Views and experiences of infertile women regarding the role of gluten in their infertility
Article Information
Justine Bold 1, 2,*, #, Dimitra Diamantopoulou1, #
1School of Allied Health and Community, University of Worcester, Worcester, WR2 6AJ, UK.
2Centre for Medical Education, School of Medicine, Cardiff University, Cardiff, CF14 4YS, UK.
*Corresponding Author: Justine Bold Senior Lecturer, The School of Allied Health & Community, University of Worcester, Worcester UK.
# Equal co-author authorship
Received: 22 November 2022; Accepted: 25 November 2022; Published: 05 December 2022
Citation: Justine Bold, Dimitra Diamantopoulou. Views and experiences of infertile women regarding the role of gluten in their infertility. Obstetrics and Gynecology Research 5 (2022): 296-310
View / Download Pdf Share at FacebookAbstract
Background
Prevalence rates for infertility have increased globally. Untreated coeliac disease (CD) and gluten sensitivity can affect fertility. Guidelines encourage testing of women with unexplained infertility for CD and case studies demonstrate pregnancies after introducing a gluten-free diet (GFD).
Aims
To understand the experiences of women diagnosed with infertility, investigating the potential role of gluten, including testing and implementation of a GFD.
Methods
Participants completed an online survey that included open and closed questions which were coded inductively and analysed using thematic and content analysis.
Findings
29 UK based women completed the survey. The majority identified as White, with one Asian/Asian British, one Mixed, and one Arab participant. Only four had not undergone fertility treatment. Twelve had unexplained infertility, while seven had no diagnosis. Five had primary infertility and five had a secondary infertility diagnosis. TA identified six themes: (1) Experience with gluten in infertility, (2) Experiences with testing for CD, (3) Health beliefs/concerns regarding gluten, (4) Other interventions to help with infertility, (5) Nutritional support for women with infertility, (6) Infertility experience. A GFD alleviated symptoms for those with both intestinal and extra-intestinal symptoms. Participants felt unprepared for CD testing and that healthcare personnel did not treat symptoms seriously.
Conclusion
Awareness of extraintestinal manifestations of CD, including unexplained infertility, should be increased amongst healthcare professionals. Women with unexplained infertility should be screened for CD, even without the presence of intestinal symptoms. Women with infertility choosing to implement a GFD need to be better supported.
Keywords
Female infertility; coeliac disease; reproductive disorders; gluten, non-coeliac gluten sensitivity; gluten free diet
Female infertility articles Female infertility Research articles Female infertility review articles Female infertility PubMed articles Female infertility PubMed Central articles Female infertility 2023 articles Female infertility 2024 articles Female infertility Scopus articles Female infertility impact factor journals Female infertility Scopus journals Female infertility PubMed journals Female infertility medical journals Female infertility free journals Female infertility best journals Female infertility top journals Female infertility free medical journals Female infertility famous journals Female infertility Google Scholar indexed journals coeliac disease articles coeliac disease Research articles coeliac disease review articles coeliac disease PubMed articles coeliac disease PubMed Central articles coeliac disease 2023 articles coeliac disease 2024 articles coeliac disease Scopus articles coeliac disease impact factor journals coeliac disease Scopus journals coeliac disease PubMed journals coeliac disease medical journals coeliac disease free journals coeliac disease best journals coeliac disease top journals coeliac disease free medical journals coeliac disease famous journals coeliac disease Google Scholar indexed journals reproductive disorders articles reproductive disorders Research articles reproductive disorders review articles reproductive disorders PubMed articles reproductive disorders PubMed Central articles reproductive disorders 2023 articles reproductive disorders 2024 articles reproductive disorders Scopus articles reproductive disorders impact factor journals reproductive disorders Scopus journals reproductive disorders PubMed journals reproductive disorders medical journals reproductive disorders free journals reproductive disorders best journals reproductive disorders top journals reproductive disorders free medical journals reproductive disorders famous journals reproductive disorders Google Scholar indexed journals gluten articles gluten Research articles gluten review articles gluten PubMed articles gluten PubMed Central articles gluten 2023 articles gluten 2024 articles gluten Scopus articles gluten impact factor journals gluten Scopus journals gluten PubMed journals gluten medical journals gluten free journals gluten best journals gluten top journals gluten free medical journals gluten famous journals gluten Google Scholar indexed journals non-coeliac gluten sensitivity articles non-coeliac gluten sensitivity Research articles non-coeliac gluten sensitivity review articles non-coeliac gluten sensitivity PubMed articles non-coeliac gluten sensitivity PubMed Central articles non-coeliac gluten sensitivity 2023 articles non-coeliac gluten sensitivity 2024 articles non-coeliac gluten sensitivity Scopus articles non-coeliac gluten sensitivity impact factor journals non-coeliac gluten sensitivity Scopus journals non-coeliac gluten sensitivity PubMed journals non-coeliac gluten sensitivity medical journals non-coeliac gluten sensitivity free journals non-coeliac gluten sensitivity best journals non-coeliac gluten sensitivity top journals non-coeliac gluten sensitivity free medical journals non-coeliac gluten sensitivity famous journals non-coeliac gluten sensitivity Google Scholar indexed journals gluten free diet articles gluten free diet Research articles gluten free diet review articles gluten free diet PubMed articles gluten free diet PubMed Central articles gluten free diet 2023 articles gluten free diet 2024 articles gluten free diet Scopus articles gluten free diet impact factor journals gluten free diet Scopus journals gluten free diet PubMed journals gluten free diet medical journals gluten free diet free journals gluten free diet best journals gluten free diet top journals gluten free diet free medical journals gluten free diet famous journals gluten free diet Google Scholar indexed journals successful pregnancy articles successful pregnancy Research articles successful pregnancy review articles successful pregnancy PubMed articles successful pregnancy PubMed Central articles successful pregnancy 2023 articles successful pregnancy 2024 articles successful pregnancy Scopus articles successful pregnancy impact factor journals successful pregnancy Scopus journals successful pregnancy PubMed journals successful pregnancy medical journals successful pregnancy free journals successful pregnancy best journals successful pregnancy top journals successful pregnancy free medical journals successful pregnancy famous journals successful pregnancy Google Scholar indexed journals fallopian tubes articles fallopian tubes Research articles fallopian tubes review articles fallopian tubes PubMed articles fallopian tubes PubMed Central articles fallopian tubes 2023 articles fallopian tubes 2024 articles fallopian tubes Scopus articles fallopian tubes impact factor journals fallopian tubes Scopus journals fallopian tubes PubMed journals fallopian tubes medical journals fallopian tubes free journals fallopian tubes best journals fallopian tubes top journals fallopian tubes free medical journals fallopian tubes famous journals fallopian tubes Google Scholar indexed journals fibroids articles fibroids Research articles fibroids review articles fibroids PubMed articles fibroids PubMed Central articles fibroids 2023 articles fibroids 2024 articles fibroids Scopus articles fibroids impact factor journals fibroids Scopus journals fibroids PubMed journals fibroids medical journals fibroids free journals fibroids best journals fibroids top journals fibroids free medical journals fibroids famous journals fibroids Google Scholar indexed journals polycystic ovarian syndrome articles polycystic ovarian syndrome Research articles polycystic ovarian syndrome review articles polycystic ovarian syndrome PubMed articles polycystic ovarian syndrome PubMed Central articles polycystic ovarian syndrome 2023 articles polycystic ovarian syndrome 2024 articles polycystic ovarian syndrome Scopus articles polycystic ovarian syndrome impact factor journals polycystic ovarian syndrome Scopus journals polycystic ovarian syndrome PubMed journals polycystic ovarian syndrome medical journals polycystic ovarian syndrome free journals polycystic ovarian syndrome best journals polycystic ovarian syndrome top journals polycystic ovarian syndrome free medical journals polycystic ovarian syndrome famous journals polycystic ovarian syndrome Google Scholar indexed journals
Article Details
1. INTRODUCTION
1.1 Infertility
Infertility affects approximately 48.5 million couples worldwide [1] with one in seven couples having difficulty conceiving in the UK [2]. It is defined as the inability of a couple to conceive after 12 months of unprotected sexual intercourse [3] (NICE, 2013). Prevalence rates have seen an increase globally [4], with female infertility increasing by 14.962% between 1990 and 2017, with the highest rate found among the 35 to 39-year-old group [5]. Infertility is categorised into primary, secondary, and unexplained. Primary infertility refers to a couple that has never conceived, whereas secondary infertility refers to prior conception that resulted in a successful pregnancy or may have resulted in a miscarriage or ectopic pregnancy, making re-conception challenging [6,7]. Finally, when no specific cause of infertility can be identified, the condition is referred to as unexplained infertility [6]. Infertility in women can be multicausal and can stem from reproductive disorders such as blocked fallopian tubes, fibroids, endometriosis, polycystic ovarian syndrome, primary ovarian insufficiency, hyperprolactinemia, pituitary cancer and hypopituitarism [7,8]. Also, there is a well-documented connection between eating disorders (ED), decreased fertility, and infertility [9,10]. Many women seeking reproductive therapy have a history of ED [11]. Severe psychological stress might also contribute to infertility [8,12].
1.2 Gluten and Infertility:
There is some literature highlighting the effects of untreated gluten-related disorders on women, including reproductive complications [6,13,14,15,16, and 17]. Many studies have suggested routine screening for coeliac disease (CD) in women with unexplained infertility, as subclinical CD or non-coeliac gluten sensitivity (NCGS) may be present [18, 19, 20, 21]. A gluten free diet (GFD) and its potential challenges have also been studied [22,23], but not in the context of infertility.
1.3 Coeliac Disease
CD is considered one of the most common lifelong food-related disorders and has a strong genetic component [14,21,24]. It is estimated that one percent of the general population in Western countries suffers from CD, and there is increasing evidence that the illness is underdiagnosed in non-Western nations that were formerly thought to be unaffected [25]. CD develops only in genetically predisposed individuals that possess alleles that encode the HLA-DQ2 or HLA-DQ8 proteins, which are the result of two HLA genes [6,21]. Populations like East and South-East Asians, African Americans, and sub-Saharan Africans do not have the high-risk HLADR3-DQ2 haplotype, so CD is uncommon compared to Europeans, people of European ancestry, as well as those from the Middle East, South Asia, Africa, and South America, where prevalence is higher [26]. Additionally, family history of CD influences the prevalence of CD, with first-degree relatives having a prevalence of one in ten and second-degree relatives having a prevalence of one in 39 [27]. The only treatment to date for CD is to avoid dietary gluten [6,28]. Gluten is a protein found in wheat, rye, barley, and triticale (a cross between wheat and rye), helping food maintaining structure by preventing crumbling. However, salad dressings, soups, and even food colouring may contain gluten as an ingredient [29]. More research is now focusing on another condition triggered by gluten ingestion known as NCGS [28]; however, there is no mention of it in the NICE guidelines in the UK, perhaps due to it being recently recognised as a new clinical entity [30].
1.4 Coeliac Disease and Infertility
A variety of female reproductive disorders, including menstrual cycle disorders, infertility, and adverse pregnancy outcomes have been described as non-classical patterns of clinical presentation of CD; however CD is scarcely considered during the evaluation of infertility [19,31]. Interestingly, a significant association has been found between endometriosis and CD [24]. These extraintestinal manifestations may be the only method for CD to be diagnosed in women although widespread consensus on which obstetric and gynaecological disorders should be investigated for CD is lacking [6]. Current estimates show eight undiagnosed cases for every diagnosed individual with CD [32], equalling approximately half a million undiagnosed cases in the UK [29]. When comparing screening studies with point prevalence data, only an estimated one in four instances of CD are detected in the UK, implyin g a sizable undiagnosed burden [33]. As for NCGS, no figures are currently available. While NICE guidelines in the UK for health professionals suggest screening for CD in unexplained infertility or recurrent miscarriages, these conditions are not considered a high-risk group for CD [3]. It remains unclear to what extent women with unexplained infertility or recurrent miscarriages are offered to test for CD by healthcare professionals or how many are provided with one upon the individual’s request. While understanding the prevalence of CD in infertile women is critical for establishing possible risk groups for CD screening, CD prevalence among these women appears to be a matter of debate, according to systematic reviews and meta-analyses on the topic [34,35,36]. Assumptions over the healthiness of a GFD by individuals are evident in research [37] potentially due to GF substitutes being higher in saturated fat, salt and sugar content and lower protein and fibre content [23,37,38]. However, increasing naturally GF foods such as fruit, vegetables, and GF grains whilst removing processed foods improves the diet's nutritional quality [39]. There does not appear to be any data currently exploring women’s experiences with GFD in relation to infertility.
1.5 Study’s Aims and Objectives
The overall aim is to understand more about the potential role of dietary gluten in female infertility and reproductive disorders and to understand the potential role, if any, that gluten has played in women’s infertility journey, whilst exploring women’s views and experiences regarding testing for CD and/or trying a GFD and identify potential motivators and barriers regarding implementing a GFD.
1.6 Value of this Research
Infertility is a growing issue that can have detrimental social and psychological consequences within a couple, often threatening their marriage and, in some cases leading to divorce [40]. Women tend to be more frequently blamed for being infertile [40]. Literature suggests this stigma is internalised and then transformed into self-stigmatization, manifesting as feelings of shame, worthlessness, inferiority, and decreased self-esteem, which can cause social isolation [7]. It is important to ensure that any underlying issues that could potentially affect natural or assisted reproduction are thoroughly investigated and addressed appropriately [7,40]. For women undergoing IVF, there is also a considerable financial burden that should be considered if they do not qualify for funded treatment. The NICE guidelines in the UK state that women who meet specific criteria are to be offered three cycles of IVF; however, not all clinical commissioning groups are complying as it is estimated that only in 2016 alone, 59% of IVF cycles done in the UK were funded privately. Even though the estimated cost of one IVF cycle is thought to be £3348, many ‘add-ons’ such as endometrial scratching, assisted hatching, and embryo glue might be required through the process can cost as much as £3500 [41] often with medication as an additional cost.
2. MATERIALS AND METHODS
2.1 Epistemological position
A postpositive perspective and a constructionist method was used. This paradigm is founded on the premise that truth, while based on data, is contextualised and formed by both the participant and researcher [42]. Qualitative research enables an in-depth exploration of events or experiences [43]. This approach is well-suited to studies of diet and lifestyle-related behaviours, such as this one, where the objective is to understand people's views and experiences for a specific population [43,44].
2.2 Data collection
An online qualitative survey was used, primarily as this research was undertaken during the COVID-19 pandemic. Although qualitative surveys have historically been used less frequently they can provide complex, in-depth data [45]. The survey was self-administered and all participants receiving the same set of questions in the same order. Participants responded by typing their responses in their own words as free text rather than selecting from pre-defined response options. Since online qualitative surveys provide a high level of perceived anonymity, they are also well-suited for research on sensitive topics [45].
2.3 Methods
The researchers adhered to the University of Worcester's ethics policies [46] and submitted a proposal for approval (approval number SAHC2021DD1). University of Worcester Data Protection Policy [47] and Data Protection Act [48] were followed. Participants were provided with a participant information sheet at the beginning of the survey, which ensured confidentiality and anonymity, and they were allowed to withdraw from the study before the final submission of the questionnaire. Prior to starting the survey, consent was obtained. Infertility is a sensitive topic that might cause distress. Within the information sheet, the participants were provided with links for organisations that provide helplines or online peer support.
2.4 Recruitment of participants and sampling
A total of 29 women from the UK who had been diagnosed with infertility or had been attempting to conceive for more than a year with no success were recruited. Recruitment was initiated online through a Charity called ‘Fertility UK’ and through an online peer support forum called ‘Fertility Friends’ and also via the University course Facebook group ‘Nutritional Therapy Students and Alumni Worcester’. So non-probability purposive sampling was used with convenience sampling, in which participants are selected based on their accessibility. Later a snowball sampling strategy was implemented by asking University postgraduate students on a nutrition course to share the study with anyone interested. The study was also featured on the Postgraduate course 'Nutrition Health Worcester' Facebook page in the same way it was promoted on the 'Nutritional Therapy Students and Alumni Worcester' Facebook closed group. Then the study was also shared on Instagram by the senior researcher, inviting people to share the study with anyone interested, again yielding snowball sampling. A post about the study was also shared on the 'British Association of Nutrition and Lifestyle Medicine (BANT) Members' Facebook group, with the BANT communications manager acting as the gatekeeper. The group members were asked to share the study with anyone who might be interested. This recruitment strategy was chosen since many nutrition professionals specialise in fertility issues and may have the required audience on social media or might have experienced it themselves.
2.5 Inclusion Criteria
Women that are UK residents were recruited according to the criteria below.
Inclusion criteria:
- • Women who have been diagnosed with primary, secondary, or unexplained infertility in the past
- • Women who have not been diagnosed with infertility but have been trying to conceive with no success for more than a year
- • Women who are currently in the process of seeking medical assistance for infertility in order to become pregnant
2.6 Exclusion criteria
Women who have received a coeliac disease, wheat allergy, gluten ataxia or dermatitis herpetiformis diagnosis before trying to conceive. No participants were excluded from the study.
2.7 Data Collection
Participant was presented with an information sheet and a consent form when they clicked on the survey link. These outlined the study's objectives and procedures as well as the participant's ability to withdraw from the study until before submitting the questionnaire. Confidentiality protocols were included in the information sheet. The survey transcripts were not accessible to anyone other than the two researchers. Qualitative data was collected and collated using the Survey Hero [49] online survey software. The survey was designed following the study's aims and objectives and was then piloted. After piloting the survey grammatical errors were amended, and the feature of selecting multiple answers for some of the closed questions was added. Questions included the effects of ingesting gluten, the accessibility of testing for CD, and the experience of following a GFD. The final questionnaire had nine open-ended questions followed by eight check-box questions; some were demographic, and others were related to the participant’s fertility journey and history of conditions linked to CD. The survey was active from the 18th of September until the 11th of October 2021.
2.8 Data analysis
Participants were assigned numbers. Through reading and re-reading the transcripts, points of interest were identified. Then an inductive approach was used to create an initial set of codes using line-by-line coding, this was then developed into a thematic map outlining superordinate and subordinate themes. The supervising researcher cross-checked the coding and set of themes and assisted in rearranging codes and reorganizing themes.
Thematic analysis (TA) is a method for deriving meaning from data by identifying and categorising themes within a data set using a qualitative paradigm [44]. Codes are classified according to overarching themes, which are then established to serve as a framework for discussing the findings. The data's most prominent features are discovered and evaluated according to the research question [50]. Researcher bias was mitigated by second member checking and keeping a reflective journal. The final codebook contained 101 codes. The codes were analysed and condensed into superordinate and subordinate themes.
3. FINDINGS AND DISCUSSION
3.1 Participants characteristics
29 women ranging in age from 24 to 45 years and over completed the survey. The majority of the women identified as White, with one Asian/Asian British, one Mixed, and one Arab participant. Twenty-five participants were from England, three from Scotland, and one from NI. Only four had not undergone fertility treatment. Twelve participants had unexplained infertility, while seven had not obtained a diagnosis but had been trying to conceive for more than a year. Five had primary infertility and five had a secondary infertility diagnosis.
|
Marker |
Number of respondents |
|
Age |
|
|
18-23 years old |
0 |
|
24-29 years old |
2 |
|
30-35 years old |
14 |
|
36-41 years old |
8 |
|
42-45 years old |
1 |
|
Over 45 years old |
4 |
|
Ethnicity |
|
|
White |
26 |
|
Asian/ Asian British |
1 |
|
Black/African/Caribbean/Black British |
0 |
|
Mixed |
1 |
|
Other1 |
1 |
|
Region |
|
|
England |
25 |
|
Scotland |
3 |
|
Wales |
0 |
|
Northern Ireland (NI) |
1 |
|
Fertility Treatment |
|
|
In the UK |
20 |
|
Outside the UK |
7 |
|
Not sought fertility treatment |
4 |
|
Fertility diagnosis |
|
|
Primary infertility |
5 |
|
Secondary infertility |
5 |
|
Unexplained infertility |
12 |
|
Not diagnosed with infertility |
7 |
1Other ethnicity included Arab
Table 1: Demographic information of participants
None of the participants had received a CD diagnosis, with only one participant having a first-degree relative with a CD diagnosis. Three participants were diagnosed with NCGS. In terms of CD comorbidities, ten participants were diagnosed with IBS, with one having an IBD diagnosis. Three of the participants had been diagnosed with endometriosis, and one participant had a thyroid disease. As for family history the mothers of six women were diagnosed with IBS and one with endometriosis.
|
Marker |
Number of respondents |
|
First degree relative with CD |
|
|
Yes |
1 |
|
No |
28 |
|
Other blood relative |
0 |
|
Diagnosis prior to infertility journey |
|
|
Coeliac disease |
0 |
|
Non-celiac gluten sensitivity |
3 |
|
Wheat allergy |
0 |
|
Gluten ataxia |
0 |
|
Dermatitis herpetiformis |
0 |
|
None of the above |
26 |
|
Diagnosis of participant |
|
|
Irritable bowel syndrome (IBS) |
10 |
|
Inflammatory bowel disease (IBD) (Ulcerative colitis or Crohn’s disease) |
1 |
|
Endometriosis |
3 |
|
Diabetes Type 1 |
0 |
|
Thyroid disease (Hashimoto’s, Grave’s disease) |
1 |
|
Systemic Lupus Erythematosus (SLE) |
0 |
|
Antiphospholipid syndrome |
0 |
|
I have not been diagnosed with any of these |
18 |
|
Maternal diagnosis |
|
|
Irritable bowel syndrome (IBS) |
6 |
|
Inflammatory bowel disease (IBD) (Ulcerative colitis or Crohn’s disease) |
0 |
|
Endometriosis |
1 |
|
Diabetes Type 1 |
0 |
|
Thyroid disease (Hashimoto’s, Grave’s disease) |
0 |
|
Systemic Lupus Erythematosus (SLE) |
0 |
|
Antiphospholipid syndrome |
0 |
|
Mother has not been diagnosed with any of these |
21 |
Table 2: Gluten-related disorders and comorbidities associated with CD within the family
3.2 Results of Thematic Analysis (TA)
TA identified six themes, with the first and second superordinate themes having seven and three subordinate themes accordingly. A graphic overview of superordinate and subordinate themes is shown below in Figure 1.
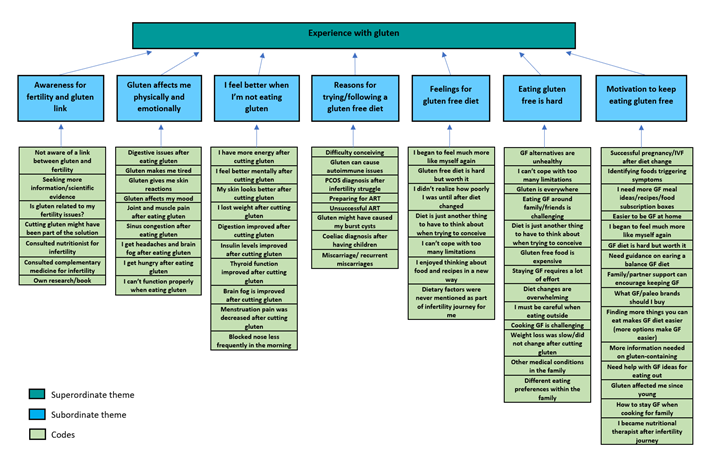
Figure 1: Part 1 of Thematic Map Showing Superordinate Themes and Subordinate Themes
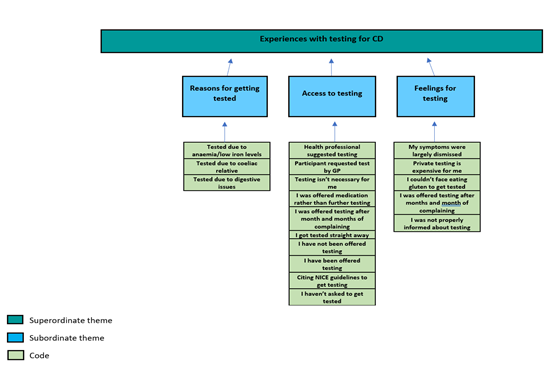
Figure 2: Part 2 of Thematic Map Showing Superordinate Themes and Subordinate Themes
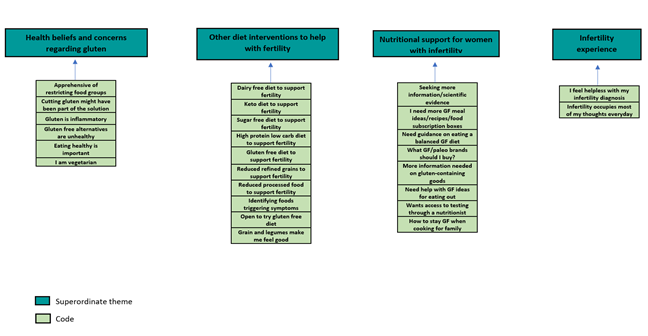
Figure 3: Part 3 of Thematic Map Showing Superordinate Themes
Participants are identified by numbers to retain anonymity. Themes pertinent to the study's purpose and objectives are the focus of the discussion, this includes experience with gluten in infertility and experience with CD testing.
4. DISCUSSION OF THE SUPERORDINATE THEMES
4.1 Superordinate theme 1: Experience with gluten in infertility journey
In this theme participants vocalised the challenges of a GFD while highlighting possible ways to overcome them and the effects of a GFD.
4.1.1 Gluten affects me physically and emotionally & I feel better when I am not eating gluten
When asked if they noticed changes after consuming gluten free (GF) foods in a closed question, almost all women reported reacting to gluten-containing foods. All participants who experienced intestinal or extra-intestinal symptoms reported improvement in at least one symptom when going on a GFD or abstaining from gluten-containing foods. Table 3 depicts most symptoms mentioned by the participants and their improvement due to following a GFD.
|
Quotations (Original text) |
|
|
Before following a GF diet |
After following a GF diet |
|
‘Eating foods like that makes me feel super sluggish. After eating food like this I almost can’t function properly’ (P27) |
‘I felt much more energetic’ (P27) |
|
‘Now that I’m mainly gluten free if I eat pizza or pastries I might get fatigue and tiredness after that I don’t normally get when I eat other foods’ (P25) |
‘I noted small changes such as having a blocked nose in the morning much less often’ (P25) |
|
‘sinus congestion, stomach problems, feeling extremely hungry for days after eating gluten’ (P17) |
‘Weight loss, felt very good, decrease in period pain’ (P17) |
|
‘Feeling bloated, constipated or diarrhoea the next day, often accompanied by a low mood.’ (P21) |
‘Initially I felt awful, like I was going through a purge but then I felt great and my skin and hair all improved, as did my overall mood, I felt less sluggish and happier. It was difficult but I enjoyed thinking about food and recipes in a new way. I also saw an improvement in my thyroid function after making these changes. I continued with the gf snd [sic] df and avoid processed food, it’s been 2.5 years now, and I on our third round of ivf, got pregnant with our little girl. The first round since making the changes.’ (P21) |
|
‘Joint and muscle pain, fatigue’ (P2) |
‘it is hard but it got rid of my symptoms and I feel well’ (P2) |
|
‘I see a definite change in my skin as well as bloating and discomfort after eating foods with gluten and wheat.’ (P26) |
‘helped with weight loss, reduced bloating, improved my skin condition but unfortunately we did not see any improvements in my fertility’ (P26) |
|
‘Bloating, sluggish feeling, blotchy skin’ (P16) |
‘Instantly within 2/3 days no reactions to food, no cramping or bloating. Felt more alert and less sluggish’ (P16) |
|
‘I felt lighter. I feel more clear headed if I'm not eating gluten.’ (P19) |
|
|
‘Before I started I was feeling very tired and low energy and as the months progressed I began to feel much more like myself again. I did loose a little bit of weight but only about half a stone. About 5 months after I started the diet i had a frozen day 5 embryo transfer and it was successful! This is my 3rd transfer and second pregnancy from that egg collection.’ (P24) |
|
|
‘Super bloated, lethargy, needing to go to the toilet quickly, heacldaches [sic]’ (10) |
‘Skin has cleared up, bloating has diminished, soreness has gone.’ (P10) |
Table 3: Quotations reflecting overall changes in perceived health of undiagnosed participants after trying a GF diet
Gastro-intestinal (GI) disturbances were the most frequently reported symptom following gluten consumption for most participants, which is consistent with literature for CD [14,21] and NCGS [17,51]. More than half of the participants reported several extraintestinal symptoms, sometimes in the absence of intestinal symptoms (see Table 3), corroborating recent findings that CD may manifest as extraintestinal symptoms in more than half of adult cases [14]. Extraintestinal symptoms are also prominent in NCGS [17,51], with recent literature highlighting a strong correlation to neurological symptoms [52]. Interestingly, the majority of the extraintestinal symptoms identified by participants in this study have been associated with CD and NCGS in the literature, including headaches, brain fog, fatigue, poor mood, skin issues, and joint pain [15,53].
Other areas where women noticed improvements after switching to a GFD were lower thyroglobulin antibodies, less pain during menstruation, and improved insulin levels. Not only is autoimmune thyroiditis the most common co-occurring autoimmune condition in CD patients [54], but infertility is quite prevalent in women with Graves' or Hashimoto's disease, affecting 50% of patients [55]. Improvements in thyroid function after following a GFD have been previously reported in studies [56,57]. Although no research on menstrual pain as a symptom of CD was found, conditions such as endometriosis, which has been linked to CD [24] present with pain during menstruation, and the alleviation of pain after a GF diet has been reported in the literature [58].
It is worth noting that none of the participants had been diagnosed with CD, and only three had been diagnosed with NCGS; however, the majority of them displayed a clinical picture that fit a CD and NCGS diagnosis, and they saw improvements in their symptoms after following a GFD. These findings are consistent with research indicating that only one in every four cases in the UK is diagnosed, indicating a significant undiagnosed burden [33].
4.1.2 Eating gluten-free is difficult
Survey results from a closed question showed women had tried a GFD, a Paleo diet and grain-free diet. More than half of the participants who tried a GFD had difficulties adhering to it. When discussing GFD, participants frequently used words like 'difficult' or 'hard'.
‘If I stay with family or away on holiday for any length of time where I'm eating more unhealthily than usual I notice a big difference. I find it hard when eating out or staying with people.’ (P19)
‘I try to be as gluten free as possible living in a family house but do eat gluten on and off suffering with the reaction I get.’ (P4)
There is widespread consensus that CD affects not only individuals but also family and social structures [23,59]. Adhering to a group norm is a pleasurable experience [60], so being unable to align our diet with that of others through changes in food preferences and liking sets you apart from the group norm [61], which is why people with CD feel that they face social exclusion over their eating habits [62]. Efforts to align the diet with that of others can impact adherence to a GFD, as participants who lived with people who did not have CD were not as competent at managing a GFD as participants who lived alone [23]. In a recent study, participants who lived with family members described cooking GF meals for themselves and gluten-containing meals for their family members as a way of adhering to the GFD [23]. Individuals with CD have also reported that bringing their own food to social gatherings helped with adherence [59].
Another participant seemed overwhelmed by the changes she had implemented by going GF.
‘Also I've already cut out some of the things I enjoy, and it is beginning to make me feel like I can't take any comfort in anything I enjoy.’ (P22)
Indeed, adopting a GFD has been associated with information overload [63], and strict adherence to a GFD has been linked to feelings of 'desperation' [64]. In patients with CD, dietary counselling and follow-up reviews have been linked to increased GF dietary adherence, remission of disease-specific symptoms, and improved quality of life (QOL) [106,65]. Furthermore, coeliac support groups can provide practical guidance and assistance to people with coeliac disease, and membership in such organisations has been consistently associated with increased adherence [66,67]. Both strategies can aid in the learning of how to follow a GF diet, and the development of coping skills can help alleviate some of the issues associated with GF diet adaptation [68].
Price was also an issue, stating that GF foods are ‘quite expensive’(P24) and that they ‘wpuld [sic] stay gluten-free if it were easier and more affordable’ (P26). Most participants in studies cite affordability of a GFD as a significant barrier to adherence [62,69]; however, only a few participants in this study expressed dissatisfaction with the price of GF products. Some studies consider the limited availability of GF foods to be a burden in adhering to the GF diet [69,70], but this was not the case in this study since none of the participants reported difficulty finding GF products. This may be due to the increased availability of GF products mentioned in studies [23,62].
4.1.3 Motivators to keep eating GF
The most common answer given by participants was that having more options makes eating GF easier. For some, trying new recipes kept them motivated, with a participant stating that she ‘enjoyed thinking about food and recipes in a new way’ (P21). Another woman mentioned that getting recommendations from their nutritionist on pulse-based pasta instead of ‘supermarket gluten-free’ (P13) pasta that is ‘highly processed’ (P13) was helpful. Eating out and an overreliance on processed foods, which are frequently high in fat and sugar and low in fibre are two of the most significant barriers [71]. Cooking at home is a GFD management strategy that addresses both of these concerns. According to non-CD research, home cooking promotes a variety of positive effects, including a healthier dietary pattern, positive self-management behaviours, an increased willingness to incorporate complex dietary adjustments, and an improved QOL [71]. Participants agreed that it was easier to eat GF at home instead of eating out. This could be for several reasons, one of them being that the internet, social media and groups related to CD have made it easier to access information about how to prepare GF meals [23]. Another reason could be fear of cross-contamination when eating out, which has been described as a barrier to adherence by others [23,62], or the challenge of finding GF food when travelling, at work, on vacation, or eating out [72,73].
One of the reasons some women were motivated to stay GF was a successful pregnancy or an up-coming IVF cycle. Three women reported getting pregnant after making dietary changes, including going GF. One woman described how her partner's support encouraged her to stay GF and eventually become pregnant.
‘Was hard to start, really missed bread. But did it with my husband so we could encourage each other. We both lost weight and I felt a lot slimmer in my midriff, and no bloated feeling. I also got pregnant after 5 rounds of IVF.’ (P14)
Indeed, partners demonstrated the strongest concordances in eating patterns over time which could explain the successful adherence to GF diet [74]. Family and social networks also impact eating habits by encouraging or discouraging individuals from making the appropriate food choices [75].
Another finding of the survey was that weight loss seemed to be a goal for some participants. Obesity rates among women of reproductive age have risen dramatically during the last few decades [76]. In the UK, one in every five pregnant women is overweight at their prenatal appointment [77,78]. Women with high body mass index (BMI) are often restricted access to fertility treatment due to health concerns and poor clinical outcomes [79, 80]. Vahratian and Smith [81] reported that obese women were more likely to seek medical care for infertility but were less likely to receive it.
4.2 Superordinate theme 2: Experiences with testing for CD
This superordinate theme encompasses participants' experiences with CD testing, including their access to testing and feelings about the test, as well as their interactions with healthcare professionals and the test itself.
4.2.1 Access to coeliac disease testing for infertile women
There was an almost even mix between women who received a test and women who did not amongst participants. Every participant who requested a test received one. Participants who requested testing from their healthcare provider outnumbered those who received testing from their healthcare provider. This reflects increased CD awareness among participants, which agrees with findings indicating that general public awareness has increased in the UK over the last decade [82]. The researcher identified two possible explanations, the first of which is increased internet access, which has resulted in an increasing number of people learning about CD from peers and non-medical personnel rather than exclusively from healthcare professionals [83]. Additionally, infertile women are more likely to consult the internet for information on the topic [84]. Second, awareness efforts by support groups such as Coeliac UK are proving effective in raising awareness among the general public, as recent findings from a UK study show a 17% increase in the number of patients who see their GP and are later diagnosed with CD [85].
Interestingly, participants who were not offered testing for CD had reported experiencing GI issues and fatigue in survey questions, both of which are classical manifestations of CD and NCGS in the literature [14,21]. Findings from studies conducted around the awareness of health practitioners had conflicting results. While CD awareness is poor among General Practitioners (GPs) in France, with gastroenterologists more likely to recognise it [86], CD awareness is increasing among GPs in Finland, who are now responsible for diagnosis [87]. The case–finding strategy implemented by general practitioners in the United States of America and Italy has demonstrated that physician awareness campaigns improve physicians' knowledge of CD and its diagnosis [88,89]. As for the UK, findings showed that case-finding in CD in primary care is no higher than the expected prevalence. However, because the vast majority of patients tested in primary care fall into the 'classic CD' category, this finding likely reflects the need to educate those expected to perform opportunistic screening about CD's associations with other processes, such as autoimmune diseases and gynaecological conditions [29].
Notably, more than half of the participants in this study with unexplained infertility were not offered testing by their healthcare professional despite the fact that it is mentioned in the CD protocol by NICE [90] and has been suggested by studies [19,21]. Findings from an Australian qualitative study highlighted low prioritisation of comprehensive preconception healthcare engagement for health screening [91]. This further indicates that awareness regarding unexplained infertility and CD needs to be increased among health providers. Shah and Leffler [19] published proposed indications of CD testing in women with infertility or of childbearing age. Only a small number of respondents did not seem interested in getting tested, stating that ‘episodes happen less often now as I've made the link to certain foods and manage my diet around them.’ (P29) and ‘I have no diagnosis but I do try to live gf’ (P10). When it comes to CD, there is concern that self-treatment might lead to a more relaxed attitude towards avoiding gluten, increasing the risk of auto-immune diseases and low fertility, among others [30,92]. The same applies to NCGS since there is a higher chance that individuals with NCGS develop autoimmune disease [93,94].
4.2.2 Testing
Despite being offered CD serological testing, many participants expressed that they were not informed on the necessary diet prior to testing, so they were asked to retake the test, which seemed unrealistic given their inability to manage gluten-related symptoms.
‘Yes, it showed as negative but they hadn't explained to me the need to be eating gluten daily for many weeks in advance. I then couldn't face the prospect of eating it for such a period so i didn't test again- cant afford more accurate, private testing’ (P11)
‘Yes again recently however they told me I would need to eat gluten for 6 weeks before testing, this seems impossible as I feel very unwell after consuming any gluten’ (P17)
These results match previous findings in the UK, where participants felt that they did not receive enough information throughout the diagnostic process [95]. Primary care physicians' initiation of a GF diet before endoscopy was also mentioned in another study, and it involved serologically positive patients; however, it was conducted in the US [96]. NICE guidelines strongly advise individuals suspected of having CD to consume gluten in more than one meal per day for at least six weeks prior to the duodenal biopsy [97]. Initiating a GF diet before testing demonstrates a lack of clinical expertise regarding the appropriate method of diagnosis, implying a degree of medical inertia toward it [96]. As a result, patients with undetected CD suffer further harm due to possible repeat endoscopies and additional time spent on a gluten-containing diet. Some participants who underwent testing but did not receive a CD diagnosis continued to experience symptoms consistent with CD or NCGS after eating gluten. However, a UK study revealed that only 40% of endoscopies included four biopsy samples, demonstrating how the bulk of duodenal biopsies do not follow BSG recommendations [98]. Also, 12.4% of patients identified with CD had previously had a gastroscopy with no biopsy samples collected in the five years preceding their diagnosis [99].
The impact of this is evident in the more than doubled diagnostic rate observed in endoscopies performed according to the guidelines [99]. Taylor et al. [96] argue that this is a clear example of how diagnostic inertia towards CD can result in missed or delayed diagnosis. Some participants in this study were not satisfied with the treatment they received from their healthcare providers:
‘I visited the dr regarding ibs type symptoms which were largely dismissed, this was in the first year of struggling to conceive.’ (P21)
‘Blood test after many trips to the doctors and months and months of complaining’ (P12)
The literature reflects the participants’ multiple visits to the doctor before getting tested, with the average number of pre-diagnosis visits to general practitioners regarding symptoms being 7 for those diagnosed after 2000 [100]. According to a recent UK study, 1/5 of the participants reported lacking confidence in their health professional because they felt that their symptoms were not taken seriously or that their health professional did not listen [95]. The dissatisfaction with healthcare professionals was evident in the Santos [62] study, in which participants were dissatisfied with the treatment they received from their medical provider as well as their ability to identify their symptoms as CD. Some findings from the UK support this viewpoint, citing long delays in diagnosing CD as a result of an early misdiagnosis of IBS in individuals with classic CD symptoms [73]. Surprisingly, as shown in Table 2, the most common diagnosis amongst participants in this study was IBS.
The following themes are discussed in outline as they do not specifically relate to the aims and objectives of this study.
4.3 Superordinate theme 3: Health beliefs and concerns regarding gluten
This theme incorporates participants' perspectives on what constitutes a healthy diet and their perceptions of the health effects of a GF, grain-free, or Paleo diet, which are listed in Figure 3. When asked if they would consider trying a gluten-free diet during their infertility journey, most participants expressed concern about removing a food group from their diet. Some participants referred to the GF diet as ‘a restrictive diet’ (P11) or ‘severe intervention’ (P8) while others described GF alternatives as ‘awful’(P27), ‘processed’ (P27), with GF being labelled as ‘quite bad and full of preservatives’ (P25). Indeed, there are concerns about the healthiness of the GF diet in the literature [23], with studies showing that GF products contain more salt, sugar, and saturated fats, as well as less fibre and protein, than non-GF counterparts [37,38].
4.4 Superordinate theme 4: Other diet interventions to help with fertility
This theme includes all of the dietary interventions that participants reported trying to support fertility, as shown in Figure 3. Overall, women appeared to avoid foods such as processed foods, refined grains, and sugar, which are staples of the Western diet and have been linked to decreased fertility [101,102].
4.5 Superordinate theme 5: Nutritional support for women with infertility
This theme incorporates how participants expressed the areas they need help with to maintain a GFD, which can be found in Figure 3. The importance of receiving nutrition care and how it promotes adherence was highlighted.
4.6 Superordinate theme 6: Infertility experiences
This theme identified feelings surrounding infertility as expressed by one of the participants. The participant expressed her frustration with the fact that ‘there isn't seemingly a 'diagnosis' to my infertility’ (P22) and how ‘infertility occupies most of your thoughts everyday’ (P22). Feelings of hopelessness and difficulty coming to terms with infertility diagnosis are prevalent in the literature [103], and healthcare professionals including allied health professionals who work with infertile clients should be aware of how mental health may affect their adherence to dietary recommendations.
4.7 Limitations
This is a qualitative study of 29 participants and the majority of participants were white British women. Purposive sampling of a broader range of ethnic backgrounds and a larger sample could have resulted in a more diverse range of perspectives, particularly among ethnic groups where the social pressure to become a mother maybe stronger [104,105]. As a result, the findings may not be applicable to other women experiencing infertility, but as this is an original study, it provided a great insight into the experiences of infertile women in the UK. The online recruitment may have recruited technology-savvy individuals only; however, this was necessary due to COVID constraints. Themes analysed addressed the research's aims and objectives, additional findings that emerged from the rest of the themes, albeit interesting, did not fit the aim and objectives and so have not been reported in depth.
4.8 Recommendations for future research
Further research is needed to determine whether the findings in this study are consistent across the UK in terms of CD and infertility, particularly in areas with the highest incidence rates of CD, such as Yorkshire and the Humber [33]. A comparable study involving a diverse group of people from various socioeconomic backgrounds would also be insightful, as CD is thought to be more common in areas with the least socioeconomic deprivation [33]. A multi-centre study across countries would also provide further useful data. The current study's findings could also be used to create a questionnaire to investigate quantitatively how many women with unexplained infertility are offered serological screening for CD and how many consult a nutrition professional throughout their infertility journey in order to make the results more generalisable. It could also be beneficial to investigate healthcare professionals' perspectives on screening for CD and NCGS in women with unexplained or other types of infertility.
5. CONCLUSIONS
Both intestinal and extraintestinal symptoms were seen in infertile women, and they reported symptom improvement after following a GFD. Successful pregnancies following dietary changes that included switching to a GFD were also reported by some participants. Many women reported difficulty sticking to a GFD, citing difficulties with social interactions as well as feeling overwhelmed. Having more GF options in stores, workplaces, and while travelling would increase GFD adherence. Many participants were not offered CD testing by their healthcare practitioners despite experiencing symptoms associated with CD and NCGS, raising concerns about medical inertia and a lack of CD awareness amongst healthcare professionals. Failure to follow CD diagnostic guidelines resulted in women lacking information for the diagnostic process and having to repeat testing, which was avoided due to consequences they would endure by eating gluten. Furthermore, some women expressed dissatisfaction with their treatment, frequently feeling that their symptoms were being dismissed. Finally, ongoing nutrition care may be supportive for infertile women choosing a GFD on their infertility journey, and it may help improve both the experience of a GFD and adherence to a GFD.
References
- Mascarenhas M N, Flaxman S R, Boerma T et al. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. 9 (2017): e1001356.
- National Health Service (NHS). Infertility [Online article]. Accessed (2021).
- National Institute for Health and Care Excellence (NICE). Fertility problems: assessment and treatment [online document] Accessed (2021)
- Inhorn M C and Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Human Reproduction Update 21(2015): 411-426.
- Sun H, Gong T-T, Jiang Y-T et al. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990-2017: results from a global burden of disease study, 2017. Aging 11 (2019): 10952–10991.
- Schiepatti A, Sprio E, Sanders D S et al. Coeliac disease and obstetric and gynaecological disorders: where are we now?. European journal of gastroenterology & hepatology 31 (2019): 425-433.
- World Health Organisation (WHO). Infertility [Online article]. Accessed (2021).
- SingleCare. Infertility Statistics 2021: How many couples are affected by infertility? Accessed (2021)
- Kimmel M C, Ferguson E H, Zerwas S et al. Obstetric and gynecologic problems associated with eating disorders. The International journal of eating disorders 49 (2016): 260-275.
- Tabler J, Utz R L, Smith K R et al. Variation in reproductive outcomes of women with histories of bulimia nervosa, anorexia nervosa, or eating disorder not otherwise specified relative to the general population and closest-aged sisters. The International journal of eating disorders 51 (2018): 102-111.
- Freizinger M, Franko D L, Dacey M et al. The prevalence of eating disorders in infertile women. Fertility and sterility 93 (2010): 72-78.
- Easter A, Treasure J and Micali N. Fertility and prenatal attitudes towards pregnancy in women with eating disorders: results from the Avon Longitudinal Study of Parents and Children. BJOG: an international journal of obstetrics and gynaecology 118 (2011): 1491-1498.
- Bold J and Rostami K. Non-coeliac gluten sensitivity and reproductive disorders. Gastroenterology and hepatology from bed to bench 8 (2015): 294-297.
- Jericho H, Sansotta N and Guandalini S. Extraintestinal Manifestations of Celiac Disease: Effectiveness of the Gluten-Free Diet. Journal of pediatric gastroenterology and nutrition 65 (2017): 75-79.
- Therrien A, Kelly C P and Silvester J A. Celiac Disease: Extraintestinal Manifestations and Associated Conditions. Journal of clinical gastroenterology 54 (2020): 8-21.
- Hall SW and Day AS. Gynæcologic Symptoms in Patients with Non-cœliac Wheat Sensitivity. Digestive Diseases and Sciences 66 (2021): 1-2.
- Soresi M, Incandela S, Mansueto P et al. Gynecological Disorders in Patients with Non-celiac Wheat Sensitivity. Digestive diseases and sciences 66 (2021): 167-174.
- Pellicano R, Astegiano M, Bruno M et al. Women and celiac disease: association with unexplained infertility. Minerva medica 98 (2007): 217-219.
- Shah S and Leffler D. Celiac disease: an underappreciated issue in women's health. Women’s health 6 (2010): 753-766.
- Lasa J S, Zubiaurre I and Soifer L O. Risk of infertility in patients with celiac disease: a meta-analysis of observational studies. Arquivos de gastroenterologia 51 (2014): 144-150.
- Tersigni C, Castellani R, de Waure C et al. Celiac disease and reproductive disorders: meta-analysis of epidemiologic associations and potential pathogenic mechanisms. Human Reproduction Update 20 (2014): 582-593.
- Burden M, Mooney P D, Blanshard R J et al. Cost and availability of gluten-free food in the UK: in store and online. Postgraduate medical journal 91 (2015): 622-626.
- Garnweidner-Holme L, Sende K, Hellmann M et al. Experiences of managing a gluten-free diet on multiple levels of society: a qualitative study. BMC nutrition 6 (2020): 65-66.
- Stephansson O, Falconer H and Ludvigsson J F. Risk of endometriosis in 11,000 women with celiac disease. Human Reproduction 26 (2011): 2896-2901.
- Green P H R, Lebwohl B and Greywoode R. Celiac disease. Journal of Allergy and Clinical Immunology 135 (2015) 1099-1106.
- Schultz M, Shin S and Coppell K J. Awareness of coeliac disease among chefs and cooks depends on the level and place of training. Asia Pacific journal of clinical nutrition 26 (2017): 719-724.
- Butler M M, Kenny LC and McCarthy F P. Coeliac disease and pregnancy outcomes. Obstetric medicine 4 (2011): 95-98.
- Roszkowska A, Pawlicka M, Mroczek A et al. Non-Celiac Gluten Sensitivity: A Review. Medicina 55 (2019): 222.
- Celiac Disease Foundation. ‘What is Gluten?’ [Online article]. Accessed (2021)
- Harper L and Bold J. An exploration into the motivation for gluten avoidance in the absence of coeliac disease. Gastroenterology and hepatology from bed to bench 11(2018): 259-268.
- Casella G, Orfanotti G, Giacomantonio L et al. Celiac disease and obstetrical-gynecological contribution. Gastroenterology and hepatology from bed to bench 9 (2016): 241-249.
- Aziz I and Sanders D S. Emerging concepts: from coeliac disease to non-coeliac gluten sensitivity. The Proceedings of the Nutrition Society 71 (2012): 576-580.
- West J, Fleming K M, Tata L J et al. Incidence and prevalence of celiac disease and dermatitis herpetiformis in the UK over two decades: population-based study. The American journal of gastroenterology 109 (2014): 757-768.
- Singh P, Arora S, Lal S et al. Celiac Disease in Women With Infertility: A Meta-Analysis. Journal of clinical gastroenterology 50 (2016): 33-39.
- Castaño M, Gómez-Gordo R, Cuevas D et al. Systematic Review and Meta-Analysis of Prevalence of Coeliac Disease in Women with Infertility. Nutrients 11 (2019).
- Glimberg I, Haggård L, Lebwohl B et al. The prevalence of celiac disease in women with infertility-A systematic review with meta-analysis. Reproductive medicine and biology 20 (2021): 224-233.
- Melini V and Melini F. Gluten-Free Diet: Gaps and Needs for a Healthier Diet. Nutrients 11 (2019): 170.
- Jamieson J A, Weir M and Gougeon L. Canadian packaged gluten-free foods are less nutritious than their regular gluten-containing counterparts. 6 (2018): e5875.
- Staudacher H M and Gibson P R. How healthy is a gluten-free diet? British Journal of Nutrition 114 (2015): 1539-1541.
- Taebi M, Kariman N, Montazeri A et al. Infertility Stigma: A Qualitative Study on Feelings and Experiences of Infertile Women. International journal of fertility & sterility 15 (2021): 189-196.
- Howard S. The hidden costs of infertility treatment. BMJ 361 (2018): k2204.
- Cuthbertson L M, Robb Y A and Blair S. Theory and application of research principles and philosophical underpinning for a study utilising interpretative phenomenological analysis. Radiography 26 (2020): 94-102.
- Tuckerman J, Kaufman J and Danchin M. How to use qualitative methods for health and health services research. Journal of Paediatrics and Child Health 56 (2020): 818-820.
- Braun V and Clarke V. Successful Qualitative Research: A Practical Guide for Beginners. (2013).
- Braun V, Clarke V, Boulton E et al. The online survey as a qualitative research tool. International Journal of Social Research Methodology 24 (2020): 1-14.
- University of Worcester. Research Ethics and policy
- University of Worcester. Data Protection Policy [Online document].
- Data Protection Act. Data Protection Act 2018 [Online document].
- Survey Hero [Online survey software].
- Braun V, Clarke V and Gray D. Innovations in qualitative methods. The Palgrave handbook of critical social psychology (2017): 243-266.
- Carroccio A, Soresi M, Chiavetta M et al. Frequency and Clinical Aspects of Neurological and Psychiatric Symptoms in Patients with Non-Celiac Wheat Sensitivity. Nutrients 13 (2021).
- Julian T, Hadjivassiliou M and Zis P. Gluten sensitivity and epilepsy: a systematic review. Journal of neurology 266 (2019): 1557-1565.
- Sergi C, Villanacci V and Carroccio A. Non-celiac wheat sensitivity: rationality and irrationality of a gluten-free diet in individuals affected with non-celiac disease: a review. BMC gastroenterology 21(2021): 5-6.
- Demirezer Bolat A, Akin F E, Tahtaci M et al. Risk Factors for Polyautoimmunity among Patients with Celiac Disease: A Cross-Sectional Survey. Digestion 92 (2015): 185-191.
- Quintino-Moro A, Zantut-Wittmann D E, Tambascia M et al. High Prevalence of Infertility among Women with Graves’ Disease and Hashimoto’s Thyroiditis. International journal of endocrinology (2014): 982705.
- Krysiak R, Szkróbka W and Okopien B. The Effect of Gluten-Free Diet on Thyroid Autoimmunity in Drug-Naïve Women with Hashimoto’s Thyroiditis: A Pilot Study. Experimental and clinical endocrinology & diabetes: official journal, German Society of Endocrinology [and] German Diabetes Association 127 (2019): 417-422.
- Rasheed J, Hassan R, Khalid M et al. Frequency of autoimmune thyroiditis in children with Celiac disease and effect of gluten free diet. Pakistan journal of medical sciences 36 (2020): 1280-1284.
- Marziali M, Venza M, Lazzaro S et al. Gluten-free diet: a new strategy for management of painful endometriosis related symptoms? Minerva chirurgica 67 (2012): 499-504.
- Bacigalupe G and Plocha A. Celiac is a social disease: family challenges and strategies. Families, systems & health?: the journal of collaborative family healthcare 33 (2015): 46-54.
- Klucharev V, Hytönen K, Rijpkema M et al. Reinforcement Learning Signal Predicts Social Conformity. Neuron 61 (2009): 140-151.
- Higgs S. Social norms and their influence on eating behaviours. Appetite 86 (2015): 38-44.
- Santos A S. Celiac patients’ perceptions about the clinical and social consequences of possible late diagnosis of celiac disease. Demetra: Alimentação, Nutrição & Saúde 14 (2019).
- Zysk W, Glabska D and Guzek D. Social and Emotional Fears and Worries Influencing the Quality of Life of Female Celiac Disease Patients Following a Gluten-Free Diet. Nutrients 10 (2018).
- Dowd A J, Tamminen K A, Jung M E et al. Motives for adherence to a gluten-free diet: a qualitative investigation involving adults with coeliac disease. Journal of Human Nutrition and Dietetics 27 (2014): 542-549.
- Gladys K, Dardzinska J, Guzek M et al. Expanded Role of a Dietitian in Monitoring a Gluten-Free Diet in Patients with Celiac Disease: Implications for Clinical Practice. Nutrients 13 (2021): 1859.
- Leffler D A, Edwards-George J, Dennis M et al. Factors that influence adherence to a gluten-free diet in adults with celiac disease. Digestive diseases and sciences 53 (2008): 1573-1581.
- Silvester J A, Weiten D, Graff L A et al. Is it gluten-free? Relationship between self-reported gluten-free diet adherence and knowledge of gluten content of foods. Nutrition 32 (2016): 777-783.
- Barratt S M, Leeds J S and Sanders D S. Quality of life in coeliac disease is determined by perceived degree of difficulty adhering to a gluten-free diet, not the level of dietary adherence ultimately achieved. J Gastrointestin Liver Dis 20 (2011): 241-245.
- Vriesekoop F, Wright E, Swinyard S et al. Gluten-free Products in the UK Retail Environment. Availability, Pricing, Consumer Opinions in a Longitudinal Study. International Journal of Celiac Disease 8 (2020): 95-103.
- Singh J and Whelan K. Limited availability and higher cost of gluten-free foods. Journal of human nutrition and dietetics?: the official journal of the British Dietetic Association 24 (2011): 479-486.
- Wolf R, Morawetz M, Lee A et al. A Cooking-Based Intervention Promotes Gluten-Free Diet Adherence and Quality of Life for Adults with Celiac Disease. Clinical Gastroenterology and Hepatology 18 (2019).
- Villafuerte-Galvez J, Vanga R R and Dennis M. Factors governing long-term adherence to a gluten-free diet in adult patients with coeliac disease. Alimentary pharmacology & therapeutics 42 (2015): 753-760.
- Violato M and Gray A. The impact of diagnosis on health-related quality of life in people with coeliac disease: a UK population-based longitudinal perspective. BMC Gastroenterology 19 (2019): 68-69.
- Pachucki M A, Jacques P F and Christakis N A. Social network concordance in food choice among spouses, friends, and siblings. American journal of public health 101 (2011): 2170–2177.
- Monterrosa E C, Frongillo E A, Drewnowski A et al. Sociocultural Influences on Food Choices and Implications for Sustainable Healthy Diets. Food and Nutrition Bulletin 41 (2020): 59-73.
- NCD-RisC. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19•2 million participants. Lancet 387 (2016): 1377–1396.
- Kanagalingam M G, Forouhi N G, Greer I A et al. Changes in booking body mass index over a decade: retrospective analysis from a Glasgow Maternity Hospital. BJOG: An International Journal of Obstetrics & Gynaecology 112 (2005): 1431-1433.
- Heslehurst N, Rankin J, Wilkinson JR et al. A nationally representative study of maternal obesity in England, UK: trends in incidence and demographic inequalities in 619 323 births, 1989–2007. International Journal of Obesity 34 (2010): 420-428.
- Zachariah M, Fleming R and Acharya U. Management of obese women in assisted conception units: a UK survey. Human Fertility 9 (2006): 101-105.
- Balen A H, Anderson R A and Policy & Practice Committee of the BFS. Impact of obesity on female reproductive health: British fertility society, policy and practice guidelines. Human Fertility 10 (2007): 195-206.
- Vahratian A and Smith Y R. Should access to fertility-related services be conditional on body mass index?. Human reproduction 24 (2009): 1532-1537.
- Aziz I, Karajeh M, Zilkha J et al. Change in awareness of gluten-related disorders among chefs and the general public in the UK: a 10-year follow-up study. European journal of gastroenterology & hepatology 26 (2014): 1228-1233.
- Ludvigsson J F, Card T, Ciclitira P J et al. Support for patients with celiac disease: A literature review. United European gastroenterology journal 3 (2015): 146-159.
- Zelkowitz P, Robins S and Grunberg P. Searching for Infertility Information Online: Differences between Men and Women. iproc 2 (2016): 36-37.
- Chandler K and Robins G. Determining whether coeliac disease case-finding in primary care is better than random testing: a retrospective study. BJGP Open 3 (2019).
- Pham B N, Musset L, Chyderiotis G et al. Celiac disease diagnosis: impact of guidelines on medical prescription in France. Journal of digestive diseases 15 (2014): 435-443.
- Fuchs V, Kurppa K, Huhtala H et al. Factors associated with long diagnostic delay in celiac disease. Scandinavian journal of gastroenterology 49 (2014): 1304-1310.
- Berti I, Della Vedova R, Paduano R, et al. Coeliac disease in primary care: evaluation of a case-finding strategy.’, Digestive and liver disease?: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver 38 (2006): 461-467.
- Catassi C, Kryszak D, Louis-Jacques O et al. Detection of Celiac disease in primary care: a multicenter case-finding study in North America. The American journal of gastroenterology 102 (2007): 1454-1460.
- National Institute for Health and Care Excellence (NICE). Coeliac Disease: Recognition, Assessment and Management [Online article] Accessed (2021).
- Khan N N, Boyle J A, Lang A Y et al. Preconception Health Attitudes and Behaviours of Women: A Qualitative Investigation. Nutrients 11 (2019): 1490-1491.
- Haines M L, Anderson R P and Gibson P R. Systematic review: The evidence base for long-term management of coeliac disease. Alimentary pharmacology & therapeutics 28 (2008): 1042–1066.
- Carroccio A, D'Alcamo A, Cavataio F et al. High Proportions of People With Nonceliac Wheat Sensitivity Have Autoimmune Disease or Antinuclear Antibodies. Gastroenterology. 149 (2015): 596-603.
- Mansueto P, Soresi M, Candore G et al. Autoimmunity Features in Patients With Non-Celiac Wheat Sensitivity. The American journal of gastroenterology 116 (2021): 1015-1023.
- Crocker H, Jenkinson C and Peters M. Healthcare experiences and quality of life of adults with coeliac disease: a cross-sectional study. Journal of Human Nutrition and Dietetics 33 (2020): 741-751.
- Taylor M A, Blanshard R J, Naylor G et al. Do gastroenterologists have medical inertia towards coeliac disease? A UK multicentre secondary care study. BMJ open gastroenterology 8 (2021): e000544.
- National Institute for Health and Care Excellence (NICE). Coeliac disease. Quality standard [QS134], 2016 [online article]. Accessed (2021).
- Ludvigsson J F, Bai J C, Biagi F et al. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut 63 (2014): 1210-1211.
- Lebwohl B, Kapel R C, Neugut AI et al. Adherence to biopsy guidelines increases celiac disease diagnosis. Gastrointestinal endoscopy 74 (2011): 103-109.
- Gray A M and Papanicolas I N. Impact of symptoms on quality of life before and after diagnosis of coeliac disease: results from a UK population survey. BMC Health Services Research 10 (2010): 105-106.
- Nazni P. Association of western diet & lifestyle with decreased fertility. The Indian journal of medical research 140 (2014): 78-81.
- Skoracka K, Ratajczak A E, Rychter A M et al. Female Fertility and the Nutritional Approach: The Most Essential Aspects. Advances in nutrition 12 (2021): 2372-2386.
- Rooney K L and Domar A D. The relationship between stress and infertility. Dialogues in clinical neuroscience 20 (2018): 41-47.
- Sheoran P and Sarin J. Infertility in India: social, religion and cultural influence. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 4 (2017).
- Zarif GY, Haniye AS and Hamidreza K. Psychosocial Consequences of Female Infertility in Iran: A Meta-Analysis. Frontiers in psychiatry 11 (2020): 518961.
- Muhammad H, Reeves S and Jeanes Y M. Identifying and improving adherence to the gluten-free diet in people with coeliac disease. Proceedings of the Nutrition Society. Cambridge University Press 78 (2019): 418-425.
