Tumid Lupus Erythematosus Following Hyaluronic Acid Filler Injection: A Case Report
Article Information
Chanisa Kiatsurayanon1,2*, Poonnawis Sudtikoonaseth1,2, Thanasri Sesavej1,2, Purich Kosidcanasup1, François Niyonsaba3,4, Mingkwan Wichaidit1
1Institute of Dermatology, Department of Medical Services, Ministry of Public Health, Bangkok, Thailand
2Graduate School of Medicine, Rangsit University, Pathumthani, Thailand
3Atopy (Allergy) Research Center, Juntendo University Graduate School of Medicine, Tokyo, Japan
4Faculty of International Liberal Arts, Juntendo University, Tokyo, Japan
*Corresponding Author: Chanisa Kiatsurayanon, Institute of Dermatology, Department of Medical Services, Ministry of Public Health, 420/7 Rajvithi Road, Phayathai District, Rajthevee, Bangkok, Thailand
Received: 04 January 2022; Accepted: 14 January 2022; Published: 31 January 2022
Citation: Chanisa Kiatsurayanon, Poonnawis Sudtikoonaseth, Thanasri Sesavej, Purich Kosidcanasup, François Niyonsaba, Mingkwan Wichaidit. Tumid Lupus Erythematosus Following Hyaluronic Acid Filler Injection: A Case Report. Archives of Clinical and Medical Case Reports 6 (2022): 57-62.
View / Download Pdf Share at FacebookAbstract
Dermal filling has increasingly become one of the most popular nonsurgical aesthetic procedures performed worldwide. Among several types of dermal fillers available, hyaluronic acid filler plays an integral role as the material of choice for treating volume reduction associated with skin aging due to its many favorable properties. Claimed to be safe, effective and biocompatible, injection of hyaluronic acid filler sometimes brings about undesirable outcomes. Adverse events from hyaluronic acid filler range from acute and temporary events such as bruising and erythema to more serious and long-lasting sequalae, including granulomas and vascular occlusion. Here, we report a case of tumid lupus erythematosus associated with hyaluronic acid filler injection. To the best of our knowledge, this is the first report of tumid lupus erythematosus following injection of a dermal filler. Treatment with oral hydroxychloroquine and topical tacrolimus resulted in gradual improvement of the skin lesions with no sign of recurrence after 3 months of followup. In summary, we highlight the potential immunologic complications of hyaluronic acid filler injection and encourage the role of intradermal pretesting in susceptible individuals.
Keywords
Adverse reaction; Cutaneous lupus erythematosus; Dermal filler; Filler complication; Hyaluronic acid
Adverse reaction articles; Cutaneous lupus erythematosus articles; Dermal filler articles; Filler complication articles; Hyaluronic acid articles
Adverse reaction articles Adverse reaction Research articles Adverse reaction review articles Adverse reaction PubMed articles Adverse reaction PubMed Central articles Adverse reaction 2023 articles Adverse reaction 2024 articles Adverse reaction Scopus articles Adverse reaction impact factor journals Adverse reaction Scopus journals Adverse reaction PubMed journals Adverse reaction medical journals Adverse reaction free journals Adverse reaction best journals Adverse reaction top journals Adverse reaction free medical journals Adverse reaction famous journals Adverse reaction Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Cutaneous lupus erythematosus articles Cutaneous lupus erythematosus Research articles Cutaneous lupus erythematosus review articles Cutaneous lupus erythematosus PubMed articles Cutaneous lupus erythematosus PubMed Central articles Cutaneous lupus erythematosus 2023 articles Cutaneous lupus erythematosus 2024 articles Cutaneous lupus erythematosus Scopus articles Cutaneous lupus erythematosus impact factor journals Cutaneous lupus erythematosus Scopus journals Cutaneous lupus erythematosus PubMed journals Cutaneous lupus erythematosus medical journals Cutaneous lupus erythematosus free journals Cutaneous lupus erythematosus best journals Cutaneous lupus erythematosus top journals Cutaneous lupus erythematosus free medical journals Cutaneous lupus erythematosus famous journals Cutaneous lupus erythematosus Google Scholar indexed journals Dermal filler articles Dermal filler Research articles Dermal filler review articles Dermal filler PubMed articles Dermal filler PubMed Central articles Dermal filler 2023 articles Dermal filler 2024 articles Dermal filler Scopus articles Dermal filler impact factor journals Dermal filler Scopus journals Dermal filler PubMed journals Dermal filler medical journals Dermal filler free journals Dermal filler best journals Dermal filler top journals Dermal filler free medical journals Dermal filler famous journals Dermal filler Google Scholar indexed journals Ultra Sound articles Ultra Sound Research articles Ultra Sound review articles Ultra Sound PubMed articles Ultra Sound PubMed Central articles Ultra Sound 2023 articles Ultra Sound 2024 articles Ultra Sound Scopus articles Ultra Sound impact factor journals Ultra Sound Scopus journals Ultra Sound PubMed journals Ultra Sound medical journals Ultra Sound free journals Ultra Sound best journals Ultra Sound top journals Ultra Sound free medical journals Ultra Sound famous journals Ultra Sound Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Lymphangioma articles Lymphangioma Research articles Lymphangioma review articles Lymphangioma PubMed articles Lymphangioma PubMed Central articles Lymphangioma 2023 articles Lymphangioma 2024 articles Lymphangioma Scopus articles Lymphangioma impact factor journals Lymphangioma Scopus journals Lymphangioma PubMed journals Lymphangioma medical journals Lymphangioma free journals Lymphangioma best journals Lymphangioma top journals Lymphangioma free medical journals Lymphangioma famous journals Lymphangioma Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals Hyaluronic acid articles Hyaluronic acid Research articles Hyaluronic acid review articles Hyaluronic acid PubMed articles Hyaluronic acid PubMed Central articles Hyaluronic acid 2023 articles Hyaluronic acid 2024 articles Hyaluronic acid Scopus articles Hyaluronic acid impact factor journals Hyaluronic acid Scopus journals Hyaluronic acid PubMed journals Hyaluronic acid medical journals Hyaluronic acid free journals Hyaluronic acid best journals Hyaluronic acid top journals Hyaluronic acid free medical journals Hyaluronic acid famous journals Hyaluronic acid Google Scholar indexed journals
Article Details
Abbreviations:
HA- Hyaluronic Acid; NASHA- Nonanimal Stabilized Hyaluronic Acid; TLE- Tumid Lupus Erythematosus
1. Introduction
The use of dermal fillers has increased dramatically in the past few decades. At present, physicians have many different types of fillers to choose from, such as bovine and human collagen, hyaluronic acid (HA), calcium hydroxyl apatite, silicone and poly-L-lactic acid. Although various manufacturers claimed dermal fillers to be safe and nonimmunogenic, several reports have addressed complications that occurred with all compounds used [1]. HA, a major component of the extracellular matrix, is a glycosaminoglycan polymer composed of repeating units of monosaccharide D-glucuronic acid and N-acetyl-D-glucosamine. Due to its hydrophilic and viscoelastic properties, HA is capable of binding to large quantities of water to maintain the structural integrity of the tissue [2]. Currently, HA filler is often regarded as one of the most popular dermal fillers used for cosmetic purposes globally. HA can be derived from both animal and nonanimal sources. Recently, nonanimal stabilized HA (NASHA), a modified HA compound with fewer contaminant proteins, has been introduced to the market and is preferred for biomedical purposes considering its superior safety profile [3]. However, an increasing number of reports on unwanted outcomes from NASHA have continuously appeared [2]. It is now well-accepted that reactions to HA fillers are limited not only to the injection techniques but also to the body’s immune responses [1, 2, 4]. Herein, we communicate a case of tumid lupus erythematosus (TLE) following NASHA filler injection.
2. Case Report
A 30-year-old healthy Thai woman presented with a 3-year history of gradually enlarging, painless, red rashes on both sides of her cheeks. She had a past history of HA injection to augment her cheeks 4 years ago. The patient had never been implanted with other fillers before. The rash started initially as small red lesions at exactly the injection points. She also noticed exacerbation of the rashes after sun exposure. The lesions were neither itchy nor tender. Physical examination revealed bilateral non-scaly erythematous edematous plaques with telangiectasias on both cheeks as shown in (Figure 1). There were no oral lesions or skin lesions elsewhere. The rest of the physical examination was within normal limits.
Incisional biopsy was performed, and the histopathological examination revealed superficial and deep perivascular infiltration with lymphocytes, histiocytes and a few eosinophils (Figure 2A, 2B). An Alcian blue special stain showed diffusely increased mucin deposition in the dermis (Figure 3A) and the immunohistochemistry staining demonstrated some CD123+ plasmacytoid dendritic cells in clusters within the infiltrate (Figure 3B). Direct immunofluorescence showed weak granular deposition of complement protein C3 but not immunoglobulin (Ig) G/IgM at the dermo-epidermal junction (data not shown). Cultures and PCR for bacteria, mycobacteria and fungi were negative. The examination of antinuclear antibodies was positive with a 1:320 titer in a homogeneous pattern; however, anti-double stranded DNA was negative. Other blood tests were unremarkable. Based on the typical clinical manifestations and histopathology, the diagnosis of TLE was established.
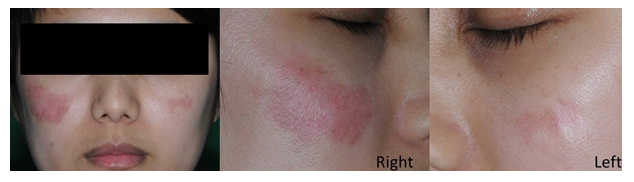
Figure 1: Tumid lupus erythematosus following hyaluronic acid filler injection. Bilateral erythematous, edematous non-scarring plaques with telangiectasias are located on both cheeks at exactly the previous injection points.
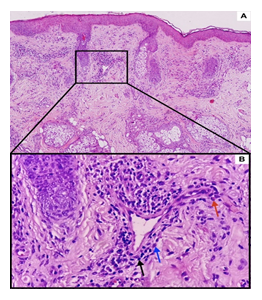
Figure 2: Hematoxylin and eosin (H&E) staining of lesional tissue (A) At 10× magnification, hematoxylin and eosin section reveals perivascular infiltration within the superficial and deep dermis. (B) Close-up view (40× magnification) of perivascular infiltration of lymphocytes (black arrow), histiocytes (blue arrow) and a few eosinophils (red arrow). There are neither epidermal abnormalities nor interface changes. No foreign body is seen.
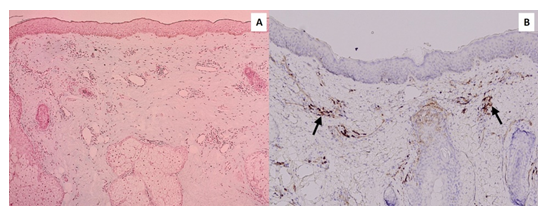
Figure 3: Alcian blue and immunohistochemical staining of lesional tissue (A) An Alcian blue special stain shows diffuse mucin deposition in the dermis (10× magnification). (B) Immunohistochemistry demonstrates some CD123+ plasmacytoid dendritic cells in clusters within the infiltrate (black arrows, 10× magnification).
The patient was treated with oral hydroxychloroquine 400 mg per day for one month, followed by 200 mg per day for two months together with topical tacrolimus. After 3 months of treatment, the erythema subsided with no sign of recurrence. Unfortunately, the patient was lost to follow-up due to COVID-19 outbreak in Thailand.
3. Discussion
After its introduction to the market in the late 1990s, HA has been considered one of the safest and most effective injections for soft tissue augmentation [4]. Since HA and NASHA fillers have no tissue or species specificity, there should theoretically be no risk of immune-mediated reactions. However, accumulated evidence has addressed delayed immunologic adverse effects related to HA and NASHA fillers, and the latency time between HA injection and development of these side effects can range from months to several years [1,2,4].
We describe a unique case of TLE that occurred approximately 1 year after NASHA filler injection. Our case presented with typical clinical findings of TLE and histopathology revealed superficial and deep infiltration in the dermis. Due to the presence of eosinophils in our skin specimen, one might militate against a diagnosis of TLE but rather a dermal hypersensitivity reaction. However, in this patient, there are also positive antinuclear antibodies in homogeneous pattern, an increased dermal mucin deposition together with the plasmacytoid dendritic cells in clusters which serve as essential diagnostic marker in differentiating various subtypes of chronic lupus erythematosus from their mimickers [5]. Furthermore, we also performed CD4 and CD8 immunohistochemical staining and detected a predominance of CD4 over CD8 lymphocytes in the inflammatory infiltrate with a ratio of 3:1 (data not shown), which correlates with the criteria of TLE classification [5]. Based on the above-mentioned characteristics together with the excellent response to hydroxychloroquine, the diagnosis of TLE was finally made.
TLE is a subset of chronic cutaneous lupus erythematosus that classically presents with erythematous, edematous plaques on sun-exposed areas of the skin. Although its pathogenesis is unclear, immune dysregulation has been postulated to contribute to the pathogenesis of TLE, especially upregulation of regulatory T-cells and plasmacytoid dendritic cells; decrease of Langerhans cells; and upregulation of type-1 interferon, tumor necrosis factor-a, and T helper 17 cells. Interestingly, a case of systemic lupus erythematosus following polyalkylimide dermal filler injection has recently been reported, suggesting a possible causal relationship between dermal fillers and autoimmune manifestations [6]. In fact, many reports have indicated that systemic lupus erythematosus can be induced by vaccines, and immune reactions secondary to fillers have been shown to be linked with delayed-type hypersensitivity reactions [1,6,7]. Therefore, similar to vaccines, dermal fillers can activate certain T-cell populations and function as immunologic adjuvants. HA, like other fillers, could elicit autoimmune responses via macrophage and T-cell response cascade stimulation [6].
Although the causal association between NASHA fillers and TLE cannot be conclusively proven in our case, increasing evidence has addressed the capability of HA compounds to develop immune-mediated reactions. Immunologic reactions from HA fillers can originate from HA itself, from hyaluronan-associated proteins or from contaminated proteins/DNA in the product [1]. Evidence of immunogenicity from HA derivatives has been described in animal models and anti-HA antibodies have been detected in patients receiving HA fillers [8,9]. In addition, HA could act as a “superantigen” and directly initiate immune responses in a murine model [10]. Furthermore, the presence of contaminating DNA in HA products could induce proinflammatory cytokines, such as interleukin-12 and tumor necrosis factor-a and potentially trigger or exacerbate inflammation [11]. Moreover, a Spanish in vitro study found that treatment of human blood mononuclear cells with NASHA could trigger a low-grade immune inflammatory response resulting in T-cell activation [2].
Taken together, via multiple mechanisms, HA preparations are capable of provoking undesirable immune-mediated adverse effects. Our case highlights the possibility of autoimmune/immunologic side effects of NASHA filler injections and calls for further robust investigations to demonstrate a plausible link to elucidate this phenomenon. Given the rising popularity of HA filler injection, physicians should be aware of potential complications related to its use.
Acknowledgments
The authors wish to thank Kamonwat Chongkoltanalab for figure illustration and Michiyo Matsumoto for secretarial assistance.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Alijotas-Reig J, Garcia-Gimenez V. Delayed immune-mediated adverse effects related to hyaluronic acid and acrylic hydrogel dermal fillers: clinical findings, long-term follow-up and review of the literature. J Eur Acad Dermatol Venereol 22 (2008): 150-161.
- Alijotas-Reig J, Hindié M, Kandhaya-Pillai R, et al. Bioengineered hyaluronic acid elicited a nonantigenic T cell activation: implications from cosmetic medicine and surgery to nanomedicine. J Biomed Mater Res A 95 (2010): 180-190.
- André P. Evaluation of the safety of a non-animal stabilized hyaluronic acid (NASHA -- Q-Medical, Sweden) in European countries: a retrospective study from 1997 to 2001. J Eur Acad Dermatol Venereol 18 (2004): 422-425.
- Bitterman-Deutsch O, Kogan L, Nasser F. Delayed immune mediated adverse effects to hyaluronic acid fillers: report of five cases and review of the literature. Dermatol Reports 7 (2015): 5851.
- Alexiades-Armenakas MR, Baldassano M, Bince B, et al. Tumid lupus erythematosus: criteria for classification with immunohistochemical analysis. Arthritis Rheum 49 (2003): 494-500.
- Haber R, Stéphan F. A case of systemic lupus erythematosus following polyalkylimide dermal filler. J Eur Acad Dermatol Venereol 30 (2016): 1420-1422.
- Alijotas-Reig J, Garcia-Gimenez V, Vilardell-Tarres M. Late-onset immune-mediated adverse effects after poly-L-lactic acid injection in non-HIV patients: clinical findings and long-term follow-up. Dermatology 219 (2009): 303-308.
- Sasaki M, Miyazaki T, Nakamura T, et al. Immunogenicity of hylan g-f 20 in Guinea pigs and mice. J Rheumatol 31 (2004): 943-950.
- Micheels P. Human anti-hyaluronic acid antibodies: is it possible? Dermatol Surg 27 (2001): 185-191.
- Wang JY, Roehrl MH. Glycosaminoglycans are a potential cause of rheumatoid arthritis. Proc Natl Acad Sci U S A 99 (2002): 14362-14367.
- Filion MC, Phillips NC. Pro-inflammatory activity of contaminating DNA in hyaluronic acid preparations. J Pharm Pharmacol 53 (2001): 555-561.
