancreatic lesions (borderline head and neck of the pancreas - 14/13 mm; head of the pancreas - 18/12 mm and 9/7mm) (Figure 3, 4). The clinical exam was normal, the patient was normotensive, with no signs or symptoms typical for pheochromocytoma.
Considering the slightly elevated value of the plasmatic normetanephrines, the presence of the bilateral adrenal masses and the presence of the heterozygote mutation VHL c.500G>A; p.Arg167Gln, the patient was referred to surgical intervention, and underwent minim-invasive partial bilateral adrenalectomy with tumor enucleation via retroperitoneal approach (histopathological exam confirmed bilateral pheochromocytoma). Postoperative evolution was favorable, with preserved adrenal function. Due to ocular complications related to the right optic nerve hemangioblastoma, the patient had another intervention with intradural and extradural decompression of the optic canal and tumor biopsy (34 years old).
The histopathological exam confirmed the nature of the tumor – optic nerve hemangioblastoma with optic canal extension (WHO grade I). Unfortunately, after one year, a surgical reintervention was needed for a complete microsurgical removal of the hemangioblastoma. Further, cerebral MRI follow-up showed the progression of a right cerebellar hemangioblastoma (25/24/20 mm) which was completely surgically resected (2019) with a good outcome. The most recent (41 years old) ophthalmologic exam showed a narrowing of the right visual field, loss of the left visual field with left eye ocular motility disorder (divergence) with pigmentary sequel lesions (right eye fundus) and undetectable left eye fundus.
Also, at the last evaluation, the CT abdomnial scan revealed a hypodense hepatic lesion in segment VIII (1.11cm /1.18cm /1.07cm, 55 UH-59 UH), and a right renal cyst.
2.2 Case number 2
The second patient, a 38 years old female, non-smoker, with positive familial history of VHL (her sister – the first patient and her father) was diagnosed with VHL when she came with a right ovarian cyst at 26 years old (she underwent minimal invasion resection of the cyst). At the age of 36 years old, MRI examination revealed right retinal hemangioblastoma and bilateral cerebellar hemangioblastomas (Figure 5). Neurosurgical resection of the right retinal hemangioblastoma and right cerebellar hemangioblastoma was performed via retro-sigmoid approach; postoperatively, the patient had hearing loss, which was resolved surgically. For the right retinal hemangioblastoma, she underwent multiple interventions for retinal depigmentation and retinal exudates, with right eye visual loss. The patient associated multiple cysts (breast, ovary) and nephrolithiasis (which was resolved via ureteroscopy–at the age of 33 years).
The most recent endocrinological evaluation showed a normal hormonal panel: plasmatic metanephrines 14.7 pg/ml (normal range: 10-90), and normetanephrines 42.9 pg/ml (20-200), and neuroendocrine markers in normal range - plasmatic chromogranin A 21.5 ng/ml (20-100), specific neuronal enolase 10.95 ng/ml (0-16.3), plasmatic serotonin 139.8 ng/ml (80-400), urinary 5-hydroxy indole-acetic acid 1.76 mg/24 hours (1-10), ACTH 17.81 pg/ml (3-66), 8 am cortisol 17.20 micrograms/dl and normal thyroid function. The pelvic sonographic exam showed a left ovarian cyst (14/16 mm), with breast ultrasonography showing bilateral multiple cysts (of maximum 7 mm). The ophthalmologic exam showed a right visual field loss, retinal depigmentation, with normal vascularization.
3. Discussions
The first patient was confirmed with VHLc.500 G>A (p.Arg167Gln), which was associated in studies with VHL type 2, and preferentially induces pheochro-mocytoma and retinal, cerebellar and spinal cord hemangioblastomas [14]. The frequency of pheochromocytomas and paragangliomas in patients with VHL has been estimated between 10% and 25% [15]. In most of the patients with this missense mutation, the pheochromocytomas were non-functional, as in our patient. Other data from genetic studies showed that p.Arg167Gln is associated with RCC and renal cysts in western populations (VHL type 1), but in Chinese populations it was associated with pheochromocytoma and VHL type 2 [14]. Missense mutations in codon 167, like p.Arg167Gln occur in all ethnic groups, and were also observed in Korean population [16]. Codon 167 was strongly associated with the development of pheochromocytoma, also missense mutations are more frequently associated with the development of pheochromocytoma, compared with other mutations [17]. Mutations in this codon have been found in approximately 43% of the American and Canadian families with VHL type 2 [17, 18, 19]. Genotype-phenotype correlations should indicate the best way of surveillance, as stated in the literature, pheochromocytoma screening should start in VHL families and children with missense mutations, before 5 years old [17]. The second patient presented at 26 years old with a right ovarian cyst and at 37 years old with right retinal hemangioblastoma and right cerebellar hemangioblastoma. She underwent multiple ophthalmologic interventions with poor outcome (vision loss of the right eye). Retinal hemangioblastomas are generally located in the peripheral temporal retina, but may rarely occur in the posterior pole and on the optic disc. Lesions are usually bilateral and multiple. The differential diagnosis should include Coat’s disease, retinal cavernous hemangioma, retinal macroaneurysm, and vaso-proliferative tumors [20]. In the retina, hemangioblastomas are slow-growing, benign tumors. Without treatment, however, they can cause complications such as macular edema, exudative and tractional retinal detachment, intravitreal hemorrhage, and neovascular glaucoma. There is no standard treatment approach to retinal hemangioblastomas. The treatment method varies depending on the location, size and related complications. Treatment options include laser photocoagulation, cryotherapy, photodynamic therapy, transpupillary thermotherapy, plaque radiotherapy, external beam radiotherapy, and vitreoretinal surgical ablation [20]. Intravitreal anti-vascular endothelial growth factor inhibitors have recently come into use for reducing exudation resulting from hemangioblastoma. Active surveillance is recommended. The likelihood of favorable outcome is lower with tumors located on or around the optic disc [20]. Both of the patients presented with ocular manifestations, and some data show that in half of the cases of VHL, ocular manifestations revealed the disease, and visual morbidity is associated with the retinal hemangioblastomas count, and with no other ocular or general characteristics and can be related with the type of germline mutation, but further genetic and clinical studies on large series are needed [21].
4. Conclusions
Clinical hallmarks of VHL syndrome are retinal and central nervous system hemangioblastomas, pheochromocytomas and a high risk of malignant transformation of the renal cysts in RCC. The wide variation of the clinical presentation, the positive diagnosis should be considered when a patient has more than one hemangioblastoma (brain, spinal cord, eye); a single hemangioblastoma (central nervous system, retina) plus a visceral manifestation (multiple renal, pancreatic, hepatic cysts, pheochromocytoma, renal cancer); positive family history plus any clinical manifestation stated; a positive mutation in the VHL gene. Hemorrhages associated with renal cancer and cerebral hemangioblastomas are the leading causes of mortality in these patients at young ages, systemic evaluation and monitoring is crucial. Routine follow-up is mandatory. The patients must avoid tobacco products, as well as chemical and industrial toxins and must avoid contact sports due to their renal and pancreatic lesions. The prognosis of the patients diagnosed with VHL depend on the number, location and complications of the tumors. Untreated, VHL may cause blindness and permanent brain damage. Early detection, genetic screening and treatment may significantly improve the prognosis. Multidisciplinary approach – ophthalmology, neurology, neurosurgery, endocrinology evaluations are necessary.
Conflict of Interest
The authors declare that they have no conflict of interest.
Declarations
Ethics approval
This manuscript is a report of two cases. All procedures performed in this case reports were in accordance with the ethical standards of the institutional research committee and the 1954 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Consent to publish: The authors affirm that the participants provided informed consent regarding publishing their data and the images in Figures 1, 2, 3, 4, 5. The participants have consented to the submission of the case report to the journal. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Funding
The authors declare no source of funding for this manuscript.
References
- Brien F J O’, Danapal M, Jairam S, et al. Manifestations of Von Hippel Lindau syndrome: a retrospective national review, QJM: An International Journal of Medicine 4 (2014): 291-296.
- Friedrich C A. Genotype-phenotype correlation in von Hippel-Lindau syndrome. Hum. Mol. Genet 10 (2001): 763-767.
- Sabine Schmid, Silke Gillessen, Isabelle Binet, et al. Management of von Hippel-Lindau Disease: an interdisciplinary review. Oncol Res Treat 37 (2014): 761-771.
- Neumann HP, Wiestler OD. Clustering of features of von Hippel–Lindau disease: Evidence of a complex genetic locus. Lancet 337 (1991): 1052-1054.
- Choyke P L, Glenn G M, Walther M M, et al. Von Hippel Lindau disease: genetic, clinical and imaging features. Radiology 194 (1995): 629-642.
- Grubb RL, Choyke PL, Pinto PA, et al. Management of von Hippel Lindau associated kidney cancer, Nat ClinPractUrol 2 (2005): 248-255.
- Maher E R, Yates J R, Harries R, et al. Clinical features and natural history of von Hippel-Lindau disease. Q. J. Med 77 (1990): 1151-1163.
- Filling-Katz M R, Choyke P L, Oldfield E, et al. Central nervous system involvement in von Hippel Lindau disease. Neurology 41 (1991): 41-46.
- Martz CH. Von Hippel Lindau disease: a genetic condition predisposing tumor formation. Oncol.Nurs. Forum, 18 (1991): 545-551.
- Maddock I R, Moran A, Maher ER, et al. A genetic register for von Hippel-Lindau disease. J. Med. Genet 33 (1996): 120-127.
- Sarah M Nielsen, Wendy S Rubinstein, Darcy L Thull, et al. Genotype–phenotype correlations of pheochromocytoma in two large von Hippel–Lindau (VHL) type 2A kindreds with different missense mutations. Am. J. Med. Genet 155 (2011): 168-173.
- Chen F, Kishida T, Yao M, et al. Germline mutations in the von Hippel–Lindau disease tumor suppressor gene: Correlations with phenotype. Hum Mutat 5 (1995): 66-75.
- Kai Ren Ong, Emma R Woodward, Pip Killick, et al. Genotype-phenotype correlations in von Hippel?Lindau disease. Hum. Mutat 28 (2007): 143-149.
- Liu Q, Yuan G, Tong D, et al. Novel genotype-phenotype correlations in five Chinese families with Von Hippel-Lindau disease. Endocrine Connections 7 (2018): 870-878.
- Stolle C, Glenn G, Zbar B, et al. Improved detection of germline mutations in the von Hippel-Lindau disease tumor suppressor gene. Hum Mutat 12 (1998): 417-423.
- Jee-Soo Lee, Ji-Hyun Lee, Kyu Eun Lee, et al. Genotype-phenotype analysis of von Hippel-Lindau syndrome in Korean families: HIF-α binding site missense mutations elevate age-specific risk for CNS hemangioblastoma. BMC Med Genet 17 (2016).
- Gustavo F C Fagundes, Janaina Petenuci, Delmar M Lourenco, Jr, et al. New Insights Into Pheochromocytoma Surveillance of Young Patients With VHL Missense Mutations, Journal of the Endocrine Society 3 (2019): 1682-1692.
- Gillian L Dalgliesh, Kyle Furge, Chris Greenman, et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature 463 (2010): 360-363.
- Dollfus H, Massin P, Taupin P, et al. Retinal hemangioblastoma in von Hippel-Lindau disease: a clinical and molecular study. Investigative Ophthalmology & Visual Science 43 (2002): 3067-3074.
- Sarah M Nielsen, Lindsay Rhodes, Ignacio Blanco, et al. Von Hippel-Lindau disease: genetics and role of genetic counseling in a multiple neoplasia syndrome. J Clin Oncol 34 (2016): 2172-2181.
- SahinAtik S, Solmaz AE, Öztas Z, et al. Von Hippel-Lindau Disease: The Importance of Retinal Hemangioblastomas in Diagnosis. Turkish Journal of Ophthalmology 47 (2017): 180-183.