Triple versus Double Orifice Valves After Transcatheter Edge-to-Edge Repair: Clinical Features and Outcomes
Article Information
Maia Eng, Lily Chen, Benjamin Stripe, Thomas W. Smith, Edris Aman, Dali Fan, Gagan D. Singh, Jason H. Rogers*
Division of Cardiovascular Medicine University of California, Davis Medical Center Sacramento, California 95817 USA
*Corresponding author: Jason H. Rogers. Division of Cardiovascular Medicine University of California, Davis Medical Center Sacramento, California 95817 USA. Email: jhrogers@ucdavis.edu
Received: 02 November 2023; Accepted: 09 November 2023; Published: 09 January 2024
Citation: Maia Eng, Lily Chen, Benjamin Stripe, Thomas W. Smith, Edris Aman, Dali Fan, Gagan D. Singh, Jason H. Rogers. Triple versus Double Orifice Valves After Transcatheter Edge-to- Edge Repair: Clinical Features and Outcomes. Cardiology and Cardiovascular Medicine. 8 (2024): 7-14.
View / Download Pdf Share at FacebookAbstract
Rationale: Procedural and clinical outcomes of triple orifice valves created by mitral valve transcatheter edge-to-edge repair (TEER) have not been previously characterized.
Methods: 201 patients undergoing MitraClip were retrospectively analyzed from 2014-2019 (before generation 4 MitraClip release). A triple orifice valve was defined as having three orifices with all diastolic inflow diameters > 4 mm (the width of a non-XTW/NTW MitraClip). We reviewed pre- and post-procedure pressure gradients, hemodynamic parameters, and mitral regurgitation (MR) grades in triple vs. double orifice valves.
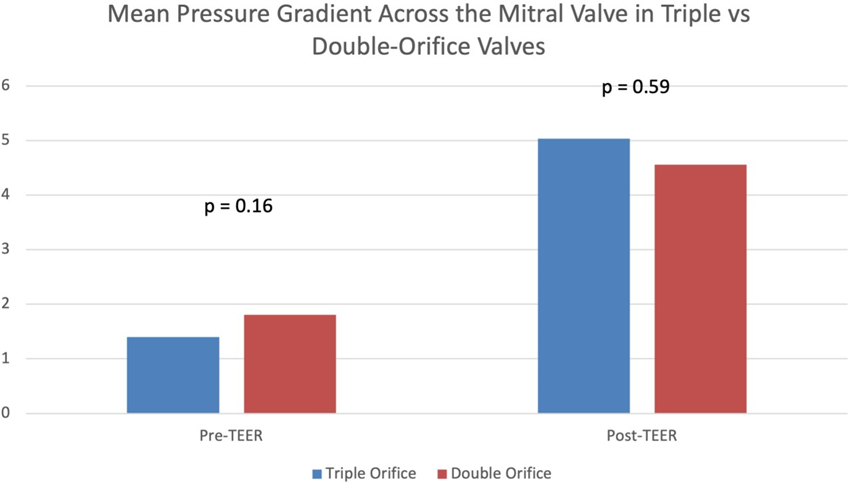
Results: Patients with triple orifice valves after MitraClip (n=13) had lower baseline mean pressure gradients across the mitral valve compared to double orifice valves, although this difference was not significant (1.4 ± 0.8 vs 1.8 ±.2 mmHg, p = 0.15). They also did not have significantly different post-procedure gradients (5.0 ± 2.8 vs 4.6 ±2.2 mmHg, p = 0.59). MR grade pre- and post-TEER was similar between the two groups. Procedural hemodynamic parameters were also similar between the two groups.
Conclusions: In this single center retrospective study, we found that the creation of a triple orifice mitral valve after MitraClip had a comparable impact on the final gradients when compared to double orifice, perhaps due to lower baseline gradients. Overall MR reduction was similar. The need to create triple orifice valves in selected patients is often driven by more complex baseline disease with multiple or wide jets.
Keywords
Transcatheter edge-to-edge repair, structural heart interventions, mitral valve
Article Details
1. Introduction
Transcatheter edge-to-edge repair (TEER) is used to treat symptomatic mitral regurgitation (MR) in patients with primary MR at high risk for conventional surgery, or in patients with secondary MR without severe left ventricular dilation or right heart dysfunction who remain symptomatic despite guideline directed medical therapy (GDMT), (1). The MitraClip TEER system corrects mitral regurgitation by creating a functional “Alfieri stitch”(2) generally resulting in a classic symmetric double-orifice mitral valve. Depending on the valve characteristics and the degree and location of regurgitation, patients may have one or more clips placed with multiple possible orifice configurations. For instance, patients may have two symmetric or asymmetric residual valve orifices, a single residual orifice, or less commonly a triple orifice valve. Triple-orifice valves have historically been seen only in the setting of congenital mitral valve disease or post-surgical valve repair. Congenital triple-orifice mitral valves are exceedingly rare and have only been described a handful of times in the literature (3). In contrast, surgically creating a triple orifice mitral valve is a technique that has been used at some centers for treatment of very myxomatous (Barlow’s type) mitral valves. These degenerative valves often have redundant valvular tissue, which allows for surgical edge-to-edge repair at two separate sites on the valve, thus creating a triple-orifice phenotype (4). Cases of triple orifice valves have previously been described after TEER (5, 6), but to our knowledge this is the first published series and systematic study of triple orifice valves after TEER. The objectives of this study are: (1) to describe the echocardiographic and clinical characteristics of patients with triple orifice valves after TEER; and (2) to compare post-procedure clinical outcomes and the degree of mitral stenosis or regurgitation in patients with triple orifice vs double orifice mitral valves.
2. Methods
This single center retrospective study was conducted using institutional procedural databases derived from the University of California Davis Medical Center (2014–2019). The Institutional Review Board approved the study protocol. As this was a retrospective study of collected clinical data, the ethics committee stated that written consents were not required by patients. We retrospectively analyzed mean pressure gradient, mitral regurgitation grade, and other hemodynamic parameters of MitraClip patients at our center over a three-year period and compared patients who had triple orifice valves to those who had double orifice valves. We used mean mitral valve gradient as a clinically relevant surrogate for mitral valve area (MVA), since calculation of MVA is challenging and of questionable validity in multi-orifice valves. An orifice was defined as a distance along the coaptation plane between the valve leaflets that is wide enough to allow placement of a clip that could theoretically close that orifice. The width of a standard MitraClip is 4mm. We therefore defined an orifice as having a diameter of at least ≥ 4 mm as measured by the vena contracta of the diastolic color jet in the midesophageal bicommissural view. This study was performed before the introduction of the generation 4 MitraClip system and therefore wider clips (NTW or XTW clips, which are 6 mm wide) were not included.
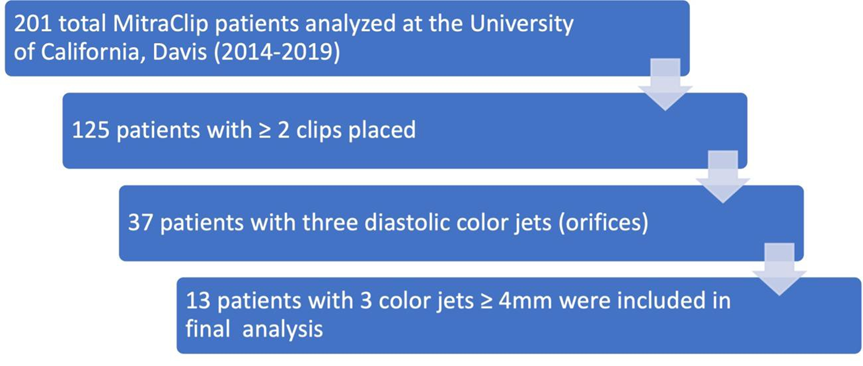
The patient selection and exclusion process is outlined in Figure 1.
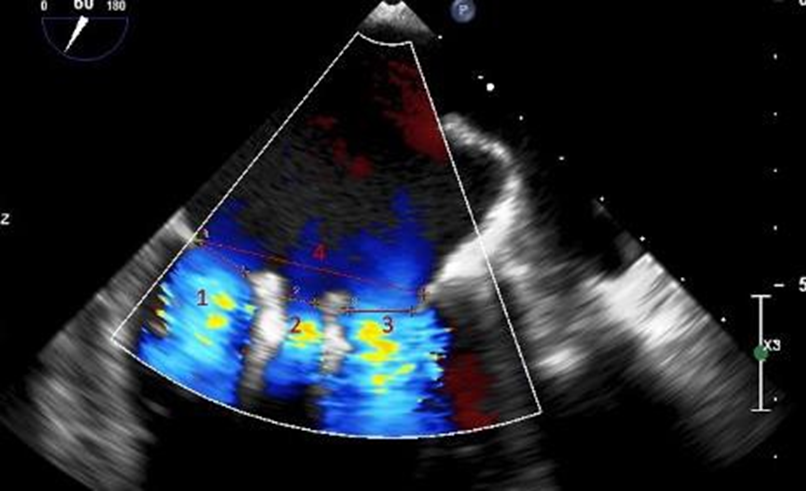
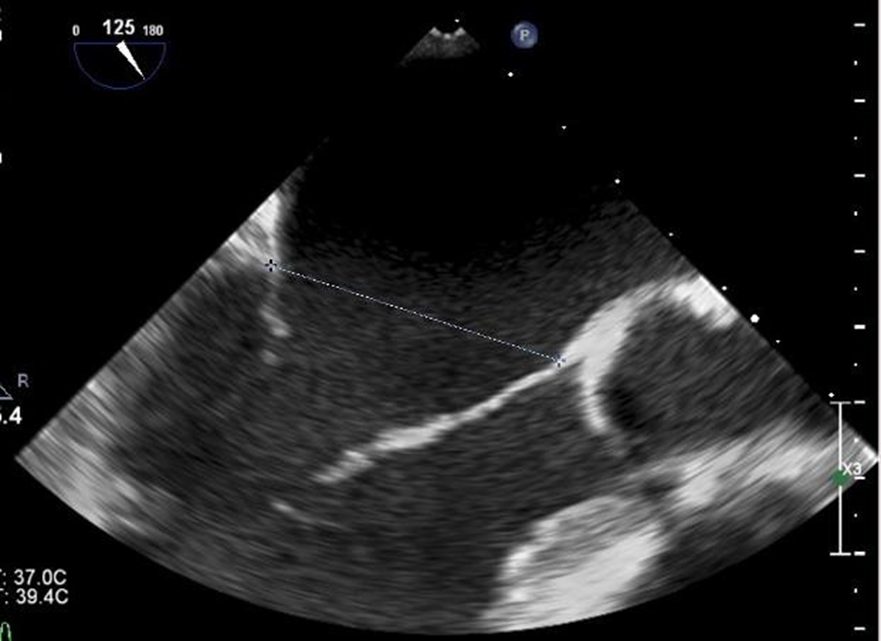
We reviewed 201 total patients at the University of California, Davis from 2014-2019 who underwent placement of MitraClip NT clips and MitraClip XT clips. We limited our analysis to patients who underwent placement of at least two clips since these are the patients who would have possible triple orifice valves (placement of a single clip will not result in a triple orifice valve). There were 125 patients with ≥ 2 clips placed. Of these, we eliminated patients who had clips placed on the same leaflet segment (e.g., both clips placed A2/P2), as they were unlikely to have a distance between them that would qualify as an orifice. We then identified 37 patients who had clips placed on non-adjacent leaflet segments (e.g., A1/P1 and A2/P2). Each of these patients’ post-procedure echocardiography was then reviewed by two independent cardiologists. Transesophageal echocardiograms were reviewed and measurements were made of the commissure-commissure distance in the mitral bicommisural view (45-60 degrees, Figure 2) and of the septal-lateral distance in the left ventricular long axis view (120-130 degrees, Figure 3). The vena contracta of the diastolic inflow jets and the distance between the jets were measured in the mitral bicommissural view (Figure 2).
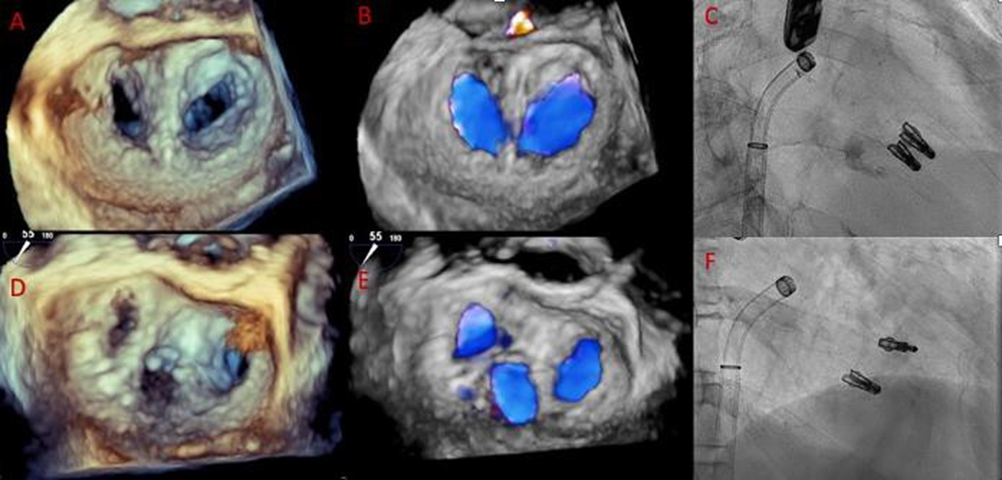
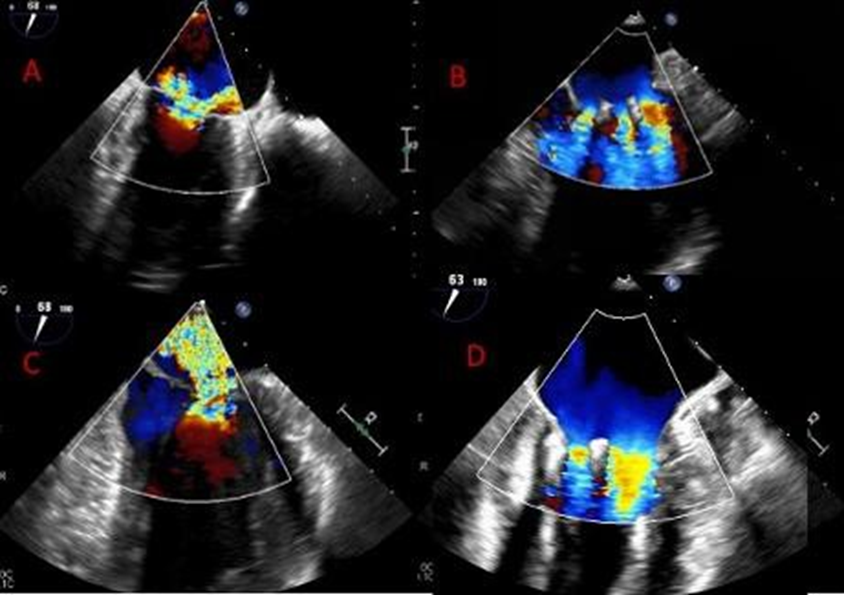
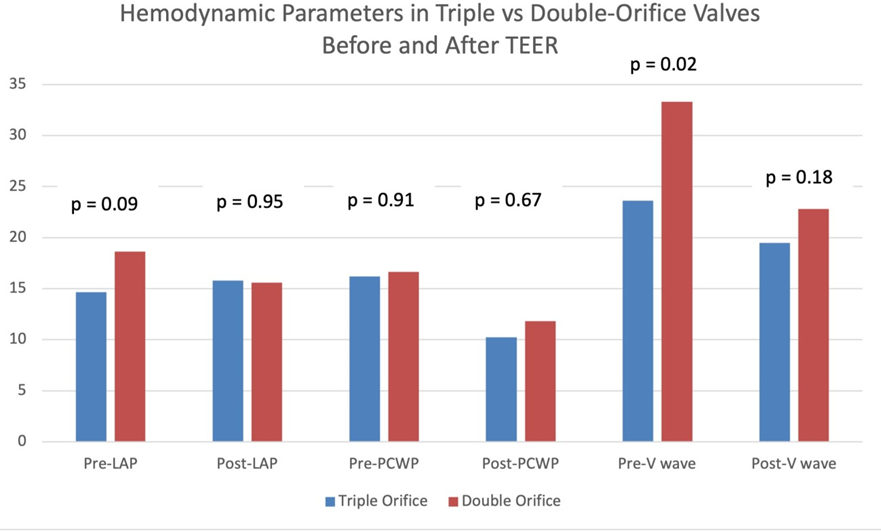
Thirteen patients were identified who met our definition of the triple orifice phenotype. Representative images of patients with triple-orifice vs double-orifice valves pre- and post-TEER are shown in Figure 4 and the Central Illustration. We then compared pre- and post-MitraClip echocardiographic variables between the triple-orifice group and the double- orifice group. We also reviewed pre- and post-MitraClip invasive hemodynamic parameters of left atrial pressure (LAP), pulmonary capillary wedge pressure (PCWP), and v-wave pressures.
2.1 Statistical Analysis
Statistical analysis was performed using Excel version 16.59 (Microsoft Corporation). Continuous data were expressed as mean ± SD and categorical data were reported as grades from 0-4. An independent-samples Student’s t test was used for the analysis of continuous variables, and the differences between nominal variables were compared using Chi-squared testing or Fisher’s exact test, when appropriate. Statistical significance was defined as p < 0.05.
3. Results
201 patients who underwent TEER at our center between 2014-2019 were reviewed. Of these, thirteen patients with triple-orifice valves and 188 patients with double-orifice valves were identified. Baseline demographic variables, including age, sex, and presence of preexisting conditions, were similar between the two groups (see Table 1).
Table 1: Baseline Demographics
|
Triple Orifice |
Double Orifice |
|
|
AGE (years) |
75 |
79 |
|
MALE (%) |
62 |
56 |
|
HTN (%) |
100 |
72 |
|
HLD (%) |
54 |
65 |
|
DM2 (%) |
38 |
25 |
|
CVA/TIA (%) |
15 |
14 |
|
AFIB (%) |
54 |
58 |
|
CHF (%) |
85 |
75 |
|
CAD (%) |
62 |
60 |
|
BMI (kg/m2) |
27 |
25 |
All values reported are means. HTN = hypertension; HLD = hyperlipidemia; DM2 = type 2 diabetes mellitus; CVA = cerebrovascular accident; TIA = transient ischemic attack; AFIB = atrial fibrillation; CHF = congestive heart failure; CAD = coronary artery disease; BMI = body mass index
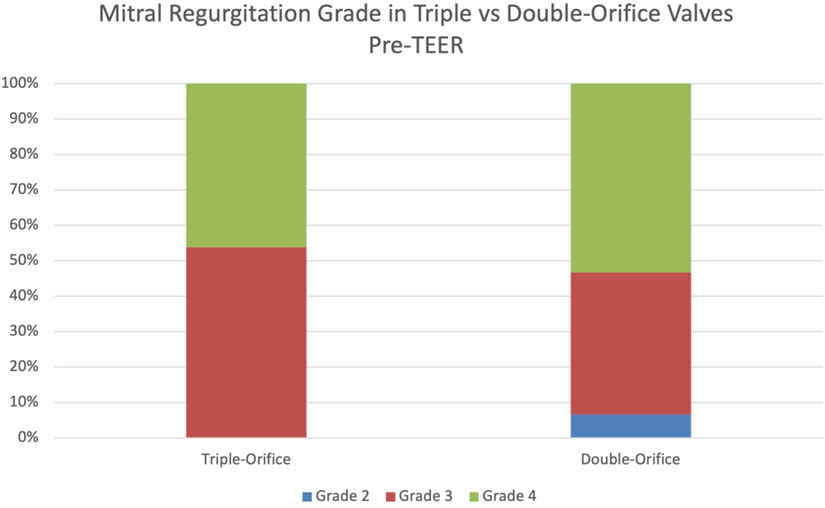
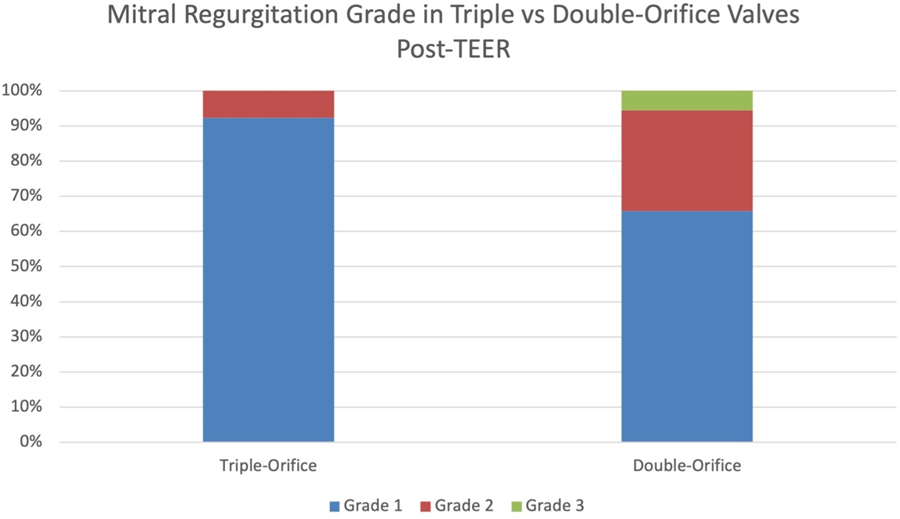
Prior to mitral TEER, there were no significant differences in echocardiographic parameters between the two groups. The pre-procedure diastolic mean mitral valve pressure gradients in patients with triple-orifice vs. double orifice valves were not significantly different. The post-procedure mean pressure gradient across the valve also was not significantly different between the two groups (Figure 5 and Table 3). MR grade pre-procedure ranged from grade 2+ to 4+; MR grade post-procedure ranged from grade 1+ to 3+. Patients in the triple-orifice group tended to have more severe MR at baseline (100% of patients in the triple-orifice group started with ≥3+ MR vs 93% in the double- orifice group). Post-procedure, patients in the triple-orifice group tended to have less MR as compared to patients in the double-orifice group (100% of patients in the triple-orifice group had ≤ 2+ MR as compared to 91% in the double-orifice group; see Figures 6-7 and Table 2). There was no significant difference in septal-lateral distance in the left ventricular outflow tract (LVOT) view between the triple-orifice and the double-orifice group (3.38 ± 0.7 cm vs 3.39 ± 0.7 cm, respectively, p = 0.98). There was also no significant difference in commissure- commissure distance as measured in the bicommissural view (4.1 ± 0.8 cm vs 3.7 ± 0.6 cm, p = 0.15, in the triple-orifice vs double-orifice group).
Table 2: Mitral Regurgitation Grading Pre- and Post-MitraClip
|
Mitral Regurgitation Grade |
||||
|
Pre (%) |
||||
|
Grade |
Triple Orifice |
Double Orifice |
p-value |
|
|
0 |
0 |
0 |
N/A |
|
|
1 |
0 |
0 |
N/A |
|
|
2 |
0 |
12 (7) |
0.34 |
|
|
3 |
7 (54) |
73 (40) |
0.28 |
|
|
4 |
6 (46) |
97 (53) |
0.7 |
|
|
Post (%) |
||||
|
0 |
0 |
0 |
N/A |
|
|
1 |
12 (93) |
119 (63) |
0.7 |
|
|
2 |
1 (7) |
52 (28) |
0.03 |
|
|
3 |
0 |
10 (5) |
N/A |
|
|
4 |
0 |
0 |
N/A |
|
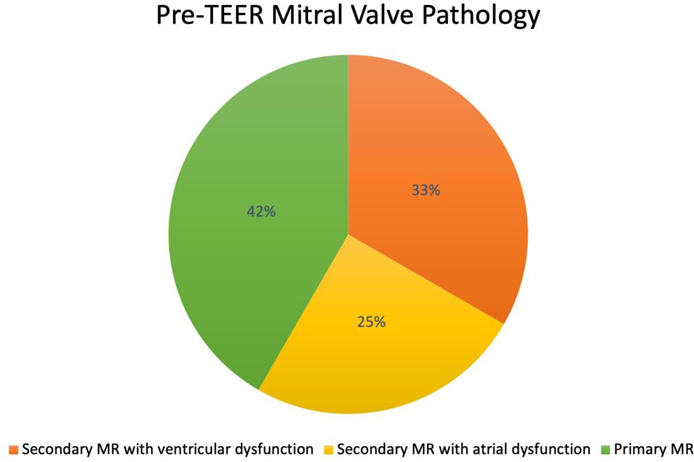
Hemodynamic values including pre-procedure LAP, post-procedure LAP, pre-procedure PCWP, and post- procedure PCWP were not significantly different between the two groups. Pre-procedure left atrial v-wave was significantly lower in the triple-orifice group; post-procedure, the v-waves were not significantly different between the two groups (see Figure 8 and Table 3). The initial presenting pathology in patients treated with a triple-orifice technique is approximately evenly distributed between secondary ventricular functional MR, secondary atrial functional MR, and primary MR (Figure 9).
|
Triple Orifice (n=13) |
Double Orifice (n=188) |
p-value |
|
|
Mean Pressure Gradient (mmHg) |
|||
|
pre |
1.4 ± 0.8 |
1.8 ± 1.2 |
0.16 |
|
post |
5.0 ± 2.8 |
4.6 ± 2.2 |
0.59 |
|
Left Atrial Pressure (mmHg) |
|||
|
pre |
15 ± 7.3 |
19 ± 7.9 |
0.09 |
|
post |
16 ± 6.5 |
16 ± 5.6 |
0.95 |
|
Wedge Pressure (mmHg) |
|||
|
pre |
16 ± 8.1 |
17 ± 8.3 |
0.91 |
|
post |
10 ± 6.5 |
12 ± 8.4 |
0.67 |
|
V-wave (mmHg) |
|||
|
pre |
24 ± 11.8 |
33± 16 |
0.02 |
|
post |
20 ± 3.7 |
23 ± 8.3 |
0.17 |
4. Discussion
In this retrospective analysis of MitraClip procedures over three years at a single center, we compared patients who underwent the procedure with creation of a triple orifice mitral valve and those who underwent creation of a double orifice valve. To our knowledge, this is the first systematic study to demonstrate that utilization of a triple orifice strategy was not associated with significantly different post-procedure valvular hemodynamics. The intraprocedural creation of a triple orifice valve is generally the result of complex baseline valvular pathology. The basic strategy in TEER is to treat the main regurgitant jet first. In some patients after placement of a first clip at the dominant jet location, there is a second major jet at some distance from the first jet. If there is adequate valve area, it may be reasonable to place a second clip at some distance from the first, creating a triple orifice valve. The development of clinically significant mitral stenosis is a potential complication of MitraClip implantation, and there is understandably particular concern in the creation of triple orifice valves. Hemodynamic studies have shown that there is a significant increase in mean pressure gradient and a decrease in valve area after double-orifice MitraClip. Herrmann et al analyzed 107 patients in the EVEREST trial registry with moderate- severe or severe MR. In these patients, mitral valve area decreased significantly and mitral valve gradient increased significantly after MitraClip, but then stayed stable at 24 month follow up (7). In the same study, there was no difference in mitral valve area or pressure gradient seen between patients who had one vs two Mitraclips placed.
Two cases of triple-orifice anatomy have been described in previous case reports, and these situations were not associated with increased mitral valve gradient or decreased valve area. Paranskaya et al in 2012 described a case of triple-orifice valve after mitral TEER which did not result in significant mitral valve stenosis immediately post-procedure nor at 3-month follow-up. The authors point out in this case that a significant number of patients with secondary MR due to advanced heart failure will have annular dilation and extensive lack of leaflet coaptation; this situation may be best handled by using three clips instead of two to better approximate the mitral leaflets and distribute tension on a larger area of the valve (5). In 2016, Wengenmayer et al also described a case with the creation of a triple-orifice mitral valve after TEER. In this case, the presence of a pseudo-cleft in the P2 segment in a patient with severe functional mitral regurgitation required a novel approach to the valvular repair. Initially, the patient was treated with a single clip to the A2/P2 region lateral to the cleft with reduction of the MR to moderate and discharged in stable condition. However, the patient was later readmitted with severe dyspnea and found to have moderate-severe MR. He underwent a second clip which was placed at a 45-degree angle to the first, with the cleft between them, in such a way that a triple-orifice valve was created. The patient’s MR was reduced to mild with improvement in his symptoms (6).
With regards intraprocedural triple orifice valve creation, standardized procedural techniques have not been formally developed and this is a dynamic process that varies from patient to patient. Although placement of MitraClip is typically guided by transesophageal echocardiography, fluoroscopy can also be used to assess clip spacing. As MitraClip availability and access continues to expand, the need to continue to refine our approach in order to achieve optimal clinical outcomes is imperative. The ability to tailor the clipping strategy to the individual patient’s mitral valve anatomy and function is an ongoing challenge. Often, patients with severe mitral regurgitation will require a multi-clip strategy in order to achieve significant reduction in MR. Based on our experience, in order to determine which patients would benefit from creation of a triple-orifice valve, each patient’s mitral valve anatomy and hemodynamics were evaluated after placement of the initial clip. If the patient was determined to have residual MR greater than mild, indicating another clip was needed, the residual jet was then targeted for placement of a second clip. If the second clip needed to be more than one clip width’s distance away in order to address the residual MR, then a triple-orifice valve was created. Interestingly, the initial presenting pathology in patients treated with a triple-orifice technique is approximately evenly distributed between secondary ventricular functional MR, secondary atrial functional MR, and primary MR. Further study is needed to determine if there is any significant difference between these groups in predicted which patients are ultimately treated with a triple-orifice strategy.
There has not previously been a review of the possible hemodynamic consequences of creating a triple orifice. In this study, we sought to define the basic differences between triple-orifice and double-orifice valvular echocardiographic parameters. Ultimately, this will inform future strategic approaches to MitraClip procedure in patients who may require the placement of two or more clips to achieve satisfactory reduction in MR. Our analysis demonstrated no significant difference between the two groups in echocardiographic or invasive assessment of the mitral valve.
Our study has several limitations. This is a retrospective review of a small number of patients at a single center. In this small cohort of patients, we do not have detailed long-term follow-up information (such as reintervention rates or development of mitral stenosis), as our intention with this manuscript was to describe the acute procedural outcomes of double vs. triple orifice valves. Given that procedural outcomes are similar, we would expect the repeat intervention rates to be similar between the two groups. Further study is needed to determine whether triple-orifice mitral valve anatomy after MitraClip has long-term clinical ramifications.
5. Conclusion
Based on our analysis, patients with triple orifice valves have a lower resting gradient across the mitral valve at baseline, but do not have an increased final gradient across the valve after clip. This initial data supports the safety and efficacy of employing a triple orifice technique at the operator’s discretion.
Ethics Statement
The Institutional Review Board approved the study protocol. As this was a retrospective study of collected clinical data, the ethics committee stated that written consents were not required by patients.
Financial Disclosures
Dr. Eng is a consultant for Abbott, Moray Medical, and MedTecX. Dr. Chen is a consultant for Phillips, Abbott, and Akura Medical. Dr. Stripe is a consultant for Abbott. Dr. Smith is a consultant to Abbott, Gore, Novartis, and Laminar and holds equity in Laminar. Dr. Aman is a consultant for or has received honoraria from Abbott, Siemens, Phillips, WL Gore, Silara MedTech, and ReValve. Dr. Fan has no disclosures. Dr. Singh is a consultant and advisory board member for Abbot, Phillips, and Gore and a consultant for Boston Scientific and Baylis. Dr. Rogers is a consultant to Abbott, Baylis, and Boston Scientific.
List of Abbreviations
Transcatheter edge-to-edge repair (TEER)
Mitral regurgitation (MR)
Guideline directed medical therapy (GDMT)
Mitral valve area (MVA)
Left atrial pressure (LAP)
Pulmonary capillary wedge pressure (PCWP)
Left ventricular outflow tract (LVOT)
References
- Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Circulation 143 (2021).
- Alfieri O, De Bonis The role of the edge-to-edge repair in the surgical treatment of mitral regurgitation. J Card Surg 25 (2010).
- Oliveira SM, Faria R, Amorim MJ & Almeida J. Triple-orifice congenital stenotic mitral valve: A unique finding characterized by multimodality echocardiography. European Journal of Echocardiography 12 (2011): 799.
- Fucci C, Faggiano P, Nardi M, D’Aloia A, Coletti G, De Cicco G, et al. Triple-orifice valve repair in severe Barlow disease with multiple-jet mitral regurgitation: Report of mid-term International Journal of Cardiology 167 (2013): 2623–2629.
- Paranskaya L, Kische S, Bozdag-Turan I, Nienaber C, Ince H. Mitral valve with three orifices after percutaneous repair with the MitraClip system: The triple-orifice technique. Clinical Research in Cardiology 101 (2012): 847–849.
- Wengenmayer T, Zhou Q, Bode C, Zehender M. Triple orifice as a novel strategy in interventional reconstruction of a mitral pseudo EuroIntervention 12 (2016): 1297.
- Herrmann HC, Kar S, Siegel R, Fail P, Loghin C, Lim S, et al EVEREST Investigators. Effect of percutaneous mitral repair with the MitraClip device on mitral valve area and gradient. EuroIntervention (2009): 437-42.