Utility of Bio-Degradable Bile Duct Stents Following Bile Leaks and Pre-Operative Stone Clearance: International, Prospective, Multi-Centre, Observational Cohort Study
Article Information
Suresh Vasan Venkatachalapathy1, Martin W James1, Deepak Joshi2, Gavin Johnson3, Simon Philpotts3, Manu Nayar4, Matthew Huggett5, Danny Cheriyan6, Christopher Clarke1, Damilola Olajide1, Aloysious D Aravinthan1, Guruprasad P Aithal1
1NIHR Nottingham Biomedical Research Centre, Nottingham University Hospitals NHS Trust, and School of Medicine, University of Nottingham, Nottingham, United Kingdom
2Institute of Liver Studies, King’s College Hospital NHS Foundation Trust, London, United Kingdom
3University College London Hospitals NHS Foundation Trust – Department of Gastroenterology, London, United Kingdom
4Population Health Sciences Institute, Newcastle University & Newcastle Upon Tyne Hospitals NHS foundation Trust, United Kingdom
5Leeds Teaching Hospitals NHS Trust – Gastroenterology, Leeds, Leeds, United Kingdom
6Department of Gastroenterology, Beaumont Hospital and RCSI, Dublin, Ireland
*Corresponding authors: Suresh Vasan Venkatachalapathy, NIHR Nottingham Biomedical Research Centre, Nottingham University Hospitals NHS Trust, and School of Medi-cine, University of Nottingham, Nottingham, United Kingdom.
Received: 06 December 2024; Accepted: 09 December 2024; Published: 21 August 2025
Citation: Suresh Vasan Venkatachalapathy, Martin W James, Deepak Joshi, Gavin Johnson, Simon Philpotts, Manu Nayar, Matthew Huggett, Danny Cheriyan, Christopher Clarke, Damilola Olajide, Aloysious D Aravinthan, Guruprasad P Aithal. Utility of Bio-Degradable Bile Duct Stents Following Bile Leaks and Pre-Operative Stone Clearance: International, Prospective, Multi- Centre, Observational Cohort Study. Journal of Biotechnology and Biomedicine. 8 (2025): 278-286.
View / Download Pdf Share at FacebookAbstract
Introduction: Endoscopic retrograde cholangio-pancreatography (ERCP) and biliary stenting provides faster resolution of bile leaks post-cholecystectomy, while its use pre-cholecystectomy remains controversial. In both scenarios, patients frequently need repeat procedures for removal of stent. Novel biodegradable stents may prevent the need for repeat procedures. We tested the utility of these in a prospective multi-centre observational study. Methods: Consecutive patients referred for ERCP, either for treatment of bile leaks or removal of bile duct stones (pre-cholecystectomy), were recruited from 6 tertiary centres in the United Kingdom and Ireland between December 2020 and August 2022. The primary objective was to assess the clinical success of biliary drainage with biodegradable stents. The secondary objectives were to assess the technical success, complications, patient discomfort, stent visibility on fluoroscopy, stent degradation, quality of life (QoL) before and after the procedure, and the health economics of these stents. Results: Sixty-five patients were included. The stent placement was feasible in all patients with a clinical success of 96.8%. Forty-three patients (88%) had complete stent degradation, 2 (4%)-partial degradation, and 4 (8%)-no degradation at day-90. QoL measure improved by 14-fold during 90 day follow up – 17-fold improvement in the bile leak and 13-fold in the bile duct stone cohort. Elimination of repeat procedures would result in a cost saving of £332 per patient for all indications. Conclusion: Biodegradable stents are associated with excellent clinical success rate and improvement in QoL post- procedure. This is also associated with cost savings to health care providers.
Article Details
Introduction
The incidence of post-cholecystectomy bile leak is 0.5–1% [1,2] and 4–7.2% following liver resection [3-5]. Endotherapy of bile leaks with stents is an accepted mode of treatment [1,6,7]. Biliary stenting achieves resolution of the leak therefore, is recommended by the European Society of Gastrointestinal Endoscopy [7]. Among patients with bile duct stones treated with endoscopic sphincterotomy, wide variability exists in the timing of cholecystectomy [8]. COVID-19 pandemic has significantly delayed non-cancer surgeries such as cholecystectomy for gallstones [9]. Around 10% have bile duct stones during cholecystectomy and it is associated with unfavourable outcomes [10,11]. Therefore endoscopists may consider prophylactic biliary stenting in patients following endoscopic clearance of bile duct while awaiting cholecystectomy [12]. A novel biodegradable bile duct stent (Archimedes®, AMG) has been designed to spontaneously biodegrade and this eliminates the need for subsequent stent removal at ERCP. We undertook a multicentre prospective observational cohort study to assess the efficacy of bio-degradable stents.
Materials and Methods
Design
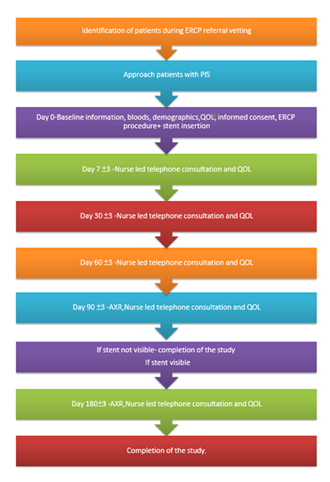
A multicentre, prospective, observational, cohort study was undertaken to assess the clinical effectiveness and utility of biodegradable stents in bile duct drainage in 6 centres across the UK and Republic of Ireland - Nottingham University Hospitals, University College London Hospital, King’s College Hospital, Newcastle upon Tyne Hospital, Leeds Teaching Hospital and Beaumont Hospital, Dublin. All consecutive patients referred for ERCP treatment for bile leak and bile duct stones (fit for cholecystectomy) were eligible for inclusion in the study (Flow chart 1).
Primary and secondary objective
The primary objective was to assess the clinical success of biodegradable stents in bile duct drainage. The secondary objectives were to assess the technical success, complications, QoL before and after the procedure and health economic assessment of placing the stent.
Inclusion criteria
All adult patients who had bile leaks (either post cholecystectomy or liver resection) and patients presenting with symptomatic bile ducts stones with intact gallbladder who would be considered for cholecystectomy were included in the study.
Exclusion criteria
Patients not fit for cholecystectomy, routine stent exchange, where bile duct stone clearance was not achieved, patients with ECOG 4 (Eastern cooperative oncology group) and female patients who were pregnant were excluded.
Eligible patients willing to participate were consented both for the study and the ERCP procedure on the day of endoscopy. During baseline visit, demographics, and quality of life (QoL; (using EQ-5D-5L) data were collected. The stent size was chosen at the discretion of endoscopist during the procedure. Seven French pushing catheter was used to deploy the stent over wire.
Biodegradable Bile duct stent (Archimedes®, AMG)
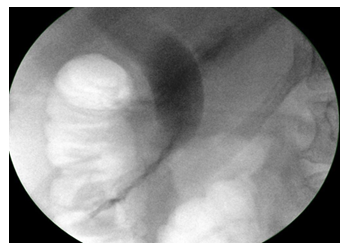
The biodegradable stents are polymeric stents manufactured with different mixtures of polydiaxonone, poly-ethylene glycol, barium sulphate and lactideco-caprolactone-co-trimethylene carbonate depending on the degradation profile of the stent. The helical design allows the bile to flow on the outer extremities of the stent, while supporting the opening of the lumen. The proximal and distal flanges minimize migration and are impregnated with barium to make them radiopaque under X-ray. They have different degradation profiles; fast degrading stents maintains structural integrity for 12 days, medium -25 days and slow degradation-11 weeks. We tested slow degrading stents in this study (Figure 3,4), based on the likely time required for bile leak resolution or interval to cholecystectomy. All patients had sphincterotomy before stent insertion. These are CE marked for the above indications.
In-vitro degradation of Stent:
In-vitro degradation was evaluated in a controlled laboratory setting. The stent was placed in a mock tube and was immersed in Sorensen buffer solution to maintain a pH of 7.4±0.2. The Sorensen buffer solution was flowed continuously. The stent degradation was assessed continuously with video monitoring at day-30, -60, -90, -120, -150, -180 and -190 time points (time lapse video 1).
Stent follow up
After the procedure, patients were followed up for 270 days (on 1, 7, 30, 60, 90, 180, and 270 ±3 days). During these appointments, data on adverse events and QoL were collected. The EQ-5D-5L questionnaire was completed by the patient unless they needed assistance. At day 90±3 days, abdominal X-ray (AXR) was performed to assess the degradation of the stent. A central radiologist blinded to the timing of index ERCP reviewed the AXR at Nottingham. X-rays from other centres were electronically transferred to Nottingham. If there was partial or no degradation, then the AXR was repeated on day 180, 270 and until, there was complete degradation of the stent. Achievement of complete degradation of stent was considered completion of the study (Flow chart 1).
Outcomes
The clinical success of treating bile leaks was defined as resolution of bile leak and removal of external drain. Clinical success for bile duct stones was defined as improvement or resolution of symptoms following the procedure. Technical success was defined as successful placement of biodegradable stent into the bile duct. Stent failure was defined as failure to resolve bile leak or readmission with symptoms in case of CBD stones. Stent visibility was defined as clear image of the stent on fluoroscopy after deployment (Figure 4). They were categorised into excellent, good, neutral and poor visibility on fluoroscopy. Stent degradation was defined as complete if <25% of stent was visible in the region of CBD; partial if 25–75% of the stent was visible and no degradation if >75% of the stent was visible [13].
Sample size calculation
With an estimated sample size of 53 patients and the quoted success rate of 77–100% [6, 14-16], we would be able to detect an overall success rate at the lower end of the range of 77% with a precision allowing an estimated 95% confidence interval of 64-88%. If the success rate was at the upper end of the range at 95%, then this would be measured with a precision allowing an estimated 95% confidence interval of 84-99%.
Statistical Analysis
Categorical variables were presented as number with percentages rounded to the nearest integer; continuous variables were presented as median and interquartile range (IQR). Statistical analyses were performed using GraphPad prism 9 (San Diego, CA). Wilcoxon matched pairs signed rank test was used to compare individual components of QoL at different time points of assessment. A p-value of <0.05 was considered significant.
Health economic analysis
The economic evaluation comprised cost analysis and assessment of QoL. For cost analysis, the direct (hospital-related) costs and indirect (patient-related) costs were compared between a counterfactual current practice plastic stenting [6, 15] and observed biodegradable stenting methods, to derive estimates of cost saving. The sensitivity of cost saving was tested at zero cost of repeated procedures for stent failure. The QoL for each patient, derived from their response to items in EQ-5D-5L questionnaire (Pain/discomfort, mobility, self-care, usual activities, and anxiety/depression), was further used to generate utilities weighted by time factors to derive the quality-adjusted life year (QALY). The QALY provided a measure of how well all the ERCP procedure improved the patients' lives as of each of the post-ERCP procedure inspection days in the year. All analyses were conducted using Microsoft Excel.
Ethical Approval
National Research Ethical Committee and NHS Health Research Authority approval was obtained for the study (REC ID: 285192). Nottingham University Hospitals NHS trust was the sponsor of the study (study ref: 20GA041).This study was registered with clinical trials.gov (NCT04477005) and was adopted as an NIHR portfolio study (CPMS ID: 46829).
Results
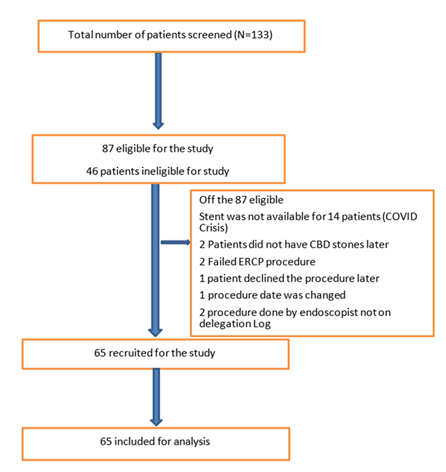
A total of 133 consecutive patients were screened; of which 65 patients were recruited (figure 2 Consort diagram). Forty (62.5%) patients were female; median age was 67.5 years (IQR 52–73); median ECOGs status was 1 (IQR 1–2). Fifty-five (85%) patients had CBD stones and 9 (14%) had bile leak (8 post-cholecystectomy and 1 liver resection) as the sole indication; one patient (2%) had both post-cholecystectomy bile leak and bile duct stone. Forty-one patients (63%) underwent the procedure under conscious sedation and 24 (37%) under propofol sedation or general anaesthesia (Table 1).
|
Patient characteristics |
Data |
|
Total number of patients recruited |
65 |
|
Males Vs Females |
25(37.5%) Vs 40 (62.5%) |
|
Ethnicity |
Caucasians 57(87%) |
|
Other 8 (13%) |
|
|
Age (median, IQR) |
67.5 (21) |
|
ECOG (median, IQR) |
1(2) |
|
CBD stones |
55 (86%) |
|
Sedation Vs GA/propofol sedation |
41 (62.5%) Vs 24 (38.5 %) |
|
Pethidine dose (median, IQR) |
50 mg (25) |
|
Fentanyl dose (median, IQR) |
62.5 mcg (68.75) |
|
Midazolam (median, IQR) |
2.5 (2mg) |
|
Rectal NSAID |
52 (81%) |
Table1: Patient demographics.
The technical success was 100% and the clinical success was 96.8%. Thirty-five (54%) had 8Fr, while 29 (45%) had 10Fr stents (information not available for one stent). The stent deployment was judged by the endoscopists as easy in 59 (91%) and neutral in 6 (9%-neither easy nor difficult). The visibility of the stent on fluoroscopy was excellent in 46 (71%), good in 13 (20%), neutral in 5 (8%) and poor in one (2%) patient (Table 2).
|
Stent Characteristics |
Data |
|
Technical success |
100% |
|
Diameter10Fr Vs 8Fr |
29(45%) Vs 35 (55%) |
|
Length 8cm Vs 6cm |
31 (47%) Vs 34 (53%) |
|
Stent deployment (Easy Vs Neutral) |
59 (91%) Vs 6 (9%) |
|
Visibility of stent on fluoroscopy Excellent |
|
|
Good Neutral Poor |
46 (70.31%) |
|
13 (20.31%) |
|
|
5 (7.81%) |
|
|
1 (1.56%) |
|
|
Patient discomfort during the procedure (median,IQR) |
1 (0) |
|
Patient discomfort in recovery (median,IQR) |
1 (0) |
|
Immediate adverse event |
1 (1.56%) mild bleeding |
Table 2: Procedure Characteristics.
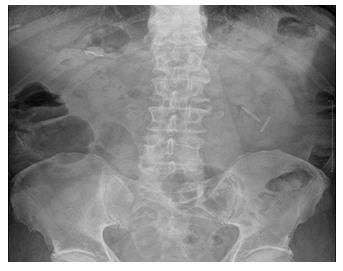
Stent degradation profile was available in 49 patients at 90 days. Forty-three patients (88%) had complete, 2 (4%) had partial, and 4 (8%) had no degradation at day-90. The 43 patients with complete degradation were discharged from the study at day-90. Of the rest, only 3 attended follow up AXR - two achieved complete degradation at day-180 and one patient at day-270. None had symptoms related to ‘delayed’ degradation. The images of stent after insertion, complete, partial and no degradation are shown in images 4–8.
Quality of life before and after the procedure
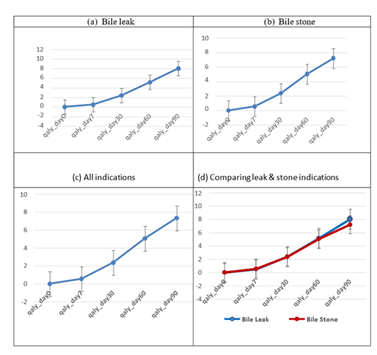
The QoL measure at day-90 was about 17 times the value of day-7. The QoL measure day 90 was relatively higher (12%) on average for the bile leak than for bile stone indication. The mean QALY increased progressively during the post-ERCP follow-up inspection days. For the bile leak cohort, the mean QALY increased from 0 at the baseline (day-0) to 0.48 at day-7 (p=0.0001; 95%CI 0.28–0.68). The mean QALY increased progressively over the remaining follow-up inspection days reaching 8.03 as at day-90 (p=0.0001; 95%CI 5.62–10.43).
For the bile duct stone cohort, the mean QALY increased from 0 at baseline (day-0) to 0.55 at day-7 (p=0.0001; 95%C) 0.49–0.61). The mean QALY also increased progressively over the remaining follow- up inspection days reaching 7.20 at day-90 (p=0.0001; 95CI 6.22–8.17).
The result for the entire cohort showed a similar pattern. The mean QALY increased progressively over the follow-up inspection days reaching 7.32 at day-90 (p=0.0001; 95%CI 6.44–8.20; Figure 9).
On examining individual components of EQ-5D-5L questionnaire (i.e., pain, mobility, self-care, usual activities, anxiety and depression), there was significant improvement in the pain and anxiety/depression scores. The mean pain score reduced following the procedure and was sustained to day-90 (2.469 Vs 1.67, p<0.0001; 95%CI 1.41–1.93). There was an increase in the mean anxiety score (1.95 Vs 1.26, p<0.0001; 95%CI 1.07–1.46).
Cost saving
Procedure cost was highly sensitive to the comorbidity status of patients. An additional comorbidity score increased procedure cost by £292. The adverse events and clinical success rate were comparable to the existing plastic stents. Complete elimination of repeat procedures could save the NHS an additional £332/patient for both indications; £222/patient for bike leak indication. Allowing for spontaneous plastic stent migration; on assumption that a repeat procedure was needed to remove the plastic stents in 90% of cases, hospitals would save £2,185/patient by placing biodegradable stent for both the indications. In this study, the estimated cost saving was £11,356 and £128,473 for bile leak and bile duct stone, respectively.
Complications
There were 9 procedure-related adverse events in the study: one (2%) bleeding (Mallory Weiss tear), 2 (3%) pancreatitis, 1 (2%) cholecystitis, 2 (3%) stent failures and 3 (5%) stent migrations. The stent failures occurred in bile leak indication - one patient already had plastic stent, which was converted to biodegradable stent, and the biodegradable stent failed to resolve the bile leak and hence a metal stent was placed. The bile leak resolved between day-30 and 60 after metal stent placement. The second patient was treated with metal stent and the bile leak resolved quickly after the metal stent placement. One patient with cholecystitis was treated with early cholecystectomy. There was no mortality during the follow-up period.
|
Adverse event |
Number of patients |
|
Bleeding |
1 (1.56%) (Mallory Weiss tear) |
|
Pancreatitis |
2 (3.12%) |
|
Cholecystitis |
1 (1.56%) |
|
Stent migration |
3 (5%) |
|
Stent failure (bile leak) |
2 (3.12%) |
Table 3: Complications.
Discussion
This is the first international, multi-centre prospective observational study assessing the utility of biodegradable stent. There were no technical challenges in placing biodegradable stents. The stent could be advanced over a guidewire with a 7Fr pushing catheter, with fluoroscopic visibility of the stent reported as either excellent or good in 91% of patients. A prospective study on 34 patients previously reported similar outcomes [13]. The overall clinical success rate was 97% [17]. The helical design of the stent allowed bile to drain along the side of the stent rather than through the stent. This allows biliary drainage even when the stent loses its tensile strength because of degradation and breaks down in fragments (Image 6). This correlate well with the in-vitro degradation. This study demonstrates that the biodegradable stent is equivalent to conventional stent but with the added advantage of not needing a repeat procedure.
The stent degradation was assessed centrally by a radiologist blinded to the patient details and time of follow up on day-90, 180 and 270 post-procedure. At day-90, there was complete degradation in 88% and partial degradation in 4% of patients. In this study, we followed them up with AXRs until there was complete degradation. The in-vivo degradation was slightly quicker compared to the in-vitro degradation done in parallel in the laboratory. This is the first multi-centre study that demonstrates how the stent degrades in-vivo during follow-up. As the stent degrades, It loses its tensile strength, breaks down in to fragments and then either migrate into the small bowel if they are small or remain in bile duct if the fragments are large (Figure 5-7). This was similar to the in-vitro degradation. They did not dissolve and disappear as it was anticipated before the study.
This study demonstrates sustained significantly improved QALY following ERCP and biodegradable stent insertion. This is the first study to demonstrate sustained improvement in QALY up to 90 days for both indications examined. The QALY provides a measure of the value that patients placed on their health status during post-ERCP follow-up. During post-ERCP follow-up (day-90 follow-up), patients QoL increased by 17-fold in the bile leak cohort compared to 13-fold in the bile stone cohort. Irrespective of the indication, QoL measure improved 14-fold during the follow-up period. The QoL measure was relatively higher (~12%) for the bile leak than for bile stone indication. Bile leaks are a significant post-operative sequela and are associated with significant morbidity, increased 1-year mortality and reduced long term QoL [18, 19]. There was stent failure in 2 patients, defined as failure to resolve bile leak. Both the patients required fully covered metal stents to resolve the bile leak following laparoscopic cholecystectomy in one and after liver resection in the other. The reported failure rate for plastic stents following hepato-biliary surgery is 23% and 12% following cholecystectomy [20-23]. Most studies showed that a proportion of patients needed sequential plastic stent placement to resolve the bile leak. Covered metal stents have higher success rate for bile leak following hepato-biliary surgery [24]. There were no stent-related adverse events and the ERCP-related adverse events were comparable to the existing literature [25]. As these stents are straight and the flare of the flanges are minimal they are prone to migration. The stent migration occurred in all three patients who had sphincteroplasty. Hence, placing these stents may not be helpful following sphincteroplasty.
The estimated cost savings was £1,136/patient and £2,379/patient for bile leak and bile duct stones, respectively. For a tertiary centre performing 400 procedures, assuming 50% would be suitable for biodegradable stent insertion and keeping stent failure at approx. 4%, this could save the NHS about £22,712, £8,816 and £6,828 for bile leak, bile duct stones, and all indications, respectively, other things being equal. For a smaller hospital performing around 100 procedures, this could save about £11,356, £4,408, and £3,414, respectively.
This study was conducted during COVID-19 crisis hence access to surgery for benign indications such as cholecystectomy was severely limited. The “CONTACT” study reported that there was significant reduction in access to cancer surgery and palliative chemotherapy during COVID crisis [26]. At least in the UK, this seems to continue even after COVID because of the long backlog caused by cancellation of benign surgery. If the interval between ERCP and cholecystectomy is longer, then placing a biodegradable stent may be helpful for the patients.The single arm observational nature of the study is an important limitation of this study. This could have led to selection bias and increased intervention effect. However, the multicentre design of this study would have minimised this. As it is a single arm study, we were unable to differentiate the effect of stent from ERCP procedure itself on the QALY of patients following procedure.
In conclusion, this international multi-centre prospective observational study demonstrates that biodegradable bile duct stents are safe, associated with high clinical success rate and significant improvement in the QALY of patients following procedure.
Acknowledgements
The authors are grateful to Samantha Warburton, Sian Parkes, Violeta Anuskievic and Rachelle Boxhall for support with data collection, recruitment and training of staff for the study.
Author Contribution
Suresh Vasan Venkatachalapathy: Chief investigator, Conceptualisation, design,Recruitment, drafting of manuscript
Martin W James: PI for Nottingham, Recruitment, review of manuscript.
Deepak Joshi: PI for Kings hospital, Recruitment, review of manuscript
Gavin Johnson: PI for UCL London, Recruitment, review of manuscript
Simon Philpotts: Recruitment, review of manuscript.
Manu Nayar: PI for Newcastle, Recruitment, review of manuscript
Danny Cheriyan: PI for Ireland, Recruitment, review of manuscript
Matthew Huggett: PI for Leeds, Recruitment, review of manuscript
Christopher Clarke: Independent radiologist, review of manuscript
Damilola Olajide: Health economic analysis
Aloysious D Aravinthan: Statistical analysis, review of manuscript
Guruprasad P Aithal: Recruitment, review of manuscript.
Ethics Statement
Approval of the research protocol by an Institutional Reviewer Board: National research ethical committee (REC) and NHS health research authority approval: 285192
Registry and the Registration No: Clinical trials.gov number: NCT04477005
NIHR portfolio adoption number
CPMS ID 46829 Informed Consent: Obtained
Animal Studies: N/A
Clinical trials.gov number
NCT04477005
National research ethical committee (REC) and NHS health research authority approval: 285192 NIHR portfolio adoption number: CPMS ID 46829
Funding
This was an investigator led industry funded study: funded by Archimedes, AMG and was adopted in to NIHR portfolio studies (CPMS ID 46829). The study was supported by NIHR Nottingham BRC (NIHR203310).
References
- Barkun AN, Rezieg M, Mehta SN, et Postcholecystectomy biliary leaks in the laparoscopic era: risk factors, presentation, and management. Gastrointestinal endoscopy 45 (1997): 277-282.
- Deziel DJ, Millikan KW, Economou SG, et al. Complications of laparoscopic cholecystectomy: a national survey of 4,292 hospitals and an analysis of 77,604 cases 165 (1993): 9-14.
- Yamashita Y, Hamatsu T, Rikimaru T, et Bile leakage after hepatic resection. Annals of surgery 233 (2001): 45-50.
- Tanaka S, Hirohashi K, Tanaka H, et al. Incidence and management of bile leakage after hepatic resection for malignant hepatic Journal of the American College of Surgeons 195 (2002): 484-489.
- Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 149 (2011): 680-688.
- Kaffes AJ, Hourigan L, De Luca N, et al. Impact of endoscopic intervention in 100 patients with suspected postcholecystectomy bile leak. Gastrointestinal endoscopy 61 (2005): 269-275.
- Dumonceau J-M, Tringali A, Blero D, et al. Biliary stenting: indications, choice of stents and results: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy 44 (2012): 277-298.
- Mador BD, Nathens AB, Xiong W, et al. Timing of cholecystectomy following endoscopic sphincterotomy: a population-based study. Surgical endoscopy 31 (2017): 2977-2985.
- Ahmed W, Obeng L, Hegazy A, et al. P138 Delays in cholecystectomy after bile duct clearance: another consequence of Covid-19? : BMJ Publishing Group (2022).
- Möller M, Gustafsson U, Rasmussen F, et al. Natural Course vs Interventions to Clear Common Bile Duct Stones: Data From the Swedish Registry for Gallstone Surgery and Endoscopic Retrograde Cholangiopancreatography (GallRiks). JAMA surgery 149 (2014): 1008-1013.
- Clout M, Blazeby J, Rogers C, et al. Randomised controlled trial to establish the clinical and cost-effectiveness of expectant management versus preoperative imaging with magnetic resonance cholangiopancreatography in patients with symptomatic gallbladder disease undergoing laparoscopic cholecystectomy at low or moderate risk of common bile duct stones (The Sunflower Study): a study protocol. BMJ Open 11 (2021): e044281.
- Kawabata H, Kawakatsu Y, Yamaguchi K, et Prophylactic Biliary Stenting Before Cholecystectomy in Patients With Gallstones and Common Bile Duct Stones. Gastroenterology Res 12 (2019): 191-197.
- Anderloni A, Fugazza A, Maroni L, et al. New biliary and pancreatic biodegradable stent placement: a single-center, prospective, pilot study (with video). J Gastrointestinal Endoscopy (2020).
- Chinnery GE, Krige JE, Bornman PC, et Endoscopic management of bile leaks after laparoscopic cholecystectomy. S Afr J Surg 51 (2013): 116-121.
- Dumonceau JM, Tringali A, Papanikolaou IS, et Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated October 2017. Endoscopy 50 (2018): 910-930.
- Yun SU, Cheon YK, Shim CS, et al. The outcome of endoscopic management of bile leakage after hepatobiliary Korean J Intern Med 32 (2017): 79-84.
- Manes G, Paspatis G, Aabakken L, et Endoscopic management of common bile duct stones: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 51 (2019): 472-491.
- Booij KAC, de Reuver PR, van Dieren S, et Long- term Impact of Bile Duct Injury on Morbidity, Mortality, Quality of Life, and Work Related Limitations. Ann Surg 268 (2018): 143-150.
- Fong ZV, Pitt HA, Strasberg SM, et al. Diminished Survival in Patients with Bile Leak and Ductal Injury: Management Strategy and J Am Coll Surg 226 (2018): 568-76.e1.
- Dechêne A, Jochum C, Fingas C, et Endoscopic management is the treatment of choice for bile leaks after liver resection. Gastrointestinal endoscopy 80 (2014): 626-33. e1.
- Ryan ME, Geenen JE, Lehman GA, et al. Endoscopic intervention for biliary leaks after laparoscopic cholecystectomy: a multicenter Gastrointest Endosc 47 (1998): 261-266.
- Kwon C-I, Gromski MA, Oh H-C, et Additional flap on plastic stents for improved antimigration effect in the treatment of post-cholecystectomy bile leak. Endoscopy International Open 6 (2018): E489-E94.
- Katsinelos P, Kountouras J, Paroutoglou G, et al. A comparative study of 10-Fr 7-Fr straight plastic stents in the treatment of postcholecystectomy bile leak. Surgical endoscopy 22 (2008): 101-106.
- Mangiavillano B, Luigiano C, Tarantino I, et Fully covered, self-expandable metal stents for first-step endoscopic treatment of biliary leaks secondary to hepato-biliary surgery: a retrospective study. Digestive and Liver Disease 45 (2013): 430-432.
- Dumonceau J-M, Kapral C, Aabakken L, et ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 52 (2020): 127-149.
- Hall LA, McKay SC, Halle-Smith J, et al. The impact of the COVID-19 pandemic upon pancreatic cancer treatment (CONTACT Study): a UK national observational cohort study. British Journal of Cancer (2023).