Umbilical Mass with Cyclical Pain: Surgical Management
Article Information
Kay Hung MD1, Lisa Marie Knowlton MD, MPH1, Peter Cha MD1, Brooke Howitt MD2, Aussama K Nassar MD, MSc1*
1Department of Surgery, Stanford University School of Medicine, Stanford, CA, USA
2Department of Pathology, Stanford University School of Medicine, Stanford, CA, USA
*Corresponding Author: Aussama Nassar, MD, MSC, Assistant Professor of Surgery, Section of Trauma, Surgical Critical Care and Acute Care Surgery, Stanford University, 300 Pasteur Drive, H3635, Stanford, CA 94305, USA, Tel: 650-725-1097
Received: 06 January 2021; Accepted: 06 April 2021; Published: 05 May 2021
Citation: Kay Hung, Lisa Marie Knowlton, Peter Cha, Brooke Howitt, Aussama K Nassar. Umbilical Mass with Cyclical Pain: Surgical Management. Archives of Clinical and Medical Case Reports 5 (2021): 393-397.
View / Download Pdf Share at FacebookKeywords
Umbilical hernia; Endometriosis; General surgery
Umbilical hernia articles; Endometriosis articles; General surgery articles
Umbilical hernia articles Umbilical hernia Research articles Umbilical hernia review articles Umbilical hernia PubMed articles Umbilical hernia PubMed Central articles Umbilical hernia 2023 articles Umbilical hernia 2024 articles Umbilical hernia Scopus articles Umbilical hernia impact factor journals Umbilical hernia Scopus journals Umbilical hernia PubMed journals Umbilical hernia medical journals Umbilical hernia free journals Umbilical hernia best journals Umbilical hernia top journals Umbilical hernia free medical journals Umbilical hernia famous journals Umbilical hernia Google Scholar indexed journals Endometriosis articles Endometriosis Research articles Endometriosis review articles Endometriosis PubMed articles Endometriosis PubMed Central articles Endometriosis 2023 articles Endometriosis 2024 articles Endometriosis Scopus articles Endometriosis impact factor journals Endometriosis Scopus journals Endometriosis PubMed journals Endometriosis medical journals Endometriosis free journals Endometriosis best journals Endometriosis top journals Endometriosis free medical journals Endometriosis famous journals Endometriosis Google Scholar indexed journals ultrasound articles ultrasound Research articles ultrasound review articles ultrasound PubMed articles ultrasound PubMed Central articles ultrasound 2023 articles ultrasound 2024 articles ultrasound Scopus articles ultrasound impact factor journals ultrasound Scopus journals ultrasound PubMed journals ultrasound medical journals ultrasound free journals ultrasound best journals ultrasound top journals ultrasound free medical journals ultrasound famous journals ultrasound Google Scholar indexed journals laparoscopic articles laparoscopic Research articles laparoscopic review articles laparoscopic PubMed articles laparoscopic PubMed Central articles laparoscopic 2023 articles laparoscopic 2024 articles laparoscopic Scopus articles laparoscopic impact factor journals laparoscopic Scopus journals laparoscopic PubMed journals laparoscopic medical journals laparoscopic free journals laparoscopic best journals laparoscopic top journals laparoscopic free medical journals laparoscopic famous journals laparoscopic Google Scholar indexed journals SARS-CoV-2 articles SARS-CoV-2 Research articles SARS-CoV-2 review articles SARS-CoV-2 PubMed articles SARS-CoV-2 PubMed Central articles SARS-CoV-2 2023 articles SARS-CoV-2 2024 articles SARS-CoV-2 Scopus articles SARS-CoV-2 impact factor journals SARS-CoV-2 Scopus journals SARS-CoV-2 PubMed journals SARS-CoV-2 medical journals SARS-CoV-2 free journals SARS-CoV-2 best journals SARS-CoV-2 top journals SARS-CoV-2 free medical journals SARS-CoV-2 famous journals SARS-CoV-2 Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Case Report articles Case Report Research articles Case Report review articles Case Report PubMed articles Case Report PubMed Central articles Case Report 2023 articles Case Report 2024 articles Case Report Scopus articles Case Report impact factor journals Case Report Scopus journals Case Report PubMed journals Case Report medical journals Case Report free journals Case Report best journals Case Report top journals Case Report free medical journals Case Report famous journals Case Report Google Scholar indexed journals Cancer articles Cancer Research articles Cancer review articles Cancer PubMed articles Cancer PubMed Central articles Cancer 2023 articles Cancer 2024 articles Cancer Scopus articles Cancer impact factor journals Cancer Scopus journals Cancer PubMed journals Cancer medical journals Cancer free journals Cancer best journals Cancer top journals Cancer free medical journals Cancer famous journals Cancer Google Scholar indexed journals CT imaging articles CT imaging Research articles CT imaging review articles CT imaging PubMed articles CT imaging PubMed Central articles CT imaging 2023 articles CT imaging 2024 articles CT imaging Scopus articles CT imaging impact factor journals CT imaging Scopus journals CT imaging PubMed journals CT imaging medical journals CT imaging free journals CT imaging best journals CT imaging top journals CT imaging free medical journals CT imaging famous journals CT imaging Google Scholar indexed journals
Article Details
1. Case Description
A 30-year-old female patient presented with a small periumbilical lump of 1.5 years duration. The mass was irreducible, gradually increased in size, and was now pea sized, non-tender, and was associated with recurrent mild pain during her menstrual cycles. She denied gastrointestinal symptoms, had no constitutional symptoms, and review of systems was otherwise unremarkable. There was no history of prior surgery, and the patient was not on any medications. Family history was noncontributory. On physical exam, vital signs were within normal limits. The firm 2 cm supraumbilical mass was non-tender and immobile. Her laboratory values were within normal range. Ultrasound imaging revealed a circumscribed heterogeneously hypoechoic lesion centered in the subcutaneous fat immediately superior to the umbilicus measuring 1.4 × 1.0 × 1.1 cm without associated vascularity. CT abdomen and pelvis with IV contrast revealed an area of focal increased soft tissue attenuation in the subcutaneous fat measuring 1.9 × 1.2 × 1.1 cm with no focal fluid collection and no communication with the abdominal cavity. Based on the above, our top differential would be: congenital mass, incarcerated hernia, and umbilical endometriosis.
- What Would You Do?
- Start with a diagnostic laparoscopic approach to rule out intraabdominal pathology
- Treat with combined oral contraceptive and NSAIDs
- FNA/Core biopsy to rule out neoplastic process
- Mass excision with possible umbilical hernia repair
- Perform an MRI with contrast to further delineate anatomy, rule out malignancy, prior to surgery.
3. What We Did And Why
Correct Answer: D
Abdominal wall mass excision with possible umbilical hernia repair.
Abdominal wall endometriosis, in which endometrium-like tissue is present above the peritoneum (i.e., skin, subcutaneous tissue, abdominal or pelvic wall musculature, or in abdominal incisions), is a rare type of endometriosis. Although endometriosis affects 6-10% of women of reproductive age, primary abdominal wall endometriosis is quite rare, with a reported incidence of 0.03% to 3.5% [1]. Secondary umbilical endometriosis can occur due to iatrogenic dissemination from prior surgery. It is occasionally misdiagnosed as incarcerated hernia, lipoma, suture granuloma, hematoma, abscess, or primary or metastatic cancer, given its clinical presentation as a mass in the abdominal wall [2]. Localized and cyclic abdominal pain without associated dysmenorrhea, as well as a history of laparotomy, are independent risk factors that should raise clinical suspicion for the diagnosis of abdominal wall endometriosis [1].
Despite inconclusive findings on ultrasound and CT imaging, the patient’s history of cyclical discomfort that localized to the peri-umbilical mass during menstruation raised the concern for endometriosis. Treatment of umbilical endometriosis consists of excision of the mass. After the induction of general anesthesia, a 2 cm firm mass was palpated in the supraumbilical area, tethered to the underlying fascia. A 2 cm transverse supraumbilical incision was made. We encountered a 1 cm nodule that was excised. On further inspection of the area, we palpated another firm 1.5 cm mass that wasn’t readily appreciated on our initial assessment and was in close proximity to the umbilical stalk and nearby fascia. In order to excise the entire mass with a healthy fascial margin, we needed to free the umbilical stalk. We then excised this mass with the involved 1 cm surrounding fascia, and specimens were sent for pathology. The fascial defect was small enough to be primarily repaired using 0- Vicryl interrupted sutures. Hemostasis was achieved. The umbilical stalk was reconstructed and anchored to the fascia using 3-0 Monocryl sutures, and the skin was closed with 4-0 Monocryl simple buried sutures. We bisected the tan-colored mass and found brownish deposits. The patient had an uneventful recovery, and the pathology confirmed the mass to be endometriosis.
Abdominal wall endometriosis more often occurs in the setting of prior pelvic surgery as it seeds in the abdominal incisions, but may also arise spontaneously, as in our case [3, 4]. History is an important aspect of the clinical assessment; a patient present with a cyclical painful mass during menses suggests the diagnosis of endometriosis, but only 57% of patients presenting with these classically described [3, 5]. Ultrasound imaging may be considered as the initial modality to reduce radiation exposure. CT or MRI can yield improved characterization of the mass and rules out primary intraabdominal pathology in order to better assist in surgical planning. In this case, the patient already had a CT done by the primary care provider and imaging was non-diagnostic.
The preferred treatment for abdominal wall endometriosis is surgical excision, ideally with 1 cm margins to reduce the chance of disease recurrence. However, one case series reported a 4.3% recurrence rate following wide local excision [5]. Medical hormonal therapy has shown some limited benefit in reducing symptoms and often does not result in permanent resolution of the underlying lesion.
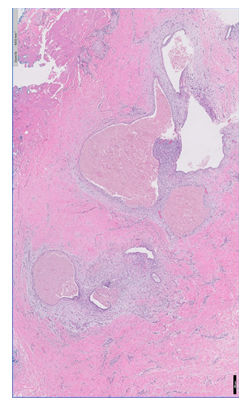
Figure 1A: Histopathology revealing endometriosis in the biopsy specimen. Endometrioid glands are surrounded by endometrial stroma (H&E, Bar = 200 µm).
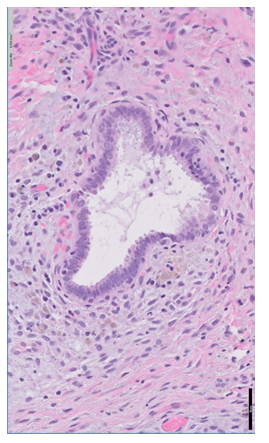
Figure 1B: Higher power view of 1A demonstrating endometriotic glandular tissue surrounded with stromal cells (H&E, Bar = 50 µm).
Conflicts of Interest
There are no conflicts of interest or disclosures to report for any of the authors.
References
- Khan Z, Zanfagnin V, El-Nashar SA, et al. Risk Factors, Clinical Presentation, and Outcomes for Abdominal Wall Endometriosis. J Minim Invasive Gynecol 24 (2017): 478-484.
- Blanco RG, Parithivel VS, Shah AK, et al. Abdominal wall endometriomas. Am J Surg 185 (2003): 596-598.
- Rindos NB, Mansuria S. Diagnosis and Management of Abdominal Wall Endometriosis: A Systematic Review and Clinical Recommendations. Obstet Gynecol Surv 72 (2017): 116-122.
- Ecker AM, Donnellan NM, Shepherd JP, et al. Abdominal wall endometriosis: 12 years of experience at a large academic institution. Am J Obstet Gynecol 211 (2014): 363.e1-363.e5.
- Horton JD, DeZee KJ, Ahnfeldt EP, et al. Abdominal wall endometriosis: a surgeon’s perspective and review of 445 cases. Am J Surg 196 (2008): 207-212.
