Value of Ivabradine in Patients with Anterior ST-Elevation Myocardial Infarction: The VIVA-STEMI Study
Article Information
Ahmed Rezq1*, Marwan Saad1,2, Ahmed Al Mahmoudy1, Mostafa El Nozahi1
1Department of Cardiology, Ain Shams University, Cairo, Egypt
2Cardiovascular Institute, Department of Medicine, The Warren Alpert Medical School at Brown University, Providence, RI, USA
*Corresponding Authors: Ahmed Rezq, MD, Department of cardiology, Ain Shams University, Cairo, Egypt
Received: 05 October 2020; Accepted: 21 October 2020; Published: 03 November 2020
Citation: Ahmed Rezq, Marwan Saad, Ahmed Al Mahmoudy, Mostafa El Nozahi. Value of Ivabradine in Patients with Anterior ST-Elevation Myocardial Infarction: The VIVA-STEMI Study. Cardiology and Cardiovascular Medicine 4 (2020): 630-639.
View / Download Pdf Share at FacebookAbstract
Background: Role of ivabradine in patients with anterior wall ST-elevation myocardial infarction (STEMI) is unknown.
Methods: This is a two-center, randomized controlled, double-blinded trial, that included patients presenting with anterior wall STEMI and eligible for primary percutaneous coronary intervention (PPCI) from June 2016 through July 2018. After PPCI, patients were randomized (1:1) to receive bisoprolol plus ivabradine (ivabradine group) versus bisoprolol plus placebo (control group). Up-titration of ivabradine and/or bisoprolol was performed over a period of 6 weeks in all patients as tolerated. The primary outcome was resting heart rate at 6 weeks. Other secondary clinical outcomes were studied.
Results: A total of 670 patients were included. Ivabradine was associated with a significant reduction in heart rate compared to placebo at 2 weeks (73.0 ± 2.8 vs 78.2 ± 1.75 bpm, respectively, p<0.001), 4 weeks (67.1 ± 2.9 vs 73.3 ± 2.2 bpm, respectively, p<0.001), and 6 weeks (62.3 ± 2.1 vs 66.9 ± 2.9 bpm respectively, p=0.001). At 6 months, post-MI angina occurred in 30 (10.7%) versus 50 patients (17.6%) in ivabradine and control groups, respectively (p=0.022). Heart Failure (HF) hospitalizations were less in ivabradine group (14.6% versus 23.2%, p=0.010). At 12 months, there was a significant reduction in post-MI angina, and HF hospitalizations (16.7% versus 27.4%, p=0.002 and 21.7% versus 34.8%, p=0.001, in ivabradine and control groups, respectively).
Conclusions: Ivabradine, in addition to beta-blocker therapy, in patients with anterior STEMI was associated with superior heart rate control and clinical outcomes compared to placebo. Larger randomized controlled trials are encouraged to confirm role of ivabradine in these patients.
Keywords
Ivabradine; STEMI
Article Details
Abbreviations:
CAD-coronary artery disease; MACE-major adverse cardiac events; PCI-percutaneous coronary intervention; STEMI-ST-elevation myocardial infarction
1. Introduction
Coronary artery disease (CAD) remains a leading cause of death in many countries worldwide [1]. Multiple efforts aim at improving outcomes after myocardial infarction (MI) including revascularization and advancements in medical therapy. Among the determinants of outcomes after MI is the resting heart rate. Prior studies have shown that elevated heart rate (usually defined as higher than 75 beats per minute) at discharge and follow up are associated with worse outcomes and even increased mortality [2-4]. At the cellular level, elevated heart rate is associated with shortening of the length of cardiac cycle, minimizing diastolic perfusion time and thus worsening of the myocyte oxygen supply [2]. Such hemodynamic effects can also lead to impaired flow through collaterals resulting in a reduction of tissue perfusion in the jeopardized myocardial regions [3, 4] with potential impairment in contractility [4-6]. Ivabradine, a drug that selectively inhibits certain ion channel in the sinoatrial node (SAN) responsible for the pacemaker or “funny” (If) mixed sodium-potassium current, leads to slowing of the sinus rate with no effect on myocardial inotropic function or coronary vasomotor tone [7]. The role of Ivabradine in stable CAD was studied in multiple randomized controlled trials (RCT) and have demonstrated an overall neutral effect on clinical outcomes. The data about the role of ivabradine after MI is more limited. In patients presenting with inferior wall MI, ivabradine was equally effective as metoprolol in a single-center study [8]. So far, the role of ivabradine after anterior wall ST-elevation myocardial infarction (STEMI) has not been studied. This randomized controlled trial aims at examining the role of Ivabradine in addition to conventional beta-blocker therapy in patients with anterior wall STEMI.
2. Methods
2.1 Inclusion criteria
This study was a two-center, prospective, double-blinded, randomized controlled study conducted from June 2016 through July 2018 at the tertiary Department of Cardiology, Ain Shams University and Al-Asafra Hospital, Egypt. The main inclusion criteria were patients between the age of 18 and 90 years presenting with anterior STEMI eligible for primary PCI. Exclusion criteria included patients with a) prior thrombolysis; b) bleeding gastric ulcer or severe gastritis within 6 months; c) bleeding diathesis (e.g. advanced liver disease); d) cardiogenic shock; and e) cardiac arrhythmias (e.g. atrial fibrillation).
2.2 Study design
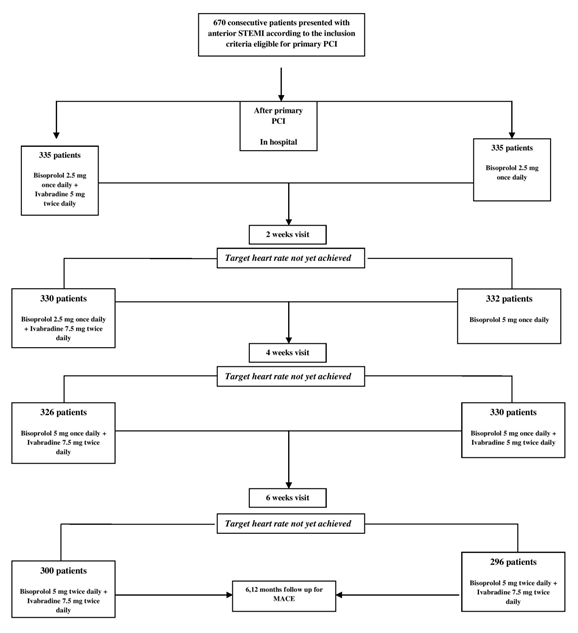
After obtaining a written consent from the patients, eligible patients willing to take part in the study were assigned in a 1:1 ratio to receive one of two treatments; bisoprolol 2.5 mg once daily plus ivabradine 5 mg twice daily (ivabradine group) or bisoprolol 2.5 mg once daily plus placebo (control group). Medical therapy was advanced during hospitalization then at 2-week intervals over the period of 6 weeks to the maximum tolerated doses aiming to reach a target heart rate of 60 beats per minute on a resting ECG. Figure 1 illustrates the study design. Randomization was performed before coronary angiography using a computer-generated list of random numbers. All patients provided written informed consent before randomization. Both the patients and physicians performing the primary PCI and follow up were blinded to the treatment.
2.3 Ethical committee approval
This study was performed according to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethical committees at both centers.
2.4 Patient and Public Involvement
Neither patients nor any public authority was involved in the study design or participated in any data collection or analysis. Patients were aware of the study after proper explanation of the protocol.
2.5 Endpoints
The primary endpoint was the resting heart rate at 6 weeks. Secondary endpoints included resting heart rate at 2 and 4 weeks, all-cause death, cardiovascular death, myocardial infarction (MI), target vessel revascularization, stroke, unstable (post-MI) angina, and heart failure hospitalization at 6 and 12 months after randomization. Clinical endpoints were defined according to guidelines [9].
2.6 Statistical analysis
Categorical variables are expressed as number and percentage of patients. Continuous data are reported as means and standard deviation as well as 95% confidence intervals when appropriate. Statistical analyses were performed with SPSS 18.0 (SPSS Inc., Chicago, Illinois).
3. Results
3.1 Baseline characteristics of the included cohort
A total of 670 patients met our inclusion criteria. The baseline characteristics were well balanced between the two treatment groups. The door-to-balloon time was similar between the ivabradine and placebo group (44.6 ± 22.7 mins vs. 53.7 ± 39.1 mins, P=0.275). No significant difference was noted between both groups regarding baseline TIMI flow (0.2 ± 0.5 vs. 0.2 ± 0.4 respectively, p=0.595) or thrombus burden (4.5 ± 1.1 vs. 4.7 ± 0.8 respectively, p=0.332). Echocardiographic data at the time of discharge were also similar between both groups. Details of the baseline, procedural and echocardiographic criteria are summarized in Tables 1-3.
3.2 Primary outcome
In-hospital resting heart rate was similar between ivabradine and control groups, with a trend towards lower resting heart rate with ivabradine (79.6 ± 2.4 vs. 81.4 ± 1.6 bpm, respectively, p=0.067). There was a significant reduction in heart rate with ivabradine at 2 weeks (73.0 ± 2.8 vs. 78.2 ± 1.75 bpm respectively, p<0.001), 4 weeks (67.1 ± 2.9 vs. 73.3 ± 2.2 bpm respectively, p<0.001), and 6 weeks (62.3 ± 2.1 vs. 66.9 ± 2.88 bpm respectively, p=0.001). All patients tolerated the maximum doses of ivabradine in the study design. No significant bradycardia or arrhythmias were observed in either group.
3.3 Secondary outcomes
At 6 months, 30 patients (10.7%) in the ivabradine group versus 50 patients (17.6%) in the control group suffered from post-MI angina (p=0.022). Hospitalization due to heart failure was also significantly less in the ivabradine group (14.6% versus 23.2%, p=0.010). Similarly, at 12 months, there was a significant reduction in post-MI angina and hospitalization due to heart failure (16.7 % versus 27.4%, p=0.002 and 21.7% versus 34.8%, p=0.001, respectively) in the ivabradine versus control groups. Cardiac death and TVR were similar between both groups at 6 and 12 months follow up.
|
Male, n (%) |
Ivabradine group (n=335) |
Control group (n=335) |
P-Value |
|
290 (86.7) |
301 (90.0) |
1.000 |
|
|
Age, years, mean ± SD |
55 ± 9.2 |
57 ± 11.6 |
0.672 |
|
Diabetes, n (%) |
134 (40.0) |
111 (33.3) |
0.789 |
|
Insulin Therapy, n (%) |
78 (23.3) |
44 (13.3) |
0.506 |
|
Hypertension, n (%) |
123 (36.7) |
123 (36.7) |
1.000 |
|
Dyslipidemia, n (%) |
44 (13.3) |
22 (6.7) |
0.671 |
|
Smoking, n (%) |
223 (66.7) |
257 (76.7) |
0.567 |
|
Ex-smoking, n (%) |
22 (6.7) |
21(6.7) |
1.000 |
|
Family history, n (%) |
33 (10) |
33 (10) |
1.000 |
|
Renal Impairment, n (%) |
0 (0) |
11 (3.3) |
0.986 |
Table 1: Baseline characteristics.
|
Duration from onset of pain till peak pain, min, mean ± SD |
Ivabradine group (n=335) |
Control Group (n=335) |
P-Value |
|
195.0 ± 203.3 |
204.2 ± 181.5 |
0.854 |
|
|
Duration from peak pain till arrival to the ER, hours, mean ± SD |
3.8 ± 2.8 |
4.2 ± 3.2 |
0.293 |
|
Door to balloon time, min, mean ±SD |
44.6 ± 22.7 |
53.7 ± 39.1 |
0.275 |
|
Culprit vessel |
|||
|
LAD, n (%) |
324 (96.6) |
335 (100) |
0.975 |
|
Diagonal, n (%) |
11 (3.3) |
0 (0) |
0.986 |
|
Site of occlusion |
|||
|
Ostial, n (%) |
22 (6.7) |
0 (0) |
0.492 |
|
Proximal third, n (%) |
201 (60) |
245 (73.3) |
0.412 |
|
Mid-segment, n (%) |
100 (30) |
90 (26.7) |
0.901 |
|
Distal third, n (%) |
12 (3.3) |
0 (0) |
0.899 |
|
Baseline TIMI flow, mean ± SD |
0.2 ± 0.5 |
0.2 ± 0.4 |
0.595 |
|
Thrombus burden, mean ± SD |
4.5 ± 1.1 |
4.7 ± 0.8 |
0.332 |
|
Stent diameter, mean ± SD |
3.2 ± 0.3 |
3.6 ± 0.4 |
0.247 |
|
Stent length, mean ± SD |
20.2 ± 3.1 |
21.4 ± 3.4 |
0.061 |
|
Gb IIb/IIIa inhibitors, n (%) |
0 (0) |
0 (0) |
|
|
Stent postdilatation using non complaint balloon, n (%) |
33 (10) |
44 (13.3) |
0.983 |
Table 2: Procedural data.
|
LV end diastolic dimension, mm, mean ± SD |
Ivabradine group (n=335) |
Control Group (n=335) |
P-Value |
|
55.4 ± 4.3 |
54.4 ± 5.8 |
0.831 |
|
|
LV end systolic dimension, mm, mean ± SD |
39.8 ± 5.1 |
40.8 ± 6.2 |
0.762 |
|
Left atrial dimension, mm, mean ± SD |
36.0 ± 3.3 |
38.3 ± 6.3 |
0.807 |
|
EF by M-mode, %, mean ± SD |
51.6 ± 8.6 |
49.7 ± 10.6 |
0.745 |
|
EF by 2D eyeballing, %, mean ± SD |
47.7 ± 8.9 |
48.2 ± 8.7 |
0.899 |
|
EF by Simpson’s technique, %, mean ± SD |
44.3 ± 7.5 |
43.6 ± 7.0 |
0.797 |
|
Pericardial effusion, n (%) |
0 (0) |
0 (0) |
EF= ejection fraction; LV= left ventricle
Table 3: Echocardiographic data.
|
In-hospital HR |
Ivabradine group |
Control group |
P-Value |
|
(n=335) |
(n=335) |
||
|
Day 1, bpm, mean ± SD |
87.9 ± 2.46 |
87.7 ± 3.1 |
0.875 |
|
Day 2, bpm, mean ± SD |
84.2 ± 2.85 |
84.8 ± 2.34 |
0.614 |
|
Day 3, bpm, mean ± SD |
79.6 ± 2.4 |
81.4 ± 1.6 |
0.067 |
|
2 weeks visit |
(n=330) |
(n=332) |
<0.001 |
|
HR at rest, bpm, mean ± SD |
73.0 ± 2.8 |
78.2 ± 1.75 |
|
|
Percentage achieving target HR, n (%) |
67 (20.3) |
58 (17.4) |
0.709 |
|
4 weeks visit |
(n=326) |
(n=330) |
<0.001 |
|
HR at rest, bpm, mean ± SD |
67.1 ± 2.9 |
73.3 ± 2.2 |
|
|
Percentage achieving target HR, n (%) |
194 (59) |
107 (32) |
0.370 |
|
6 weeks visit |
(n=300) |
(n=296) |
0.001 |
|
HR at rest, bpm, mean ± SD |
62.3 ± 2.1 |
66.9 ± 2.88 |
|
|
Percentage achieving target HR, n (%) |
242 (80.6) |
157 (53) |
0.07 |
BPM= beats per minute; HR= heart rate
Table 4: Primary outcome of resting heart rate.
|
In-hospital |
Ivabradine group |
Control Group |
P-Value |
|
(n=335) |
(n=335) |
- |
|
|
Bleeding, n (%) |
0 (0) |
0 (0) |
|
|
Stroke, n (%) |
0 (0) |
0 (0) |
- |
|
Death, n (%) |
0 (0) |
0 (0) |
- |
|
Cardiac death, n (%) |
0 (0) |
0 (0) |
- |
|
ST-segment Re-elevation (MI) , n (%) |
0 (0) |
0 (0) |
- |
|
TLR, n (%) |
0 (0) |
0 (0) |
- |
|
TVR, n (%) |
0 (0) |
0 (0) |
- |
|
Total MACE, n (%) |
0 (0) |
0 (0) |
- |
|
Re-catheterization, n (%) |
0 (0) |
0 (0) |
- |
|
6 months follow up |
(n=280) |
(n=284) |
|
|
Stroke, n (%) |
0 (0) |
0 (0) |
|
|
MI, n (%) |
0 (0) |
0 (0) |
- |
|
Death, n (%) |
0 (0) |
0 (0) |
- |
|
Cardiac death, n (%) |
0 (0) |
0 (0) |
- |
|
TLR, n (%) |
0 (0) |
0 (0) |
- |
|
TVR, n (%) |
0 (0) |
0 (0) |
- |
|
Post MI angina, n (%) |
30 (10.7) |
50 (17.6) |
0.022 |
|
Hospitalization for HF, n (%) |
41 (14.6) |
66 (23.2) |
0.010 |
|
12 months follow up |
(n=262) |
(n=276) |
- |
|
Stroke, n (%) |
0 (0) |
0 (0) |
|
|
MI, n (%) |
0 (0) |
0 (0) |
- |
|
Death, n (%) |
0 (0) |
0 (0) |
- |
|
Cardiac death, n (%) |
3 (0.8) |
5 (1.49) |
0.725 |
|
TLR, n (%) |
32 (11.4) |
41 (14.4) |
0.263 |
|
TVR, n (%) |
32 (11.4) |
41 (14.4) |
0.263 |
|
Post MI angina, n (%) |
47 (16.7) |
78 (27.4) |
0.002 |
|
Hospitalization for HF, n (%) |
61 (21.7) |
99 (34.8) |
0.001 |
MI: myocardial infarction. TLR: target lesion revascularization. TVR: target vessel revascularization. MACE: major adverse cardiac events
Table 5: Secondary outcomes.
4. Discussion
In this prospective, randomized controlled, double-blinded study comparing ivabradine versus placebo in addition to beta-blocker after anterior wall STEMI, we report an important finding. Compared with placebo, the use of ivabradine in addition to bisoprolol was associated with better control of resting heart at 2, 4, and 6 weeks, and was further associated with a lower risk of hospitalization for unstable angina or heart failure at 6 and 12-month follow up. Normally, heart rate is determined by the rate of spontaneous diastolic depolarization in the SA node [10]. Spontaneous diastolic depolarization is influenced by a mixed sodium–potassium current (If) across f-channels. If channels are directly and selectively inhibited by ivabradine, which results in reduced diastolic depolarization rates and the slowing of heart rate [11-14]. The beneficial role of ivabradine in patients with stable CAD was inconsistent across multiple randomized controlled trials. The anti-ischemic effects of ivabradine were compared with atenolol in the INITIATIVE study [15]. After 16 weeks of treatment, patients receiving 7.5 mg twice daily of ivabradine and those receiving atenolol (100 mg/day) had comparable advantageous effects on the exercise time and the occurrence of angina attacks per week. In addition to ivabradine’s non-inferiority in this study, data at 4 months of follow-up showed that all stress test variables, including time to limiting angina, time to angina onset and time to 1 mm ST-segment depression, were improved with ivabradine. Furthermore, Ivabradine was of value in patients with angina who had undergone coronary revascularization [16-18]. In a post hoc analysis from ADDITIONS [16] in 1193 patients with angina and history of PCI treated with ivabradine 5.0 mg or 7.5 mg twice daily for 4 months, the frequency of angina attacks was reduced from 1.9 ± 2.4 per week to 0.5 ± 1.5 per week and the rate of nitrate utilization was reduced from 2.7 ± 3.7 per week to 1.0 ± 1.9 per week (P<0.0001).
Lower resting heart rates was associated with improved outcomes after myocardial infarction. Despite that, the role of ivabradine in post-MI patients was even less studied, with some of the studies being on animal models [19]. A single-center study examined the role of ivabradine in patients with inferior wall STEMI and demonstrated its safety and efficacy when compared with metoprolol [8]. The current study is the first to challenge ivabradine vs placebo in addition to bisoprolol in patients with anterior wall STEMI and demonstrate the potential benefit of adding ivabradine in better controlling resting heart rate and lowering the risk of recurrent hospitalization for unstable angina or heart failure.
The role of beta-blocker therapy after MI cannot be underestimated, especially on the cardiac muscle performance, in addition to their mortality benefit. In patients where beta-blocker therapy is not enough to achieve target heart rates, ivabradine can play a beneficial role. It is important to note, however, that despite its role in reducing the risk of unstable angina and heart failure, that was not translated into a reduction in all-cause or cardiovascular mortality. Few explanations exist. First the sample size was relatively small, and the study was not powered to detect a difference in mortality. Second, the left ventricular systolic function, a major determinant of long-term mortality after STEMI, was comparable in both groups. Further studies are encouraged to examine the survival benefit of ivabradine in patients with anterior STEMI and heart failure.
We acknowledge some limitations in the current study. First, the majority of our patient population were of middle age, with single-vessel disease, and absence of multiple comorbidities. This limits the extrapolation of our results to more elderly and sicker patients where aggressive heart rate control may be associated with side effects. Second, the mean left ventricular EF after STEMI was 40-50% range, which did not allow the study of ivabradine in patients with significant systolic dysfunction.
5. Conclusions
Ivabradine in addition to bisoprolol in anterior STEMI offers better control of heart rate with potential benefit in reducing risk of post MI angina and heart failure hospitalization. Studies including more diverse cohorts of patients with anterior STEMI are encouraged to confirm our results.
References
- Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 139 (2019): 56-58.
- Giannoglou GD, Chatzizisis YS, Zamboulis C, et al. Elevated heart rate and atherosclerosis: an overview of the pathogenetic mechanisms. Int J Cardiol 126 (2008): 302-312.
- Heusch G, Yoshimoto N. Effects of heart rate and perfusion pressure on segmental coronary resistances and collateral perfusion. Pflugers Arch 397 (1983): 284-289.
- Heusch G. Myocardial ischemia: lack of coronary blood flow or myocardial oxygen supply/demand imbalance? Circ Res 119(2016): 194-196.
- Gallagher KP, Matsuzaki M, Koziol JA, et al. Regional myocardial perfusion and wall thickening during ischemia in conscious dogs. Am J Physiol 247(1984): 727-738.
- Heusch G. The regional myocardial flow-function relationship: a framework for an understanding of acute ischemia, hibernation, stunning and coronary microembolization. 1980. Circ Res 112 2013): 1535-1537.
- Koruth JS, Lala A, Pinney S, et al. The Clinical Use of Ivabradine. J Am Coll Cardiol 70 (2017): 1777-1784.
- Priti K, Ranwa BL, Gokhroo RK, et al. Ivabradine vs metoprolol in patients with acute inferior wall myocardial infarction-"Expanding arena for ivabradine". Cardiovasc Ther 35 (2017).
- Hicks KA, Tcheng JE, Bozkurt B, et al. 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol 66 (2015): 403-469.
- DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol 55 (1993): 455-472.
- Bois P, Bescond J, Renaudon B, et al. Mode of action of bradycardic agent, S 16257, on ionic currents of rabbit sinoatrial node cells. Br J Pharmacol 118 (1996): 1051-1057.
- Bucchi A, Baruscotti M, DiFrancesco D. Current-dependent block of rabbit sino-atrial node I(f) channels by ivabradine. J Gen Physiol 120 (2002): 1-13.
- Canet E, Lerebours G, Vilaine JP. Innovation in coronary artery disease and heart failure: clinical benefits of pure heart rate reduction with ivabradine. Ann N Y Acad Sci 1222 (2011): 90-99.
- Niccoli G, Borovac JA, Vetrugno V, et al. Ivabradine in acute coronary syndromes: Protection beyond heart rate lowering. Int J Cardiol 36 (2017): 107-112.
- Tardif JC, Ford I, Tendera M, et al. Efficacy of ivabradine, a new selective I(f) inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J 26 (2005): 2529-2536.
- Werdan K, Ebelt H, Nuding S, et al. Ivabradine in combination with Beta-blockers in patients with chronic stable angina after percutaneous coronary intervention. Adv Ther 32 (2015): 120-137.
- Zarifis J, Grammatikou V, Kallistratos M, et al. Antianginal Efficacy of Ivabradine in Patients With History of Coronary Revascularization.Angiology 68 (2017): 10-18.
- Mangiacapra F, Colaiori I, Ricottini E, et al. Heart Rate reduction by IVabradine for improvement of ENDothELial function in patients with coronary artery disease: the RIVENDEL study. Clin Res Cardiol 106 (2017): 69-75.
- Cao X, Sun Z, Zhang B, et al. The Effects of Ivabradine on Cardiac Function after Myocardial Infarction are Weaker in Diabetic Rats. Cell Physiol Biochem 39 (2016): 2055-2064.