Treat to Target ASCVD Risk with Oral Non-statins and Bempedoic Acid: DELPHI Statement
Article Information
JPS Sawhney1, Saumitra Ray2, Prakash Hazra3, Sunil Sathe4, B C Srinivas5, Vijay Pathak6, Jay Shah7, Kamal Sharma8, Ashutosh Kakkad9*, Krishnaprasad K9, on behalf of CLEAN panel members
1Department of Cardiology, Sir Ganga Ram Hospital, New Delhi, India.
2Woodlands Multispeciality Hospital, Kolkata, India.
3AMRI Hospitals, Kolkata, West Bengal, India.
4Cardiac Care and Counselling Centre, Pune, India
5Department of Cardiology, Sri Jayadeva Institute of Cardiovascular Sciences and Research, Bengaluru, India
6Department of Cardiology, Sawai Man Singh (SMS) Medical College, Jaipur, India
7Department of Cardiology, Life Care Institute, Ahmedabad, India
8Dr. Kamal Sharma Cardiology Clinic, Ahmedabad, India
9Medical Services, Torrent Pharmaceuticals Limited, Ahmedabad, India
*Corresponding author: Ashutosh Kakkad, Medical Services, Torrent Pharmaceuticals Limited, Ahmedabad, Gujarat, 380009, India
Received: 18 May 2023; Accepted: 25 May 2023; Published: 8 July 2023
Citation: JPS Sawhney, Saumitra Ray, Prakash Hazra, Sunil Sathe, B C Srinivas, Vijay Pathak, Jay Shah, Kamal Sharma, Ashutosh Kakkad, Krishnaprasad K. Treat to Target ASCVD Risk with Oral Non-statins and Bempedoic Acid: DELPHI Statement. Cardiology and Cardiovascular Medicine. 7 (2023): 265-272.
View / Download Pdf Share at FacebookAbstract
Background: In Asian countries, the high endemicity of Coronary Artery Disease (CAD) has been associated with an increase in premature deaths requiring a multi-interventional or multidisciplinary approach to control the cardiovascular risk traits associated with most of these cases.
Objective: ESC recommendations on lipid goals of ≤55 mg/dl in Atherosclerotic cardiovascular disease (ASCVD) with or without Type 2 Diabetes Mellitus qualify for a complementary approach with nonstatin in most cases. Bempedoic acid (BA) amongst the non-statins offers differentiated mechanistic to regulate the atherogenic lipids and markers including LDL-C, non-HDL-C, and hs-CRP that may hold relevance in cases with chronic stable angina (CSA) and chronic coronary syndromes (CCS). To further understand the role and relevance of Bempedoic acid as an oral non-statin approach in ASCVD management, a Knowledge, Attitude, and Practices (KAP) survey was conducted between Aug and Oct '22 with 75 HCPs involving a multidisciplinary team with relevant academic and clinical standing on Dyslipidemia management in ASCVD cases across India.
Material/Methods: The RAND/UCLA modified Delphi consensusgenerating methodology was used to develop the Statement with Clinical Recommendations on the role of Non-statins and High-intensity statins for the management of ASCVD involving CSA and CCS. The KAP responses were corroborated with literature review and synthesis in the next round of meetings to develop the Delphi Statement based on Agency of Health Care and Quality Systems (AHRQ) criteria for the Strength and Quality of the evidence and experience shared by the specialist panel.
Results: Treatment lipid goals for ASCVD as suggested by ESC guidelines remain a clinical challenge with clinical inertia on combination therapy with statins especially for baseline LDL-C levels >/= 120 mg/dl. Nonstatin oral therapy including Bempedoic acid offers differentiated modulation of ATP Citrate Lyase (ACL) & AMP-activated protein kinase (AMPK) pathway unlike ezetimibe (EZE). CLEAR-Harmony, CLEAR-Wisdom, and CLEAR-Outcomes suggest BA offer complementary lipid-lowering, anti-inflammatory and cardioprotective effects in ASCVD cases with T2D, Left Ventricular Dysfunction (LVD), Chronic kidney disease (CKD) (Stage 3b/4) and Peripheral arterial disease (PAD).
Conclusion: The Delphi-mediated KAP recommendations provide a reallife approach to the management of ASCVD with non-statins including Bempedoic acid as an initial add-on approach with Moderate or Highintensity statins in outpatient settings of India
Keywords
Coronary artery disease; Ischemic nonobstructive coronary artery disease; Atherosclerotic cardiovascular disease; Acute Coronary Syndrome; Bempedoic acid; Type 2 diabetes; Knowledge Attitude Perception; Delphi
Coronary artery disease articles; Ischemic non-obstructive coronary artery disease articles; Atherosclerotic cardiovascular disease articles; Acute Coronary Syndrome articles; Bempedoic acid articles; Type 2 diabetes articles; Knowledge Attitude Perception articles; Delphi articles
Article Details
1. Introduction
An alarming increase in the prevalence of CVD over the past two decades has been observed in India. CVDs such as ischaemic heart disease and cerebrovascular such as stroke account for 17.7 million deaths and are the leading cause [1,2]. The course of ASCVD also appears to be more fulminant with higher mortality. Around 10- 25% of myocardial infarctions (MI) occur before the age of 40 in India, and more than half of all CAD-related deaths occur before the age of 50 [3,4,5]. The increased incidence of CAD in Asian Indians cannot be attributed directly to the common risk factors of dyslipidemia or hypertension. It is estimated that 60% of CAD cases in Indians have residual risk that may be explained by the high inflammatory indices including hs-CRP [3]. Guideline-directed medical therapy (GDMT) with statins has been widely considered the initial and preferred approach for the majority of patients with ASCVD. Though the guidelines have evolved for the varied lipid goals suggested across the American College of Cardiology/American Heart Association (ACC/AHA) [6,7] European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) [8] and the Lipid Association of India (LAI) [9] guidelines for ASCVD, the pertinent need for effective and safe therapies as oral options remains for ASCVD management despite the availability and guidance on the use of high–intensity statin. This was further highlighted by real-world evidence involving the South East population wherein <60% of participants achieved LDL-C targets of 70 mg/dL despite the induction of high-intensity statin therapy in most cases [10].
1.1 ASCVD and Lipid goals
ESC recommendations for ASCVD with T2D or End-Organ damage (EOD) including LVD, and CKD as <55 mg/dl remain relevant, especially for cases with additional risk traits including hypertension, obesity, smoking, and dyslipidemia. Other risk-enhancing determinants are belonging to race or ethnicities at high risk, increased ASCVD risk related to persistently elevated primary hypertriglyceridemia or other biomarkers like elevated high-sensitivity C-reactive protein (hs-CRP), elevated Lp(a), elevated apoB, or ankle-brachial index <0.9 [6,8]. On the other hand, ASCVD cases with ACS requiring revascularization, recurrent ischemic events within the last two years, and/or familial hypercholesterolemia require an aggressive approach with LDL-C goals as <40 mg/dl [8]. The DA-VINCI study [11] highlighted the great disparity in the lipid goal achievement for ASCVD and ACS despite the induction with moderate or high–intensity statins in 75% of cases. In established ASCVD (eASCVD) cases, only 21% of patients receiving high-intensity statins or Ezetimibe combination regimens achieved LDL-C targets of <55 mg/dL further accentuating the need for novel complementary agents with multimodal actions to cut the primary and residual cardiovascular risk associated with high inflammatory (hs-CRP), apo B and non-HDL-C. In this line, though statins reduce the hs-CRP level, neither PCSK9 inhibitors nor ezetimibe monotherapy have been shown to significantly reduce the biomarkers associated with inflammation.
1.2 Non-Statin Strategies & Bempedoic Acid
Bempedoic acid is a novel first-in-class oral inhibitor of adenosine triphosphate (ATP)-citrate lyase (ACL) approved worldwide as an adjunctive to maximally tolerated statin therapy to lower LDL-C for patients with ASCVD, Mixed hyperlipidemia and for patients with heterozygous familial hypercholesterolemia (HeFH). In the EU, it is also approved for the treatment of patients who are unable to take any dose of a statin (statin intolerant) or for whom a statin is contraindicated. Recently Gunn et al [12] published a simulation study estimating the 10-year risk of three-point major adverse cardiovascular events in ASCVD patients with participants from the four-phase III CLEAR trials of bempedoic acid. The 10-year CV event risk reduction was further reduced by 3.3% in statin-treated (cohort 1) and 6% in statin-intolerant (cohort 2) patients. The CLEAR-OUTCOMES [13] again demonstrated equivocal 13% risk reduction in 4P-MACE that is construed to be similar with PCK9i trials suggesting incremental benefits for the first time with a non-statin including Bempedoic acid. In this line to further assess the scope, potential, and current contemporary practices towards management of high-risk ASCVD patients in real-world settings of India with non-statins and bempedoic acid, a Knowledge, Attitude, Perception (KAP) survey was planned to be conducted.
2. Materials and Methods
A KAP survey questionnaire was developed to discuss some of the pertinent issues in the management of ASCVD with special emphasis on high-risk patients in the Indian context. This questionnaire was validated by an expert panel of eight members that included eminent interventional and practicing cardiologists with national academic and/or clinical standing with relevant experience in the development of consensus statements. The KAP survey questionnaire was shared electronically with seventy-five HCPs across India with relevant experience in the management of ASCVD before its analyses and consolidation during the phygital meet conducted between Aug-Oct ‘2022. A second round of meetings in the Dec ’22 discussed the responses while reviewing the literature and evolving treatment recommendations on the choice and role of Bempedoic acid in ASCVD management. The steering panel of eight specialists and experts responded, and reviewed the literature before developing clinical recommendations on the clinical role and positioning of non–statins in the management of ASCVD patients. The Level of Evidence (LoE) and Level of Agreement (LoA) was developed after a review of literature and publications featuring in indexed databases of Medline, PubMed, SCOPUS, INDEX MEDICUS, and COCHRANE Systemic reviews using the keywords as Cardiovascular disease, Atherosclerotic cardiovascular disease, low-density lipoprotein cholesterol, Bempedoic Acid, Statins, Chronic coronary syndrome, Type 2 diabetes. A systemic review of 12 RCTs involving >23000 cases for BA with eASCVD was conducted using PubMed, Google Scholar, and Cochrane Database of Systematic Reviews search while including the topline results from CLEAR OUTCOMES. Treatment recommendations based on a successful problem-based clinical assessment method were evolved while assessing the Strength and Quality of evidence as per Agency of Health Care and Quality Systems (AHRQ) criteria and general expert opinion shared by the panel. Levels of evidence were assigned for meta-analyses, randomized, case-cohort longitudinal, cross-sectional case-control, and Case reports as Level I, II, III, IV, and V respectively. The expert opinion was accepted as a recommendation in case of acceptance by 80 % of the panelist based on a LIKERT scale score (1: strongly disagree; 2: disagree; 3: neither agree nor disagree; 4: agree; 5: strongly agree) conducted during the meet. Descriptive statistics for mean, median, and proportion analyses were carried out for each of the responses generated for the 10-point questionnaire.
3. Results:
Seventy-five interventional and practicing Cardiologists provided clinical insights on the contemporary positioning of recently introduced non-statin therapy i.e. bempedoic acid as oral formulations in the management of Chronic stable angina, Chronic Coronary Syndrome (CCS), & Acute Coronary Syndrome (ACS) undergoing or not undergoing revascularization procedures including Percutaneous Intervention (PCI) using structured KAP based questionnaire. Responses received from HCPs based in different geographic locations across India were collated and analyzed.
Table 1: KAP questionnaire with responses on the clinical role and positioning of Non-statins and Bempedoic acid
|
No. |
Structured questionnaire |
Response |
% |
|
1 |
What % of ASCVD cases require PCI with a stent? |
<10% |
12% |
|
10-20% |
24% |
||
|
21-40% |
33% |
||
|
41-60% |
21% |
||
|
>60% |
10% |
||
|
2 |
In managing Mixed dyslipidemia with TG between 150- 500mg/dL for ASCVD, what is the choice of drugs? |
High-intensity Statins |
42% |
|
Fibrates |
32% |
||
|
Bempedoic Acid |
12% |
||
|
Ezetimibe |
14% |
||
|
3 |
LDL-C targets advisable for ASCVD cases |
<30 mg/dL |
1% |
|
<55 mg/dL |
53% |
||
|
<70 mg/dL |
37% |
||
|
<100 mg/dL |
9% |
||
|
4 |
Non-HDL-c targets advisable for ASCVD |
<85 mg/dL |
53% |
|
<130 mg/dL |
42% |
||
|
<200 mg/dL |
5% |
||
|
5 |
Bempedoic Acid as initial combination therapy with High-intensity Statin in ASCVD cases |
Strongly Agree |
24% |
|
Agree |
58% |
||
|
Neutral |
11% |
||
|
Disagree |
7% |
||
|
Strongly Disagree |
0% |
||
|
6 |
Bempedoic Acid as the initial add-on therapy choice to Moderate intensity statin in CCS cases |
Strongly Agree |
21% |
|
Agree |
56% |
||
|
Neutral |
17% |
||
|
Disagree |
5% |
||
|
Strongly Disagree |
1% |
||
|
7 |
In Mixed dyslipidemia with CCS and multiple risk factors, Bempedoic acid can be offered as an add-on to Moderate dose Statin |
Strongly Agree |
25% |
|
Agree |
67% |
||
|
Neutral |
8% |
||
|
Disagree |
0% |
||
|
Strongly Disagree |
0% |
||
|
8 |
Bempedoic acid add-on to Statin / Ezetimibe can be suggested for Very high risk or ACS cases with persistent LDL-C levels of >55 mg/dl? |
Strongly Agree |
37% |
|
Agree |
59% |
||
|
Neutral |
3% |
||
|
Disagree |
1% |
||
|
9 |
In the 52 W, CLEAR HARMONY trial with Bempedoic acid as add-on therapy to maximally tolerated statin, the risk of New onset diabetes or Worsening was significantly reduced making it ideal for ASCVD with T2DM |
Strongly Agree |
28% |
|
Agree |
57% |
||
|
Neutral |
15% |
||
|
Disagree |
0% |
||
|
Strongly disagree |
0% |
||
|
10 |
Bempedoic acid with its metabolic and anti-inflammatory actions, makes it the ideal candidate for co-therapy with High-intensity Statin in PCI cases of ASCVD requiring modulation of atherogenic lipids including LDL-C and non-HDL-C. |
Strongly Agree |
20% |
|
Agree |
72% |
||
|
Neutral |
7% |
||
|
Disagree |
1% |
||
|
Strongly Disagree |
0% |
4. Discussion
The residual risk with statin therapy in ASCVD remains a clinical challenge with recurrent Angina and impaired QoL. Nearly half of the cardiac coronary tomography (CCTA) and Angiography cases for Angina or stable CAD (SCAD) demonstrate little or non-significant central artery stenosis that requires aggressive lipid-lowering therapy in line with the stiff lipid targets of <70 mg/dl in most of these cases [14]. However, despite the advances in GDMT, up to 40% of statin-treated patients continue to suffer from life-threatening CV events even if high-dose statin treatment achieves the LDL-C target. This is likely to be caused by unresolved 'residual risk' that may be related to non-HDL-C and/or underlying inflammation (hs-CRP) as corroborated by landmark trials for icosapent ethyl and canakinumab [15]. In addition, the safety signal on the induction or progression of Type 2 diabetes with high dose statins [16] seems significant concern as highlighted by WOSCOPS, SPARCL, and JUPITER trials warranting the need for novel approaches like bempedoic acid. Third, the high rate of recurrence after the first episode of ACS often depending on non-culprit lesions, some of them initially appearing as angiographically mild and inconspicuous at the index procedure [17] makes the case curious yet pertinent for the complementary induction of the nonstatin approach as a pre-emptive strategy.
4.1 BA: Beyond LDL-C reduction for complementary Synergy with Statins
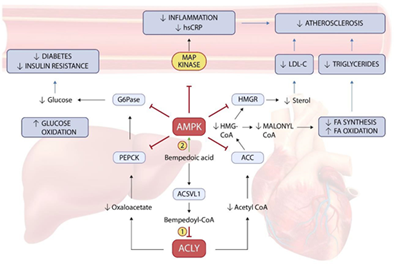
Bempedoic acid is a novel non-statin compound with selective action in hepatic tissues acting on the upstream cholesterol pathway of Acetyl-CoA synthesis inhibition leading to a significant drop in cholesterol, triglycerides, apo-B, and hs-CRP due to LDL-C receptor upregulation, fatty acid (FA) synthesis, and oxidation inhibition, unlike statins and ezetimibe. The actions are further complemented by AMPK activation-led control of intracellular glucose- and lipotoxicity with inhibition of Glucose-6-Phosphate dehydrogenase (G-6-PD) and FA entry or oxidation leading to anti-ischemic effects in heart and myocardium musculature [18].
The ancillary actions of BA in mitigating residual risk associated with statin therapy highlighted by the KAP responses were corroborated by CLEAR-WISDOM [19] and CLEAR-HARMONY [20] trials that suggested incremental anti-inflammatory actions of hs-CRP (-22.4 vs. 2.6 with placebo, p<.001) modulation despite background therapy of High-intensity statin (≤50%) in most cases of ASCVD. Systematic review and meta–analyses of 10 RCTs (n = 3,788) further demonstrated change in atherogenic lipids as non-HDL (-18.2%, p<.001) and hs-CRP (-27.0%, p<.001). Patients with background CV risk receiving maximally tolerated statin further demonstrated the differential impact of BA on atherogenic markers of non-HDL-C (-17.8 % vs. -12.1%), hs-CRP (-31.9% vs. -8.2%) and Total–C (-14.2% vs. -10.4%) when compared with EZE respectively [21].
4.2 BA: Ideal Nonstatin for ASCVD
KAP responses for the initial choice of oral nonstatin including BA were in line with the recent publication of CLEAR Trials pooled analyses and CLEAR OUTCOMES trial for ASCVD cases that is novel and first-of-kind for any oral nonstatin strategy. The dual mechanisms for BA offer incremental action in control of LDL-C, non-HDL-C, and hs-CRP through novel pathways of ACL and AMPK regulation that are independent unlike EZE [22]. The CLEAR OUTCOMES mediated ARR of 1.6 with BA over 4-y follow-up for ASCVD cases with baseline LDL-C >/=139 mg/dl was complemented by McQueen RB et al [23] for cases with differing LDL-C levels and risk assessment. The study estimated the risk reduction (RR) in 16,926 patients who were divided into two groups based on the baseline LDL-C of 101–110 mg/dL (n = 15,237) and LDL-C of > 200 mg/dL (n = 1689). The study predicted an incremental or additional CV risk reduction with BA/EZE (-10.9%) versus EZE. Taking into consideration the cardiovascular outcomes observed with Ezetimibe in the IMPROVE-IT trial of (-6%), the findings of McQueen RB et al draw inspiration for BA/EZE as a pragmatic oral strategy for implementation in ACS cases requiring LDL-C target of <55 or <40 mg/dl despite background therapy with maximally tolerated statin dose while ensuring compliance and adherence to therapy. The ARR and NNT with BA were equivocal with the landmark/pivotal Cardiovascular Outcome Trial (CVOT) of Proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9is) and ezetimibe suggesting the clinical consideration as a rational choice for initial combination therapy in cases with high CV risk or ASCVD with baseline LDL-C 110 to 185 mg/dl [24] (Table 2)
Table 2: CVOTs with Nonstatin drugs including Bempedoic acid, Evolocumab, and Ezetimibe [13,25,26]
|
CLEAR -OUTCOMES |
FOURIER |
RACING |
|
|
Intervention |
Bempedoic Acid |
Evolocumab |
Ezetimibe |
|
N |
13970 (prior ASCVD as MI, Stroke, or PAD; OR additional CV risk factors) |
27,564 (prior ASCVD: MI, non-hemorrhagic stroke, PAD, and ≥1 major/2 minor additional risk factors) |
1398 (ASCVD with T2D) |
|
Posology |
180 mg OD |
140 mg Q2W/ 420 mg Q4W |
ROSU 10 / EZE 10 vs ROSU 20 OD |
|
Inclusion criteria |
LDL-C ≥70 mg/dL or non-HDL-C ≥100 mg/dL that were either statin intolerant or receiving low-dose statins (22.7%); ezetimibe (11.5%) |
LDL-C ≥70 mg/dL or non-HDL-C≥100 mg/dL on background high-intensity statin with/ without ezetimibe |
LDL-C ≥70 mg/dL, Documented CVD, previous MI, ACS, coronary revascularization and other arterial revascularization procedures, ischemic stroke, or PAD |
|
Statin - intensity & dosage |
Statin Intolerant (66.6%), Statin (22.7%); EZE (11.5%) |
69% (atorvastatin ≥40 mg, rosuvastatin ≥20 mg, or simvastatin 80 mg) |
Mod. Intensity Statin/EZE (701, 50%); High-intensity Statin (697, 50%) |
|
Median FU |
3.8 y |
2.2 y |
3 y |
|
Primary Endpoint |
CV death, Nonfatal MI, Nonfatal stroke, or revascularization |
CV death, MI, stroke, revascularization, or hospitalization for UA |
CV Death, MACE (Coronary or Peripheral vascular disease revascularization or hospitalization) |
|
RRR |
13% (p = .004) |
15% (p<.0001) |
11% (p=NS) |
|
ARR |
1.6 |
1.5 |
p=NS |
|
NNT |
62.5 |
67 |
p=NS |
RRR: Relative risk reduction; ARR: Absolute risk reduction; NNT: Number needed to treat; FU: follow-up
5. Recommendations on Bempedoic Acid in ASCVD
- LDL-C goals for ASCVD cases with at least one additional risk factor including T2D would qualify for achievement of </=55 mg/dL (LoA, 100%)
- LDL-C, non-HDL-C, apo B, Lp (a), and hs-CRP assessment and target goal achievement remain relevant for ASCVD cases with MI history within the past < 12 mo (LoA, 100%)
- Non-statin oral therapy remains a pragmatic or pertinent strategy in real-life outpatient settings for the management of ASCVD with recent MI history within or >12 mo; ischemic stroke, or symptomatic PAD (history of claudication with ABI <0.85) (LoA, 100%)
- In ASCVD cases requiring aggressive LLT, Statin intensity-associated muscle symptoms (SAMS) may switch to a non-statin approach involving Bempedoic and/or Ezetimibe (LoA, 100%)
- In ASCVD with T2D or coronary artery calcium (CAC) >300 HU or Non-stenotic coronary lesion cases requiring >50% LDL-C reduction while targeting LDL-C levels of </=55 mg/dl, initial add-on strategy with BA may be considered as a pragmatic strategy (LoA, 100%)
- In ASCVD with revascularization history (Recent MI <12 mo) with HeFH, BA, or EZE as an initial add-on strategy with High-intensity statins can be considered (LoA, 100%)
6. Conclusion
The growing burden of ASCVD in India is accentuated by high disease progression leading to premature CVD and events. There is a significant residual risk requiring the addition of non-statin drugs to ESC-recommended targets. This makes bempedoic acid a welcome addition to the existing non-statin therapies such as ezetimibe, bile acid sequestrants, and PCSK9 inhibitors. A low frequency of muscle-related side effects, minimal drug interactions, a significant reduction in high-sensitivity C-reactive protein (hs-CRP), and a lower incidence of new-onset or worsening diabetes make it a useful adjunct for LDL-C lowering. The CLEAR OUTCOMES corroborate ancillary actions of bempedoic acid that are likely to offer incremental CV event risk reduction, particularly in ASCVD cases.
Acknowledgment
The author would like to thank the following CLEAN panel for their responses to KAP survey: Dr. KP Pramod Kumar Chennai, Dr. Vidyut Jain Indore, Dr. B Yugandhar Tirupati, Dr. Sanjay Sharma New Delhi, Dr. Bhaskar P Shah Mumbai, Dr. P Manokar Chennai, Dr. Dharmesh Solanki Rajkot, Dr. Tarun Dave Ahmedabad, Dr. Girish Navasundi Bangalore, Dr. Nagamalesh U M Bangalore, Dr. J Kannan Bangalore, Dr. Anupam Jena Bhubaneswar, Dr. Ashok Kumar Patna, Dr. Aniruddha Chandorkar Pune, Dr. Rajesh Badani Pune, Dr. Jayagopal PB Palakkad, Dr. G R Kane Mumbai, Dr. SS Binu Thiruvananthapuram, Dr. Ramesh Natarajan Anamugam, Dr. Mukesh Sharma Udaipur, Dr. S K Kaushik Udaipur, Dr. D K Garg Jaipur, Dr. Ajay Kumar Pandey Varanasi, Dr. Guru Prakash A Hyderabad, Dr. K Sarat Chandra Hyderabad, Dr. K Ravinder Reddy Karimnagar, Dr. Rajiv Agarwal New Delhi, Dr. Tushar Roy New Delhi, Dr. DK Baruah Visakhapatnam, Dr. Jayal Shah Ahmedabad, Dr. Thomas Alexander Coimbatore, Dr. KA Sambasivam Coimbatore, Dr. Debasis Ghosh Kolkata, Dr. Dhiman Kahali Kolkata, Dr. Niraj Prasad Ranchi, Dr. Dilip Ratnani Durg, Dr. C S Srinivasa Raju Nellore, Dr. N Murali Krishna Vijayawada, Dr. P C Sarma Silchar, Dr. Arindam Pande Kolkata, Dr. Spandan Bhadury Kolkata, Dr. Mahesh Fulwani Nagpur, Dr. BB Chanana New Delhi, Dr. Ashwani Mehta New Delhi, Dr. Subhash Chandra New Delhi, Dr. Dilip Kumar Kolkata, Dr. Viveka Kumar New Delhi, Dr. M Srinivasa Rao Hyderabad, Dr. Abhinav Bhagat Patna, Dr. BB Bharti Patna, Dr. Rishi Gupta Faridabad, Dr. Naveen Bhamri New Delhi, Dr. Rohit Parti Panchkula, Dr. Jitendra Singh Makkar Jaipur, Dr. Sandipta Ray Kalyani, Dr. Sujay J. Chitradurga, Dr. Sunip Banerjee Kolkata, Dr. Peeyush Jain New Delhi, Dr. Soumitra Kumar Kolkata, Dr. Prasant Kumar Sahoo Bhubaneswar, Dr. S. N. Routray Cuttack, Dr. Devanu Ghosh Roy Kolkata, Dr. Santanu Guha Kolkata, Dr. Pramod Mundra Nagpur, Dr. Rajiv Karnik Mumbai, Dr. T. Sashikant Secunderabad.
Disclosure
The authors and panelists did not receive any honorarium for the scientific meeting or development of the manuscript. The authors and panelists would like to thank Torrent Pharmaceuticals Ltd, for the unrestricted educational grant provided for the meeting.
Conflict of Interest
Krishnaprasad K, and Ashutosh Kakkad declare(s) being employees of Torrent Pharmaceuticals Ltd. These listed authors are paid employees of Torrent Pharmaceuticals Ltd.
The authors report no other potential conflicts of interest in this work.
References:
- Reddy KS, Yusuf S. Emerging epidemic of cardiovascular disease in developing countries. Circulation 97 (1998): 596–601.
- Global Health Estimates (2015): Deaths by Cause, Age, Sex, by Country and by Region, 2000e2015. Geneva: World Health Organization; 2016.
- Zhao D. Epidemiological features of cardiovascular disease in Asia. JACC: Asia 1 (2021): 1–13.
- Indrayan A. Forecasting vascular disease cases and associated mortality in India. Reports of the National Commission on Macroeconomics and Health, Ministry of Health and Family Welfare, India (2005): 197-215.
- Iyengar SS. Premature coronary artery disease in India: coronary artery disease in the young (CADY) registry. Indian Heart J 69 (2017): 211-216.
- 2018AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA.Guideline on the Management of Blood Cholesterol. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 139 (2019): 1082–1143.
- Writing Committee, Donald M. Lloyd-Jones, Pamela B. Morris, et al. 2022 ACC Expert Consensus Decision Pathway on the Role of Nonstatin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 80 (2022): 1366–1418.
- Mach F. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). European Heart Journal 41 (2020): 111–188.
- Vimal Mehta, Raman Puri, P Barton Duell, et al. Unmet Need for Further LDL-C Lowering in India despite Statin Therapy: Lipid Association of India Recommendations for the Use of Bempedoic Acid. J Assoc Physicians India 70 (2022): 11-12.
- Yuttana Wongsalap, Arom Jedsadayanmata. Trends and predictors of high-intensity statin therapy and LDL-C goal achievement among Thai patients with acute coronary syndrome. J Cardiol 75 (2020): 275-281.
- Ray KK. EU-Wide Cross-Sectional Observational Study of Lipid-Modifying Therapy Use in Secondary and Primary Care: the DAVINCI study. European Journal of Preventive Cardiology 28 (2021): 1279–1289.
- Gunn LH. Estimated cardiovascular benefits of bempedoic acid in patients with established cardiovascular disease. Atheroscler Plus 49 (2022): 20–27
- Nissen SE. Bempedoic Acid and Cardiovascular Outcomes in Statin-Intolerant Patients. N Engl J Med 388 (2023): 1353-1364
- Kohsaka S. State of the Art Review. Diagnostic and Therapeutic Strategies for Stable Coronary Artery Disease Following the ISCHEMIA Trial. JACC: Asia 3 (2023): 15–30.
- Kyoung Im Cho, Jongwook Yu, Toshio Hayashi, et al. Strategies to Overcome Residual Risk During Statins Era. Circ J 83 (2019): 1973– 1979.
- Sattar N. Statins and diabetes: What are the connections?. Best Pract Res Clin Endocrinol Metab 37 (2023): 101749.
- Chang HJ, Lin FY, Lee SE, et al. Coronary atherosclerotic precursors of acute coronary syndromes. J Am Coll Cardiol 71 (2018): 2511–2522.
- Biolo G. Mechanism of action and therapeutic use of bempedoic acid in atherosclerosis and metabolic syndrome. Front Cardiovasc Med 9 (2022): 1028355.
- Goldberg AC. Effect of Bempedoic Acid vs Placebo Added to Maximally Tolerated Statins on Low-Density Lipoprotein Cholesterol in Patients at High Risk for Cardiovascular Disease: The CLEAR Wisdom Randomized Clinical Trial. JAMA 322 (2019): 1780-1788.
- Kausik K Ray, Harold E Bays, Alberico L Catapano, et al. Safety and Efficacy of Bempedoic Acid to Reduce LDL Cholesterol. N Engl J Med 380 (2019): 1022-1032.
- Ballantyne CM. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol 27 (2020): 593-603.
- Min Seok Oh, Yun Joo Min, Jee Eun Kwon, et al. Effects of Ezetimibe Added to Ongoing Statin Therapy on C-Reactive Protein Levels in Hypercholesterolemic Patients. Korean Circ J 41 (2011): 253–258.
- McQueen RB. Potential Cardiovascular Events Avoided with Bempedoic Acid Plus Ezetimibe Fixed-Dose Combination Compared with Ezetimibe Alone in Patients with Atherosclerotic Cardiovascular Disease Taking Maximally Tolerated Statins. American Journal of Cardiovascular Drugs 23 (2023): 67–76.
- Duprez DA. Cardiovascular Outcomes and Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors: Current Data and Future Prospects. Vasc Health Risk Manag 16 (2020): 403-418.
- Sabatine MS. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med 376 (2017): 1713-1722.
- Lee YJ, Cho JY, You SC, et al. Moderate-intensity statin with ezetimibe vs. high-intensity statin in patients with diabetes and atherosclerotic cardiovascular disease in the RACING trial. Eur Heart J 44 (2023): 972-983.