Thrombotic Thrombocytopenic Purpura after COVID-19 Vaccination: A Case Report
Article Information
Lisa Hasselbach, Andreas Voß*
Department of oncology and hematology, Klinikum Oldenburg AöR, European Medical School, Germany
*Corresponding author: Voß Andreas, Department of oncology and hematology, Klinikum Oldenburg AöR, European Medical School, Germany
Received: 18 August 2021; Accepted: 25 August 2021; Published: 21 September 2021
Citation: Lisa Hasselbach, Andreas Voß. Thrombotic Thrombocytopenic Purpura after COVID-19 Vaccination: A Case Report. Archives of Clinical and Biomedical Research 5 (2021): 737-741.
View / Download Pdf Share at FacebookAbstract
We present the, to our best knowledge, first case of a 25-year old female who developed thrombotic thrombocytopenic purpura after receiving the COVID-19 vaccination with Spikevax? (COVID-19 Vaccine Moderna). She presented with petechia, acute kidney injury and neurological symptoms. After treatment with plasma exchange, Caplacizumab and Prednisolone she first recovered but relapsed after early discontinuation of Caplacizumab.
Acquired thrombotic thrombocytopenic purpura after SARS-CoV2 vaccination is a rare phenomenon that has now been seen in all of the four vaccines currently approved by the European Medicines Agency.
Keywords
COVID-19; Vaccination; Spikevax; Thrombotic thrombocytopenic purpura; aTTP
COVID-19 articles; Vaccination articles; Spikevax articles; Thrombotic thrombocytopenic purpura articles; aTTP articles
Article Details
1. Introduction
Acquired thrombotic thrombocytopenic purpura (aTTP) is characterized by a low platelet count, hemolytic anemia and patients typically present with signs of bleeding, renal insufficiency and neurological abnormalities. Autoantibodies against ADAMTS13, the von Willebrand factor (vWF) cleaving protease are produced leading to long vWF-multimers that form thrombosis in small vessels.
The cause for aTTP is often not found but it can develop after administration of drugs, vaccinations, infections, cancer, and pregnancy or due to stress. aTTP is a rare disease with an annual incidence of 1 new case/million people. With initiation of plasma-exchange (PEX) the mortality was reduced from about 90% to < 10% [1].
2. The Case
A 25-year old female was transferred to our department of oncology and hematology for further diagnostics and treatment because of thrombocytopenia, anemia and acute kidney injury. She presented with fever, nausea, vomiting, fatigue, petechia, arthralgia, cephalgia, dyspnea and vertigo. The patient received her first vaccination against SARS-CoV2 with VaxzevriaÒ (AstraZeneca-Oxford COVID-19 vaccination) one month prior to the admission and the second shot with SpikevaxÒ (COVID-19 Vaccine Moderna) two days prior to the admission. The medical history of our patient included hypothyroidism treated with 50 µg L-thyroxine per day as well as obesity, depression, a no longer continued abuse of marijuana and a continued nicotine abuse. She discontinued an oral contraceptive prior to admission leading to mild vaginal bleeding.
The lab-results showed a platelet count of 7.000*109/L, hemolytic anemia (Hb 9.8 mg/dl, 7% fragmentocytes, haptoglobin <0.1 g/l) and a calculated GFR of 32 ml/min. Fibrinogen derived factor and d-dimers were elevated. Because of the intermediate risk PLASMIC-Score (5 Points) we looked for differential diagnosis while simultaneously starting on the therapy with PEX. Coombs test as well as HIT-diagnostic came back negative. No antibodies against platelet- factor-4 (PF4) were detected. We thereby ruled out vaccine-induced immune thrombotic thrombocytopenia (VITT), which has been reported frequently after COVID-19 vaccination [2]. Hemolytic uremic syndrome was ruled out after Shiga toxin in the stool came back negative.
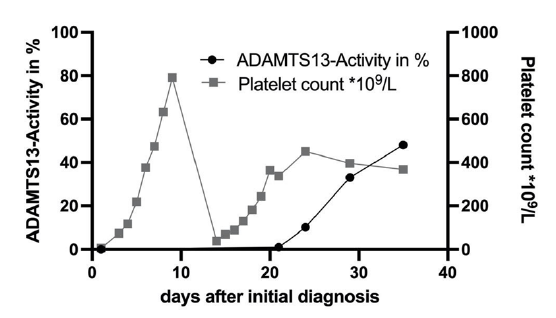
One day after the admission we demonstrated a non-detectable ADAMTS13-activity thereby proving the diagnosis of aTTP (reference range 50-100%). ADAMTS13-Antigen was strongly reduced with 0.03 IU/ml (0.35-1.2 IU/ml). The ELISA showed antibodies against ADAMTS13 with 59 Units/ml (positive >16 Units/ml). The patient stayed in the hospital for 9 days and received four PEXs. The therapy was complemented by subcutaneous application of Caplacizumab as well as 1 mg/kg Prednisolone. Symptoms resolved promptly after the first PEX, after the fourth PEX platelet count was normalized without signs of hemolysis (Figure 1). Upon dismissal Caplacizumab was discontinued. One week later our patient returned with a relapse (visual impairment and tingling paresthesia) and needed to stay for another 8 days. During that time she received four more PEXs and the therapy with Caplacizumab s.c. was reinduced. It was given for a total of 21 days after the relapse leading to a stable remission. The ADAMTS13-Activity has steadily increased (Figure 1) and the count of ADAMTS13- antibodies has normalized after 31 days. At the time of the last outpatient contact the patient was doing well without any symptoms of disease or sequelae.
3. Discussion
There have been few cases where aTTP has been linked to vaccinations, mainly against viral pathogens like influenza or H1N [3-5]. In May 2021 a case report of a 37-year-old man with aTTP after administration of VaxzevriaÒ (AstraZeneca-Oxford COVID-19 vaccination) was published in the European journal of Haematology. This patient presented with aTTP at about two weeks after the first vaccination [6]. Also after receiving the Ad26.COV2-S COVID-19 vaccine from Janssen Biotech one case of aTTP in a 62-year old patient was reported 37 days after the vaccination [7]. Two cases of aTTP after a vaccination with ComirnatyÒ (BNT162b2 anti-COVID19 vaccine, Pfizer-BioNTech) have recently been published. In one patient symptom started at about two weeks after the first shot and intensified one week after the second dose [8]. The other patient developed fatigue and shortness of breath one week after the second dose [9]. Two more patients have been identified in Israel with onset of symptoms 8 and 28 days after the second dose [10].
There have also been three published cases of a aTTP relapse shortly after the second shot with ComirnatyÒ [10, 11]. In the UK a total of 13 cases of aTTP linked to a COVID-19 vaccination have been reported to the Medicines and Healthcare products Regulatory Agency via the yellow card scheme (until August 4th 2021) [12]. This case is, to our best knowledge, the first case of aTTP linked to a vaccination with SpikevaxÒ (COVID-19 Vaccine Moderna).
In the end it remains unclear whether the development of the aTTP is due to the first vaccination from AstraZeneca or the second shot from Moderna. The symptoms just started after the second shot but they showed an earlier onset than in the other reported cases of aTTP after COVID-19 vaccinations.
There is no definite answer yet as to whether vaccination against COVID-19 correlates with the development of aTTP or not. Due to the strong medial presence of COVID-19 vaccination these days one would expect to see more published cases of consecutive aTTP to back up a correlation. With now 23 published cases so far in more than 4.6 billion of vaccinations done worldwide done until 12th August 2021 [13] development of aTTP is far less frequently reported than thrombosis-thrombocytopenia-syndrome (range: 1,2:100000 to 8,1:100000) [14] or Immune Thrombocytopenia (ITP) after vaccination [15].
On the other hand not all cases might be published or even reported, because the onset of symptoms can occur weeks after the vaccination. Still the reported rate is far below the estimated incidence of aTTP in the healthy population. In the end only time will tell whether COVID-19 vaccination leads to a peak in the incidence for aTTP.
4. Conclusion
Acquired TTP after SARS-CoV2 vaccination is a rare phenomen that can be seen in all of the vaccines currently approved by the European Medicines Agency. If signs of thrombotic microangiopathy (low platelet count, coombs-negative hemolysis, elevetation of fragmentocytes) is observed after vaccination, ADAMTS-13-Activity should be determined to check for aTTP. Secondly, our case demonstrates that relapses after early cessation of plasma-exchange occur frequently in aTTP and require thorough follow-up in the first days to weeks [16].
Conflict of Interest
Lisa Hasselbach and Andreas Voß declare no conflict of interests.
References
- Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood 129 (2017): 2836-2846.
- Tsilingiris D, Vallianou NG, Karampela I, Dalamaga M. Vaccine induced thrombotic thrombocytopenia: The shady chapter of a success story. Metabol Open 11 (2021): 100101.
- Dias PJ, Gopal S. Refractory thrombotic thrombocytopenic purpura following influenza vaccination. Anaesthesia 64 (2009): 444-446.
- Hermann R, Pfeil A, Busch M, et al. [Very severe thrombotic thrombocytopenic purpura (TTP) after H1N1 vaccination]. Med Klin (Munich) 105 (2010): 663-668.
- Yavasoglu I. Vaccination and Thrombotic Thrombocytopenic Purpura. Turk J Haematol 37 (2020): 218-219.
- Al-Ahmad M, Al-Rasheed M, Shalaby NAB. Acquired thrombotic thrombocytopenic purpura with possible association with AstraZeneca-Oxford COVID-19 vaccine. EJHaem (2021).
- Yocum A, Simon EL. Thrombotic Thrombocytopenic Purpura after Ad26.COV2-S Vaccination. Am J Emerg Med (2021).
- de Bruijn S, Maes MB, De Waele L, Vanhoorelbeke K, Gadisseur A. First report of a de novo iTTP episode associated with an mRNA-based anti-COVID-19 vaccination. J Thromb Haemost 19 (2021): 2014-2018.
- Waqar SHB, Khan AA, Memon S. Thrombotic thrombocytopenic purpura: a new menace after COVID bnt162b2 vaccine. Int J Hematol (2021).
- Maayan H, Kirgner I, Gutwein O, et al. Acquired thrombotic thrombocytopenic purpura: A rare disease associated with BNT162b2 vaccine. J Thromb Haemost (2021).
- Sissa C, Al-Khaffaf A, Frattini F, et al. Relapse of thrombotic thrombocytopenic purpura after COVID-19 vaccine. Transfus Apher Sci 103 (2021):103145.
- Coronavirus vaccine - weekly summary of Yellow Card reporting. Medicines and Healthcare products Regulatory Agency (2021).
- Hannah Ritchie EO-O, Diana Beltekian, Edouard Mathieu, Joe Hasell, Bobbie Macdonald, et al. Coronavirus Pandemic (COVID-19). Our World in Data (2020).
- Pau-Ehrlich-Institut. SICHERHEITSBERICHT Verdachtsfälle von Nebenwirkungen und Impfkomplikationen nach Impfung zum Schutz vor COVID-19 seit Beginn der Impfkampagne am 27.12.2020 bis zum (2021).
- Bhattacharjee S, Banerjee M. Immune Thrombocytopenia Secondary to COVID-19: a Systematic Review. SN Compr Clin Med (2020): 1-11.
- Page EE, Kremer Hovinga JA, Terrell DR, Vesely SK, George JN. Thrombotic thrombocytopenic purpura: diagnostic criteria, clinical features, and long-term outcomes from 1995 through 2015. Blood Adv 1 (2017): 590-600.