Thyrotropin Secreting Adenoma in a Patient with Autoimmune Hypothyroidism: Case Report of a Challenging Diagnosis
Article Information
Sala E1, Carosi G1, 2, Montefusco L3, Ferrante E1, Losa M4, Mantovani G1, 5, Arosio M1, 5*
1Endocrinology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
2Department of Experimental Medicine, Sapienza University of Rome, Rome, Italy
3Division of Endocrinology, ASST Fatebenefratelli-Sacco, Milan, Italy
4Department of Neurosurgery, IRCCS San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milan, Italy 5Department of Clinical Sciences and Community Health, University of Milan, Milan, Italy
*Corresponding Author: Maura Arosio, Endocrinology Unit, Fondazione IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, pad Zonda, Department of Clinical Sciences and Community Health, University of Milan, Via F. Sforza, 35, 20122 Milan, Italy
Received: 09 August 2021; Accepted: 02 November 2021; Published: 17 November 2021
Citation: Sala E, Carosi G, Montefusco L, Ferrante E, Losa M, Mantovani G, Arosio M. Thyrotropin Secreting Adenoma in a Patient with Autoimmune Hypothyroidism: Case Report of a Challenging Diagnosis. Archives of Clinical and Medical Case Reports 5 (2021): 846-854.
View / Download Pdf Share at FacebookAbstract
Clinical presentation: We describe a case of a 47-year-old woman with a longtime primary autoimmune hypothyroidism with a progressive rising of TSH levels despite increasing of LT4 therapy. LT4 dose was increased up to 2.9 mcg/kg/day causing an excess of circulating fT4 a minor increase of fT3 concentrations without, however, achieving TSH normalization. The patients was asymptomatic and during the long observation period she had two term pregnancies. A withdrawal of thyroid hormone replacement therapy appropriately induced a further increase of TSH levels and a decrease of fT3 and fT4 below the normal range. LT4 therapy was then resumed and, because TSH levels failed to normalize, central hyperthyroidism or thyroid hormone resistance were suspected. Thyroid levels of first grade relatives were normal, and TSH response to TRH was positive, leaving the diagnosis unclear. The magnetic resonance imaging (MRI) of sellar region revealed a pituitary macroadenoma. The remaining pituitary function was normal. The patient underwent transphenoidal surgery (TNS) and histological examination showed positive immunostaining for TSH, and a mild positivity for GH. After surgery, TSH levels quickly fall down and her replacement therapy could finally be optimized.
Conclusions: TSH-secreting pituitary adenomas are rare. Up to now there are few case reports describing their onset in patients with primary hypothyroidism. The coexistence of primary hypothyroidism can make more challenging the diagnosis of TSH-secreting pituitary adenoma.
Keywords
Pituitary; TSHoma; Thyroid; Hypothyroidism; Autoimmune hypothyroidism
Pituitary articles; TSHoma articles; Thyroid articles; Hypothyroidism articles; Autoimmune hypothyroidism articles
Pituitary articles Pituitary Research articles Pituitary review articles Pituitary PubMed articles Pituitary PubMed Central articles Pituitary 2023 articles Pituitary 2024 articles Pituitary Scopus articles Pituitary impact factor journals Pituitary Scopus journals Pituitary PubMed journals Pituitary medical journals Pituitary free journals Pituitary best journals Pituitary top journals Pituitary free medical journals Pituitary famous journals Pituitary Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals TSHoma articles TSHoma Research articles TSHoma review articles TSHoma PubMed articles TSHoma PubMed Central articles TSHoma 2023 articles TSHoma 2024 articles TSHoma Scopus articles TSHoma impact factor journals TSHoma Scopus journals TSHoma PubMed journals TSHoma medical journals TSHoma free journals TSHoma best journals TSHoma top journals TSHoma free medical journals TSHoma famous journals TSHoma Google Scholar indexed journals Thyroid articles Thyroid Research articles Thyroid review articles Thyroid PubMed articles Thyroid PubMed Central articles Thyroid 2023 articles Thyroid 2024 articles Thyroid Scopus articles Thyroid impact factor journals Thyroid Scopus journals Thyroid PubMed journals Thyroid medical journals Thyroid free journals Thyroid best journals Thyroid top journals Thyroid free medical journals Thyroid famous journals Thyroid Google Scholar indexed journals Hypothyroidism articles Hypothyroidism Research articles Hypothyroidism review articles Hypothyroidism PubMed articles Hypothyroidism PubMed Central articles Hypothyroidism 2023 articles Hypothyroidism 2024 articles Hypothyroidism Scopus articles Hypothyroidism impact factor journals Hypothyroidism Scopus journals Hypothyroidism PubMed journals Hypothyroidism medical journals Hypothyroidism free journals Hypothyroidism best journals Hypothyroidism top journals Hypothyroidism free medical journals Hypothyroidism famous journals Hypothyroidism Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals lymphadenopathy articles lymphadenopathy Research articles lymphadenopathy review articles lymphadenopathy PubMed articles lymphadenopathy PubMed Central articles lymphadenopathy 2023 articles lymphadenopathy 2024 articles lymphadenopathy Scopus articles lymphadenopathy impact factor journals lymphadenopathy Scopus journals lymphadenopathy PubMed journals lymphadenopathy medical journals lymphadenopathy free journals lymphadenopathy best journals lymphadenopathy top journals lymphadenopathy free medical journals lymphadenopathy famous journals lymphadenopathy Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals Hypoxia articles Hypoxia Research articles Hypoxia review articles Hypoxia PubMed articles Hypoxia PubMed Central articles Hypoxia 2023 articles Hypoxia 2024 articles Hypoxia Scopus articles Hypoxia impact factor journals Hypoxia Scopus journals Hypoxia PubMed journals Hypoxia medical journals Hypoxia free journals Hypoxia best journals Hypoxia top journals Hypoxia free medical journals Hypoxia famous journals Hypoxia Google Scholar indexed journals
Article Details
1. Introduction
Thyrotropin secreting adenomas (TSHoma) are rare, they account less than 1% of all pituitary adenomas [1] with an incidence of 0.15 per million of inhabitants per year [2, 3]. The immunostahining analysis is purely positive for TSH only in less than 50% of patients. GH (Growth hormone) and PRL (prolactin) co-secretion are common, with a positive immunostaining for GH in 36%, GH and PRL in 27% and PRL in 10%; however, clinical signs of GH and PRL hypersecretion are infrequent (less than 20%) [4]. Diagnosis of a TSHoma could be incidental during magne-tic resonance imaging (MRI)/computed tomography (CT) scan or, more often, can be suggested by symptoms of hyperthyroidism and/or by the presence of a goiter associated with the signs and symptoms of pituitary compression of the surrounding anatomical structures (i.e. headache or visual field impairment or partial or complete hyopopituitarism). Symptoms of hyperthyroidism are mild-to-moderate, usually milder than expected on the basis of circulating thyroid hormones levels [5].
The hormonal diagnosis is based on unsuppressed TSH levels in association with elevated thyroid hormones levels (both free triiodothyronine: fT3; and free thyroxine: fT4), indicating a central hyperthyroidism. In most cases the TSH is unresponsive to TRH stimulus and to T3 inhibition [1, 6]. The main differential diagnosis is with another rare disease: resistance to thyroid hormone syndrome (RTH). Unfortun-ately, even today, patients are often misdiagnosed with Graves’s disease or with a toxic goiter, leading to inappropriate therapy and further delay in diagnosis [1, 6, 7]. Moreover, the diagnosis of a TSHoma could even be more challenging due to the presences of concomitant diseases, such as autonomous thyroid adenoma (ATA), Graves’ disease or autoimmune hypothyroidism [1]. Here we describe a patient with a long-time primary autoimmune hypothyroidism where the replacement therapy masked for many years the presence of a TSH secreting pituitary macroadenoma.
2. Case Report
We described the story of a female patient, born in 1973. She first came at our observation at age of 41, after a long-standing history of thyroidal disease with inadequate treatment. Her family history was positive for autoimmune thyroiditis in a sister. She reported normal growth and physiological pubertal development. Menarche occurred at the age of 13 years and menses were regular. Two term pregnancies (2006, 2011) and one miscarriage (2009) were also reported. She did not report any other illness or medical problem. In 1988, at the age of 15 years, she was diagnosed with autoimmune thyroiditis with the presence of high title serum anti thyroid peroxidase antibodies (AbTPO) and subclinical hypothyroidism. The neck ultrasound exam (US) showed a gland of normal dimension and signs of chronic thyroiditis without nodulations. On the basis of these findings, she started LT4 replacement therapy (dosage 100 mcg five days a week and 125 mcg 2 days a week, 1.6 mcg/Kg/day) achieving normal TSH and fT4 levels. In the following years, the patient underwent regular follow up with a pediatric endocrinologist and the replacement therapy to achieve normal TSH levels needed successive increases over the years up to 2.0 mcg/kg/day.
In 1998, at the age of 25 years, for the first time the control tests showed high fT4 concentrations (34 ng/l, reference range 11-24 ng/L), despite the fact that the blood sample had been correctly drawn before the LT4 morning dose, and unsuppressed TSH (2.4 mIU/L,) but normal fT3 concen-trations. The findings were confirmed few months later: fT4: 30 ng/l, fT3 within the normal range and TSH 5 mIU/L. Despite this, she continued the usual therapy, with no further testing for several years. In 2006, at the age of 33 years, the patient had a pregnancy without any complication. Unfortunately, medical reports of thyroid replacement therapy taken during pregnancy are not available. In 2007, after delivery, due to the increase of TSH levels up to 5.5 mIU/L, despite persistent elevated fT4 levels, the LT4 dose was further increased and settled at 2.1 mcg/kg/day. In 2009, due to the recurrence of non-suppressed TSH values with elevated fT4, a syndrome of inappropriate TSH secretion due to resistance to thyroid hormone (RTH) was suspected and the replacement therapy stopped. Two months after LT4 withdrawal the patient showed clinical and biochemical picture of an overt hypothyroidism with TSH= 67.2 mIUL, fT3= 3.3 ng/L (3.8-8) and fT4=7.5 ng/L (9-20). The diagnosis of primary hypothyroidism was, therefore, confirmed and daily therapy with LT4 at the previous dosage of 2.1 mcg/kg/day was reintroduced without further ascertainments. In the following years TSH levels progressively increased to 7.2 mIUL (in 2009), 7.55 mIUL (in 2011), and 8.06 mIU/L (in 2012).
Furthermore, in 2011 another pregnancy occurred. LT4 dosage was maintained at 2.1 mcg/kg/day during pregnancy despite the abnormal hormone concentrations recorded (TSH 8.0 mIU/L, fT3 4.99 ng/L, and fT4 20.26 ng/L in the second trimester). The patient continued the regular follow up with her endocrinologist and, in September 2014, despite the progressive increase of LT4 up to 2.35 mcg/kg/day (175 mcg/day) TSH was 12.0 mIU/L, fT3 5.7 ng/L (3.1-6.8) and fT4 25 ng/L (7-15). In 2015, at the first evaluation in our Centre, the currently LT4 therapy was 200 mcg/day (about 2.7 mcg/Kg/day) and the hormonal evaluation showed TSH 8 mIU/L, fT4 24 ng/L (9-18), and fT3 4.5 pg/ml (2.3-4.2). At physical examination, no alterations were noted. Menses were regular. She denied typical symptoms of thyroto-xicosis (such as insomnia, hyperhidrosis, and weight loss), headache or visual impairment. She complained mild evening asthenia. Weight was 74 kg (stable from 2006) and height 171 cm with a body mass index (BMI) of 21.3 kg/m2, blood pressure was 90/55 mmHg and heart rate 74 bpm. Thyroid US showed a small thyroid gland of 4.7 ml with the classical aspect of chronic thyroiditis without nodulations. The elevated hormonal levels had been confirmed in several laboratories, thus excluding dosage interferences. We evaluated all the thyroid function in the patient history starting from 2006. TSH levels ranged from 5.5 to 8.1 mU/ml with persistently ft4 levels above the normal range and ft3 in the high normal range (Table 1). Due to the presence of an inappropriate secretion of TSH, the first-degree relatives were screened for thyroid hormone levels and a stimulus test with TRH was planned.
Thyroid function was shown to be normal in both parents. After TRH administration, TSH concentrations appropri-ately increased from 11 to 29 mU/L at 20 min (263%) without clarifying the origin of the inappropriate levels of the TSH. A pituitary MRI was then performed, showing a pituitary macroadenoma with a maximum diameter of 12 mm. The lesion was isointense in T1 with no contrast enhancement and hyperintense in T2-weigthed images. The lesion was located in the left part of the pituitary gland, diverting the pituitary stalk. The optical chiasm was apparently not involved despite the proximity of the lesion (Figure 1). Antero-pituitary function was assessed by measuring basal hormone levels for insulin-like growth factor-1 and GH (IGF-1; 182.5 ng/ml with normal range 93-245, random GH 4.85 ng/ml) and PRL (10.3 ng/ml) and by both basal and dynamic testing for adrenal reserve (Baseline: cortisol 7.55 mg/dl, adrenocorticotropin (ACTH) 12.9 pg/ml; peak of cortisol after low-dose ACTH testing : 23.9 mcg/dl, normal > 18 mcg/dL) [8]. Normal function of gonadal axis was confirmed by the presence of regular menses. As far as peripheral marker of thyroid function are concerned, both sex hormone binding globulin (SHBG) and Angiotensin Converting Enzyme (ACE) measured twice in different lab resulted in the normal range (SHBG 125 and 72 nmol/L with normal range 18-144, and ACE 27 and 31 U/L with normal range 8-52) [5].
The patient underwent transsphenoidal surgery (TNS) in June 2016 with no complications. The histological report confirmed a pituitary adenoma with a positive immune-staining for TSH (90%) and GH (15%). Two months after surgery and LT4 withdrawal, she showed central hypothy-roidism with both low free thyroid hormones and TSH concentrations. The LT4 replacement therapy was then reintroduced at a dose of 1.5 mcg/kg/day, with normali-zation of thyroid hormone concentrations, but TSH persistently suppressed (TSH 0.07, fT4 1.12 normal range 0.9-1.7 ng/dl). The remaining pituitary function proved normal and remained stable throughout all the follow-up. A reduction in the dose of LT4 led to the complete normalization of the thyroid picture, as expected on the basis of the original diagnosis of primary hypothyroidism (in 2019 TSH 1.8 µIU/ml, ft4 13 ng/L with normal range 7-15.3, ft3 4.36 ng/L with normal range 3.1-6.8, while taking LT4 at a dose of 1.0 mcg/kg/die). After 5 years follow up, at last control available in January 2021, the patient has stable negative MRI, well substituted primary hypothy-roidism with a LT4 dosage of 1.08 mcg/kg/day.
|
Start LT 4 |
Pregnancy |
TNS |
|||||||
|
1998 |
1998 |
2006 |
2009 |
2012 |
2014 |
2015 |
2016 |
2019 |
2021 |
|
TSH U/l |
2.4 |
5.5 |
7.2 |
8.1 |
7.1 |
2.4 |
0.07 |
1.8 |
2.0 |
|
FT3 pg/ml |
5.8* |
6.62¥ |
4.99¥ |
5¥ |
5.9× |
4.36¥ |
1.9µ |
||
|
FT4 pg/ml |
34 γ |
30 α |
25.01β |
20.26β |
25.24β |
34γ |
1.12∞ |
13β |
10.6≠ |
|
LT4 mcg/kg/die |
2.0 |
2.1 |
2.5 |
2.5 |
2.6 |
2.9 |
1.5 |
1.0 |
1.0 |
Table 1: Timeline with most relevant data for the patient’s history and thyroidal status. Thyroid function in different years during LT4 therapy.
FT3 normal ranges: * 3.8-8 pg/ml. ¥ 3.1-6.8 pg/ml. × 3.4-7.2 pg/ml. α 2.3-4.2 pg/ml, µ2-5 ng/L.
FT4 normal ranges: α 9-20 pg/ml. β 7-15.3 pg/ml. γ 11-24 pg/ml, 0.9-1.7 ∞ ng/dl, ≠8-17 ng/L
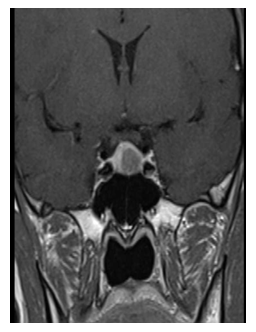
Figure 1: MRI at diagnosis. The pituitary macroadenoma had a maximum diameter of 12 mm.
3. Discussion
TSHomas are rare, and their diagnosis may be challenging. The association with autoimmune hypothyroidism and the unusual presence of a TSH response to TRH test have increased the diagnostic difficulties in our patient [1]. A recent review of 535 adult cases of TSHoma [9] describes in details some cases of concomitant Graves‘ disease and even of thyroid storm, but no cases of concomitant primary hypothyroidism. The presence of a TSHoma in patients with severe primary hypothyroidism has been described in previous studies, supposing that the protracted pituitary stimulation could be involved in the development of a pituitary adenoma/irreversible hyperplasia [10, 11]. We know that a long-standing hypothyroidism leads to thyro-troph hyperplasia [5]. It has been suggested that this condition could be a trigger for the development of a pituitary adenoma [5, 6].
Reviewing the literature, we confirm that the diagnosis of a TSH-secreting adenoma could be more difficult when coexists primary hypothyroidism. It is important to note that this condition is very common, while TSHoma is a very rare disease [12]. Nevertheless, the long delay in the diagnosis could even be helpful in defining the natural history of TSHoma. Reviewing the patient’s data we can date back the insurgence of the TSHoma to 1998, but the correct clinical diagnosis was done after more than 20 years, thus suggesting that the tumor growth in our patient was very slow. At the time of diagnosis, the pituitary adenoma was larger than 10 mm, but did not cause compressive symptoms and did not show an invasive growth. The presence of a TSH secreting adenoma in patients with a primary autoimmune hypothyroidism has been previously described in a few papers only [13-15]. Only in the patient described by Li and colleagues, who had a giant pituitary adenoma, symptoms of hypopituitarism were present at diagnosis [15].
A common point to all is the impossibility to achieve normal TSH concentrations during LT4- replacement therapy (Table 2). Moreover, and differently from what usually occurs in patients with TSH-oma, only in one patient with autoimmune hypothyroidism and coexisting TSH-oma a goiter was present [15]. In all the other cases and in our patient as well, the thyroid US showed a normal gland, with no nodulations. But the most particular findings that distinguishes these patients from other cases of TSHomas are the fT3 concentrations, which remain in the normal range or are only moderately elevated with a reduced fT3/fT4 ratio (Figure 2 and Table 2). Concordant with this observation, our patient was not reporting any symptoms of thyrotoxicosis, despite the frankly high fT4 concentrations. In the other patients reported, there were no or only mild symptoms of hyperthyroidism too [13-15]. Indeed a lower fT3/fT4 ratio has been described to be responsible of the presence of scarce symptoms of thyrotoxicosis [17]. The presence of normal levels of both circulating ft3 and peripheral markers of tissue hyperthy-roidism despite the overtreatment with exogenous L-T4 and the elevated blood fT4 concentrations could reflect an alterations of the deiodinases, and in particular of D1 and D3 that characterize the condition of hypothyroidism of our patient. In fact, D1 activity in liver and kidney is stimulated in hyperthyroidism but decreased in hypothy-roidism, thus causing a T3/T4 ratio lower than expected in a patient with hyperthyroidism which is what we observed (Figure 2).
In addition, it is intriguing that Tannahil and colleagues [16] showed that , at least at pituitary level, also D2 mRNA expression is significantly reduced and D3 mRNA is significantly overexpressed in TSHoma tissue compared to normal pituitary and other secreting or non-secreting pituitary tumors. On the other hand the absence of evident clinical symptoms of thyrotoxicosis, was probably one of the causes of the long delay in diagnosis. Moreover, in this particular case, investigations for the correct diagnosis were misleading. In fact, TRH tests showed a normal response of TSH levels that is not characteristic for a TSH-oma, since only 10% to 20% of patients with a TSH-oma do have a positive response to TRH testing [6, 7, 18]. The European Guidelines for the diagnosis and treatment of TSHoma [6] suggest with a moderate level of evidence the usefulness of the T3 suppression test and with a low level of evidence the octreotide suppression test to differentiate between a TSHoma and RTH. The decision in our patient to proceed to surgical treatment without performing additional tests can be discussed. In this choice played a role the absence of familiarity for thyroid biochemical abnormalities, making unlikely a picture of hormonal resistance on the one hand, and on the other the presence of a pituitary macroadenoma, which in any case posed a surgical indication. Our case highlights the difficulty of diagnosing a TSHoma in patients with primary hypothyroidism, but also points out that, when laboratory results are unclear, we must not forget the possibility of coexistence of a rare disease with a more common one.
|
Present Report |
Iudiculla; 2003 |
Losa; 2006 |
Li; 2018 |
|
|
Goiter |
N |
N |
N |
Y |
|
Elevated ft4 |
Y |
Y |
Y |
N |
|
Elevated ft3 |
N |
M |
N |
N |
|
TSH unsuppressed |
Y |
Y |
Y |
Y |
|
TSH response to TRH |
Y |
N |
N |
NA |
|
Symptoms of hyperthyroidism |
N |
N |
M |
N |
Table 2: comparison between our data and other similar cases reported in literature. Y= Yes; N=No, M= Mild, NA= Not available
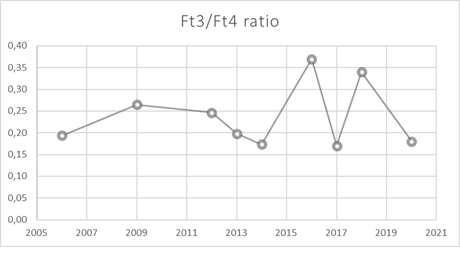
Figure 2: Ft3/Ft4 ratio during LT4 therapy. TNS occured in 2016.
Conflict of Interest
The authors declare that there are not commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Tjörnstrand A, Nyström HF. Diagnosis of Endocrine Disease: Diagnostic approach to TSH-producing pituitary adenoma. Eur J Endocrinol 177 (2017).
- Melmed S. Thyrotropin-secreting pituitary tumors. 3rd ed. elsevier, Oxford, UK (2011).
- Ónnestam L, Berinder K, Burman P, et al. National incidence and prevalence of TSH-secreting pituitary adenomas in Sweden. J Clin Endocrinol Metab 98 (2013).
- Yamada S, Fukuhara N, Horiguchi N, et al Clinicopathological characteristics and therapeutic outcomes in thyrotropin-secreting pituitary adenomas: a single-center study of 90 cases. J Neurosurg 121 (2014).
- Beck-Peccoz P, Brucker-Davis F, Persani L, et al. Thyrotropin-secreting pituitary tumors. Endocr Rev 17 (1996).
- Beck-Peccoz P, Lania A, Beckers A, et al. 2013 European thyroid association guidelines for the diagnosis and treatment of thyrotropin-secreting pituitary tumors. Eur Thyroid J 2 (2013).
- Socin H, Chanson P, Delemer B, et al. The changing spectrum of TSH-secreting pituitary adenomas: diagnosis and management in 43 patients. Eur J Endocrinol 148 (2003): 433-442.
- Kazlauskaite R, Evans AT, Villabona CV, et al. Corticotropin Tests for Hypothalamic-Pituitary- Adrenal Insufficiency: A Metaanalysis. J Clin Endocrinol Metab 93 (2008): 4245-4253.
- De Herdt C, Philipse E, De Block C. Thyrotropin-secreting pituitary adenoma: a structured review of 535 adult cases. Eur J Endocrinol 185 (2021): R65-R74.
- Ghannam NN, Hammami MM, Muttair Z, et al. Primary hypothyroidism-associated TSH-secreting pituitary adenoma/hyperplasia presenting as a bleeding nasal mass and extremely elevated TSH level. J Endocrinol Invest 22 (1999): 419-423.
- Fatourechi V, Gharib H, Scheithauer B W, et al. Pituitary thyrotropic adenoma associated with congenital hypothyroidism: Report of two cases. Am J Med 76 (1984): 725-728.
- Giorda CB, Carnà P, Romeo F, et al. Prevalence, incidence and associated comorbidities of treated hypothyroidism: an update from a European population. Eur J Endocrinol 176 (2017).
- Idiculla J M, Beckett G, Statham PF, et al. Autoimmune hypothyroidism coexisting with a pituitary adenoma secreting thyroid-stimulating hormone, prolactin and alpha-subunit. Ann Clin Biochem 38 (2001).
- Losa M, Mortini P, Minelli R, et al. Coexistence of TSH-secreting pituitary adenoma and autoimmune hypothyroidism. J Endocrinol Invest 29 (2006): 555-559.
- Li J, Jiang J, Yu R, et al. Case report of a pituitary thyrotropin-secreting macroadenoma with Hashim-oto thyroiditis and infertility. Medicine (Baltimore) 97 (2018).
- TannahillL A, Visser TJ, McCabe CJ, et al. Dysregulation of iodothyronine deiodinase enzyme expression and function in human pituitary tumours: Deiodinase enzymes in the human pituitary. Clin Endocrinol (Oxf) 56 (2002): 735-743.
- Gullo D, Latina A, Frasca F, et al. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PloS One 6 (2011): e22552.
- Beck-Peccoz P, Giavoli C, Lania A. A 2019 update on TSH-secreting pituitary adenomas. J Endocrinol Invest. 42 (2019): 1401-1406. Eur J Endocrinol 185 (2021): R65-R74.
