Varying Transvenous Pressure Gradients in Different Entities of Aortic Stenosis
Article Information
Verena Veulemans1, Oliver Maier1, Kathrin Klein1, Amin Polzin1, Christian Jung1, Ralf Westenfeld1, Alexander Blehm2, Artur Lichtenberg2, Malte Kelm1,3, Tobias Zeus1
1Division of Cardiology, Pulmonology and Vascular Medicine, Heinrich Heine University, Medical Faculty, Moorenstr. 5, Düsseldorf 40225, Germany
2Division of Cardiovascular surgery, Heinrich Heine University, Medical Faculty, Moorenstr. 5, Düsseldorf 40225, Germany
3CARID (Cardiovascular Research Institute Düsseldorf), Moorenstr. 5, Düsseldorf 40225, Germany
*Corresponding author: Verena Veulemans, Division of Cardiology, Pulmonology and Vascular Medicine, Heinrich Heine University, Medical Faculty, Moorenstr. 5, Düsseldorf 40225, Germany
Received: 20 July 2019; Accepted: 29 July 2019; Published: 19 August 2019
Citation: Verena Veulemans, Oliver Maier, Kathrin Klein, Amin Polzin, Christian Jung, Ralf Westenfeld, Alexander Blehm, Artur Lichtenberg, Malte Kelm, Tobias Zeus. Varying Transvenous Pressure Gradients in Different Entities of Aortic Stenosis. Cardiology and Cardiovascular Medicine 3 (2019): 270-276.
View / Download Pdf Share at FacebookAbstract
Aims: Enhanced left ventricular end-diastolic pressure (LVEDP) has been shown to be associated with worse outcome after acute myocardial infarction, cardiac surgery and in patients with severe aortic stenosis (AS) undergoing transcatheter aortic valve replacement (TAVR). Nowadays, pulmonary artery wedge pressure (PCWP) has largely replaced direct measurement of LVEDP but several patient series have demonstrated a poor agreement between both methods. Different AS-entities by the meaning of normal-flow high-gradient (NFHG), paradoxical and true low-flow lowgradient ((p)LFLG) AS may be also linked to high left ventricular filling pressures that can be measured by LVEDP and PCWP. Therefore, we analyzed 1) role and agreement of LVEDP and PCWP in patients with highgrade AS and 2) influence of AS-entities on LVEDP/PCWP pressure gradients.
Methods and Results: From 2009 to 2018, a total of 788 patients with highgrade AS prior to TAVR, completed hemodynamic status and echocardiographic data were retrospectively enrolled. LVEDP was significantly higher as the PCWP (23.3 ± 8.4 vs. 19.0 ± 8.9; p<0.0001) and over-all LVEDP and PAWP showed medium correlation (r=0.37, 95%-CI=0.30-0.43; p<0.0001*). Surprisingly, patients with NFHG- (6.2 [4.8-7.5] mmHg) and pLFLG-AS (4.2 [3.2-5.1] mmHg) had a significantly higher transvenous pressure gradient than the LFLG-AS cohort (1.4 [-0.1-2.9] mmHg; p<0.0001 between LFLG- and NFHG-AS; p=0.0336 between LFLG- and pLFLG-AS). However, several influencing factors as main drivers for the transvenous pressure gradient were found in NFHG- and pLFLG-AS but not LFLG entity by multivariate analysis.
Conclusion: Our data indicate, that the influence on LVEDP/PCWP pressure gradients differ according to several AS-entities and the underlying pathologies with smallest LVEDP/PCWP pressure gradients in LFLG-AS, supposing that the use o
Keywords
Aortic Stenosis; Pulmonary artery wedge pressure; Myocardial infarction
Aortic Stenosis articles, Pulmonary artery wedge pressure articles, Myocardial infarction articles
Article Details
Abbreviations
AS=aortic stenosis
cpcPHT=combined postcapillary pulmonary hypertension
ipcPHT= isolated postcapillary pulmonary hypertension
LVEDP=left ventricular end-diastolic pressure
mPAP=mean pulmonary artery pressure
NFHG=normal-flow high-gradient
PCWP=pulmonary artery wedge pressure
(p)LFLG=(p) low-flow low-gradient
TAVR=Transcatheter Aortic Valve Replacement
Introduction
Enhanced left ventricular end-diastolic pressure (LVEDP) has been shown to be associated with worse outcome after acute myocardial infarction [1], cardiac surgery [2] and in patients with severe aortic stenosis (AS) undergoing transcatheter aortic valve replacement (TAVR) [3]. Nowadays, pulmonary artery wedge pressure (PCWP) has largely replaced direct measurement of LVEDP but several patient series have demonstrated a poor -potentially multifactorial- agreement between both methods [4-6]. Looking at AS hemodynamics, different AS-entities by the meaning of normal-flow high-gradient (NFHG), paradoxical and true low-flow low-gradient ((p)LFLG) AS are well-known to be associated with different outcomes. These findings may be also linked to high left ventricular filling pressures that can be measured by LVEDP and PCWP. In this single-center study, we analyzed 1) role and agreement of LVEDP and PCWP in patients with high-grade AS and 2) influence of AS-entities on LVEDP/PCWP pressure gradients.
Material and Methods
From 2009 to 2018, a total of 788 patients with high-grade AS prior to TAVR, completed hemodynamic status and echocardiographic data were retrospectively enrolled. The study procedures were in accordance with the Declaration of Helsinki and the institutional Ethics Committee of the Heinrich-Heine University and is registered at clinical trials (NCT01805739). Patients underwent coronary angiography prior to TAVR according to current recommendations. Mean pulmonary artery pressure (mPAP), PCWP and LVEDP were recorded. Pulmonary hypertension (PHT) was classified as pre-capillary (precPHT), isolated post-capillary (IpcPHT) or combined entity (cpcPHT) according to current recommendations. Normal-flow high-gradient AS (NFHG-AS), low-flow low-gradient aortic stenosis (LFLG-AS) and paradoxical LFLG-AS (pLFLG-AS) were classified according to current guideline definitions. Continuous variables were compared using a Student’s t-test or Kolmogorov-Smirnov. Analysis of variance (ANOVA) was calculated with appropriate post-hoc tests comparing more than 2 groups. For correlations of interest, Spearman correlation coefficients were calculated. Correlation coefficients of 0.8 to 1.0 and 0.5 to 0.8, respectively, indicate a very strong and strong positive correlation between two variables, whereas coefficients between 0.2 to 0.5 and 0.0 to 0.2 suggest medium and small correlations, respectively. The influence on LVEDP/PCWP pressure gradients was tested by univariate and multivariate regression analysis. The data analysis was performed using the statistical software SPSS (version 23.0, SPSS Inc., Chicago, IL, USA) and GraphPad Prism (version 6.0, Graphpad Software, San Diego, CA, USA). All statistical tests were 2-tailed, and a value of p < 0.05 was considered statistically significant.
Results
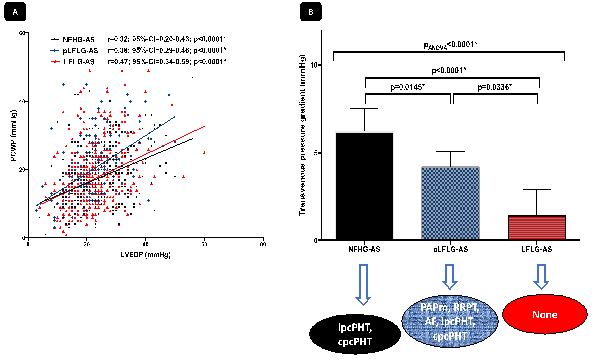
Over-all LVEDP was significantly higher as the PCWP (23.3 ± 8.4 vs. 19.0 ± 8.9; p<0.0001) and over-all LVEDP to PCWP showed only medium correlation in agreement (r=0.37, 95%-CI=0.30-0.43; p<0.0001). All patients were stratified into subgroups of AS-entity (NFHG-AS, pLFLG-AS and LFLG-AS). Surprisingly, patients with NFHG- (6.2 [4.8-7.5] mmHg) and pLFLG-AS (4.2 [3.2-5.1] mmHg) had a significantly higher transvenous pressure gradient than the LFLG-AS cohort (1.4 [-0.1-2.9] mmHg; p<0.0001 between LFLG- and NFHG-AS; p=0.0336 between LFLG- and pLFLG-AS). However, correlation of LVEDP and PCWP gradients within the several AS-entities remained at a medium level (Figure 1A).
LVEDP/PCWP gradients showed varying dependencies related to the particular AS-entities (please see Table 1): by multivariate regression analysis, ipcPHT (OR 0.27; 95%-CI 0.14-0.54; p<0.0001*) and cpcPHT (OR 0.34; 95%-CI 0.14-0.78; p=0.010*) were independently and inversely related to a pressure difference ≥5 mmHg between PCWP and LVEDP in NFHG-AS. Atrial fibrillation and multivalvular disease failed to reach significance. In pLFLG-AS, the LVEDP/PCWP pressure gradient was predominantly influenced by PAPm (OR 1.04; 95%-CI 1.01-1.07; p=0.013*), renal replacement therapy (OR 0.27; 95%-CI 0.10-0.74; p=0.011*), atrial fibrillation (OR 0.37; 95%-CI 0.23-0.59; p=<0.0001*), ipc- (OR 0.30; 95%-CI 0.16-0.55; p<0.0001*) and pcpPHT (OR 0.22; 95%-CI 0.10-0.59; p<0.0001*), whereas in LFLG-AS no relation to several influencing factors as main drivers for the transvenous pressure gradient were found (Figure 1B). Neither arterial hypertension, aortic valve area, cardiac index or previous surgical procedures took influence in this analysis.
(A) Correlation curves are shown for PCWP and LVEDP stratified for subgroups of AS-entities (NFHG-AS, pLFLG-AS and LFLG-AS). (B) Cumulative transvenous pressure gradients (LVEDP/PCWP) according to AS-entity and influencing factors based on multivariate regression analysis. NFHG=normal-flow high-gradient; (p)LFLG=(paradoxical) low-flow low-gradient.
|
Univariate analysis |
Multivariate analysis |
|||
|
A) NFHG-AS |
Regression slope (95-CI) |
p-value |
Regression slope (95-CI) |
p-value |
|
mPAP |
0.96 (0.94-0.99) |
0.006* |
- |
- |
|
AF |
0.42 (0.23-0.77) |
0.005* |
0.55 (0.29-1.05) |
0.068 |
|
Mv disease ≥ II° |
0.45 (0.25-0.82) |
0.009* |
0.56 (0.30-1.06) |
0.076 |
|
precPHT |
2.83 (0.81-9.89) |
0.103 |
- |
- |
|
ipcPHT |
0.37 (0.21-0.67) |
0.001* |
0.27 (0.14-0.54) |
<0.0001* |
|
cpcPHT |
0.50 (0.25-0.99) |
0.048* |
0.34 (0.15-0.77) |
0.0010* |
|
A) pLFLG-AS |
Regression slope (95-CI) |
p-value |
Regression slope (95-CI) |
p-value |
|
mPAP |
0.98 (0.96-1.00) |
0.063 |
1.04 (1.01-1.07) |
0.013* |
|
RRPT |
0.29 (0.11-0.73) |
0.009* |
0.27 (0.10-0.74) |
0.011* |
|
DM |
0.67 (0.44-1.02) |
0.060 |
- |
- |
|
AF |
0.34 (0.22-0.52) |
<0.0001* |
0.37 (0.23-0.59) |
<0.0001* |
|
ipcPHT |
0.53 (0.34-0.83) |
0.005* |
0.30 (0.16-0.55) |
<0.0001* |
|
cpcPHT |
0.55 (0.33-0.92) |
0.024* |
0.22 (0.10-0.59) |
<0.0001* |
|
B) LFLG-AS |
Regression slope (95-CI) |
p-value |
Regression slope (95-CI) |
p-value |
|
- |
- |
- |
- |
- |
Table 1: Parameters associated with the LVEDP-to-PCWP gap in patients with different AS-entities
AF=atrial fibrillation; aHT=arterial hypertension; DM=diabetes mellitus; mPAP=mean pulmonary artery pressure; cpcPHT=combined postcapillary pulmonary hypertension; ipcPHT=isolated postcapillary pulmonary hypertension; precPHT=precapillary pulmonary hypertension; mv=multivalvular
Discussion
The present study evaluating invasive hemodynamics of patients with severe AS revealed several findings:
- Patients with LFLG-AS had the smallest LVEDP/PCWP pressure gradient.
- Influence on LVEDP/PCWP pressure gradients differed according to several AS-entities and the underlying pathologies.
Clinically meaningful disagreement between PCWP and LVEDP is common, especially in patients with atrial fibrillation, valve diseases, and changing stroke volumes [7]. Just in patients with AS, ventricular filling pressures are often multifactorial influenced [8]. Therefore, we stratified all patients prior to TAVR into subgroups of AS-entity. In NFHG- and pLFLG-AS patients, LVEDP and PCWP pressures were significantly different, resulting in high pressure gradients between 4 to 6 mmHg, supposing that under normal and mildly reduced flow-conditions covariables exert more influence on LVEDP than on PCWP leading to a higher difference. Surprisingly, patients with LFLG-AS had the smallest transvenous pressure (2 mmHg), so in this cohort highest PCWP with nearly equal LVEDP based on the interaction of multiple and different weighted influencers seems to equalize this difference. Weber et. al confirmed, that cpcPHT, atrial fibrillation and severe mitral regurgitation were characterized by a smaller difference between LVEDP and PCWP indicating that left ventricular pressure is more strongly reflected backwards into the pulmonary circulation [8]. These findings are fundamental, suggesting multiple influencing hemodynamic, functional and structural factors, that may result in a landscape of complex interactions. To our knowledge, this is the first study on LVEDP/PCWP gradients that shows varying dependencies related to the particular AS-entities. However, our study has confirmed previous data that LVEDP and PCWP may significantly differ and added new value on differences between several AS-entities, supposing that the use of PCWP should not be used unthinkingly as a surrogate for LVEDP.
Conclusions
Our data indicate, that the influence on LVEDP/PCWP pressure gradients differ according to several AS-entities and the underlying pathologies with smallest LVEDP/PCWP pressure gradients in LFLG-AS.
Limitations
Several limitations have to be addressed: Data on cardiac catheterization were obtained from reports and database platform, facilitating mistakes in documentation. The calculation of cardiac output based on indirect Fick-method is error-prone and may have had impact on all cardiac output- and stroke volume-derived measurements. Furthermore, LVEDP and PCWP were not measured simultaneously, possibly affecting accuracy of the measurements. However, this is one of the largest studies on invasive hemodynamics in patients with AS and our center has a long experience in hemodynamic assessment.
Data Availability
The research data used to support the findings of this study are available from the corresponding author upon request.
Conflict of Interest (COI) statement
Verena Veulemans, Tobias Zeus, and Ralf Westenfeld have received consulting fees, travel expenses, or study honoraries from Medtronic and Edwards Lifesciences. All other authors have nothing to disclose with regard to this project.
Funding Statement
None.
References
- Mielniczuk LM, Lamas GA, Flaker GC, et al. Left ventricular enddiastolic pressure and risk of subsequent heart failure in patients following an acute myocardial infarction. Congest Heart Fail 13 (2007): 209-214.
- Salem R, Denault AY, Couture P, et al. Left ventricular end-diastolic pressure is a predictor of mortality in cardiac surgery independently of left ventricular ejection fraction. Br J Anaesth 97 (2006): 292-297.
- Conte L, Fabiani I, Pugliese NR, et al. Left ventricular stiffness predicts outcome in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. Echocardiography 34 (2017): 6-13.
- Bitar A, Selej M, Bolad I, Lahm T. Poor agreement between pulmonary capillary wedge pressure and left ventricular end-diastolic pressure in a veteran population. PloS One 9 (2014): e87304.
- Halpern SD, Taichman DB, Bitar A, Selej M, Bolad I, Lahm T. Misclassification of pulmonary hypertension due to reliance on pulmonary capillary wedge pressure rather than left ventricular end-diastolic pressure poor agreement between pulmonary capillary wedge pressure and left ventricular end-diastolic pressure in a veteran population. Chest 136 (2009): 37–43.
- Oliveira RK, Ferreira EV, Ramos RP, et al. Usefulness of pulmonary capillary wedge pressure as a correlate of left ventricular filling pressures in pulmonary arterial hypertension. J Heart Lung Transplant 33 (2014): 157–162.
- Mascherbauer J, Zotter-Tufaro C, Duca F, et al. Wedge Pressure Rather Than Left Ventricular End-Diastolic Pressure Predicts Outcome in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail 5 (2017): 795-801.
- Weber L, Rickli H, Haager P, et al. Haemodynamic mechanisms and long-term prognostic impact of pulmonary hypertension in patients with severe aortic stenosis undergoing valve replacement. Eur J Heart Fail 21 (2019): 172-181.