The Superselective β1-Blocker Landiolol Enhances Inotropy of Endogenous and Exogenous Catecholamines in Acute Heart Failure
Article Information
Thomas J Feuerstein1, Günther Krumpl2*
1Sektion für Neuroelektronische Systeme, Klinik für Neurochirurgie, Universität Freiburg, Breisgau, Germany
2Medical Research Network, 1010 Vienna, Austria
*Corresponding author: Günther Krumpl, , Medical Research Network, 1010 Vienna, Austria.
Received: 21 August 2022; Accepted: 06 September 2022; Published: 09 October 2022
Citation: Thomas J. Feuerstein, Günther Krumpl. The Superselective β1-Blocker Landiolol Enhances Inotropy of Endogenous and Exogenous Catecholamines in Acute Heart Failure. Cardiology and Cardiovascular Medicine 6 (2022): 502-511.
View / Download Pdf Share at FacebookAbstract
β1-Adrenoceptors (β1-AR) blocker are an established therapy for the treatment of chronic left ventricular dysfunction. In the acute setting, however, the administration in patients with left ventricular failure is seen controversial, specifically as a potential negative inotropic effect and antagonism of the applied inotropic agents may possibly worsen the clinical situation of the patient. Recently the super selective short acting β1-AR Landiolol has been used in patients with acute left ventricular decompensation and, in conjunction with inotropic agents, did not deteriorate but improved the cardiovascular status of the patients. The present work summarizes the theories how a β1-AR blocker may act additive to inotropic agents in patients with acute cardiac failure. Specifically, receptor bindings models are presented in which the β1-AR blocker Landiolol can induce a positive inotropic response. These models are based on the fact that in patients with left ventricular dysfunction the plasma levels of catecholamines exceed their dissociation constants and rather decrease than improve the inotropic response due to negative cooperativity at the occupied receptor dimers. Low distinct Landiolol concentrations then reduce the negative cooperation and shift the receptor response curve into a more positive inotropic range. This article may thus help to minimise the reservations to the treatment of acute left ventricular deterioration with the super selective beta blocker Landiolol and positive inotropic agents. More so as the dose range calculated for Landiolol in these models and the one’s used in the intensive care setting prove to be identical.
Keywords
Acute Heart Failure; Binominal Distribution; β1–Adrenoceptors; Landiolol; Negative Cooperativity; Positive Inotropic Agents
Acute Heart Failure articles; Binominal Distribution articles; ?1?Adrenoceptors articles; Landiolol articles; Negative Cooperativity articles; Positive Inotropic Agents articles
Article Details
1. Introduction
β-Adrenoceptors (β-AR) are G protein coupled receptors on which endogenous catecholamines such as noradrenaline (NA) and adrenaline (A) act as agonists. β-AR can be subcategorized into β1, β2 and β3 receptors, all three acting via the guanine nucleotide-binding regulatory protein GS to activate an adenylate cyclase which creates cAMP [1]. At the non-failing myocardium β1-receptors represent 75–80 % while β2 represent 15-18 % of beta receptors [1]. Classically these receptors mediate positive chrontropy, inotropy, dromotropy and bathmotropy [2]. In the failing heart the percentage of β1 and β2 receptors achieves a 50/50 relation [1,3,4]. In the 70es and 80es and even early 90es the use of β-blockers in patients with reduced cardiac function was considered contraindicated [5]. In the absence of representative clinical studies at that time, the rationale to use a negative inotropic agent in case of contractile problems was hard to understand. Groundbreaking results from studies investigating low dose metoprolol, bisoprolol and later carvedilol and nebivolol have indicated that in patients with reduced left ventricular function β1-AR blockade can improve the hemodynamic situation and eventually prolong survival under long term administration [3]. However, despite that, the clinically beneficial effects of β1-AR antagonism in chronic heart failure (CHF) has long been doubted. A leading textbook has stated still in 1996 that it is unclear whether β-blockers improve survival in heart failure patients [6], which is nowadays undisputed provided that the patients are not under atrial fibrillation [7].
Several mechanisms are ascribed to the beneficial effect of β-blockers in CHF patients, all based on the antagonism of elevated endogenous catecholamine levels:
- Heart rate reduction and thus improvement of performance economics [8]
- Reduction of arrhythmias, specifically atrial fibrillation [9]
- Decrease of renin angiotensin levels [10]
- Decrease of ANP (atrial natriuretic peptide) and BNP ( b-type natriuretic peptide) levels [11]
- Anti-apoptotic and antioxidative activity [12]
- Improvement of cardiac myocyte metabolism [13]
- Reduction of β-receptor stimulation via anti β-receptor antibodies [14]
- Reduction of or reversion of ventricular remodeling [15]
- Differential blockade of cell-membrane and intracellular located β-receptors [16]
- Specific effects on myocardial filaments [17]
While in the chronic treatment the use of β-blockers in CHF can now be considered as accepted, this is not the case in the acute setting. Specifically, since some of the beneficial effects of a β-blocker, such as the reduction of remodeling, are the consequences of chronic administration. Moreover, positive inotropic agents are used intravenously for the treatment in the acute situation and β-blockers may inhibit or reduce their effects. Nevertheless, except for the remodeling all the arguments raised for chronic treatment also qualify to explain why an acute intervention can be beneficial.
Moreover, there are further arguments for the co-administration of positive inotropic agents and selective β-blockers in the acute setting:
- Selective β-blockers might antagonize the β1-receptor mediated tachycardia and tachyarrhythmias but leave β2-receptors unblocked for an improvement of the cardiac work.
- In case of downstream intervention below the β-receptor level with agents such as levosimendan or milrinone, β-blockers might still block special effects of endogenous catecholamines such as tachycardias and/or tachyarrhythmias but leave positive inotropic subreceptor-level-interventions unblocked [18].
Classic intravenous β-blockers are not easy to dose in such situations, since, as shown for instance with metoprolol, they may lose their selectivity in the upper standard dose range or when given intravenously [19,20]. In case of unwanted side effects their longer duration of action may also lead to significant troubles in acute situations [20-22]. Ultra-short acting β-blockers such as Esmolol and Landiolol provide significant advantages in these circumstances since their effect can be terminated in very short time. Both agents differ as far as their selectivity [23] and their action on ion channels is concerned [24-27]. Esmolol (β1/β2 selectivity 30 fold) is also blocking Na, Ca and K channels [24-27] while Landiolol (β1/β2 selectivity 210-254 fold) is devoid of membrane activity [28,29] and is thus described as a superselective agent [30,31] with less negative inotropy [32-36] and better blood pressure preservation [36-39] and absence of chaperoning and thus rebound effects [23,38]. It is thus obvious that among i.v. β-blockers Landiolol is best qualified for the acute treatment of patients with left ventricular dysfunction, also since it is the only i.v. β-blocker with a specific dose recommendation for these patients [40,41]. Consequently, Landiolol has been used in intensive care patients in conjunction with positive inotropic agents with positive outcome [42-53]. Beside the above-described positive effects which speak for the use in the acute situation, we try to establish another rationale why a superselective β-blocker such as Landiolol may not act as a negative inotropic agent, but have a positive inotropic effect in the acute setting, as detailed in the following:
2. Methods
Surprisingly, the consideration of a discrete probability distribution, the binomial distribution, of agonist binding to β-AR is helpful to explain the above-mentioned rationale. To understand how the binomial distribution solves the riddle of negative inotropic β1-blockers inducing positive inotropic effects, two fundamental properties of β1-AR in human heart must be recalled. First, β1-AR have a receptor reserve [spare receptors, 54-57]. In other words, the concentrations of catecholamines (the endogenous agonists at β1-AR) producing their half-maximum effects (EC50s) are lower than their agonist dissociation constants KAs at the β1-ARs. Note, that during CHF the concentration of the endogenous agonists at the β1-AR may even exceed their KAs [57-59]. For the sake of simplicity, endogenous noradrenaline and adrenaline are combined as single endogenous agonist below, with a single KA only. When an exogenous agonist, i.e. a β-adrenergic stimulant like dobutamine, is administered in acute heart failure (AHF) patients, as usual in LV dysfunction, this increase in adrenoceptor activation even enhances the distance between the quasi-single concentration of endogenous agonists plus that of the “normalized” exogenous agonist and their common KA at the β1-AR. In this context, “normalized” means that the exogenous agonist concentration is expressed in affinity units of the endogenous agonist. Second, β1-ARs occur as homodimers [60,61]. In addition, agonist activation of one subunit (protomer) is sufficient to maximally induce the inotropic response obtained from this dimer and the binding of the endogenous agonist to the second protomer is negatively influenced after the first one has been occupied [negative cooperativity, 60]. Thus, negative cooperativity means that the binding of an agonist to the first protomer decreases the affinity of the agonist for the second one: Both protomers of a homodimer contribute to the allosteric modulation, and the same agonist molecule is the allosteric modulator (binding to the first protomer) and the modulated target (binding to the second protomer).
Regarding the fractional agonist receptor occupation,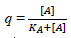 , [A] represents the concentration of all β1-AR agonists and KA their common (also “normalized”) agonist dissociation constant at the β1-AR. To make an example: Let us assume that the concentration of the endogenous agonist is 10-7 M with a KA of 10-8 M. What is the normalized concentration of the (often added) exogenous agonist dobutamine?
, [A] represents the concentration of all β1-AR agonists and KA their common (also “normalized”) agonist dissociation constant at the β1-AR. To make an example: Let us assume that the concentration of the endogenous agonist is 10-7 M with a KA of 10-8 M. What is the normalized concentration of the (often added) exogenous agonist dobutamine?
The normalization expresses the (rather low) affinity of dobutamine in the (much higher) affinity of the endogenous agonist:
According to Sanders Williams and Bishop [62] the KA of dobutamine at the β1-AR is 0.5 μM (= 10-6.30103 M). Thus, its affinity is (10-6.30103+8 =) 50-fold lower than that of the endogenous agonist. The dobutamine plasma concentration may be 592 nM [63] or 497.7 nM [64]. The “normalized” concentration, e.g. 497.7 nM, is then 497.7/50 = 9.95 nM = 10-8.0022 M.
Assuming that dobutamine is a pure agonist, the fractional agonist receptor occupation may be written as
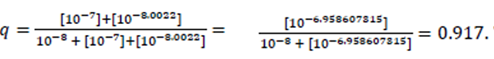
This means that 91.7 % of β1-ARs of all dimers are occupied by the endogenous β1-AR agonist or the exogenous β1-AR agonist dobutamine, either by only one agonist molecule or by two agonist molecules.
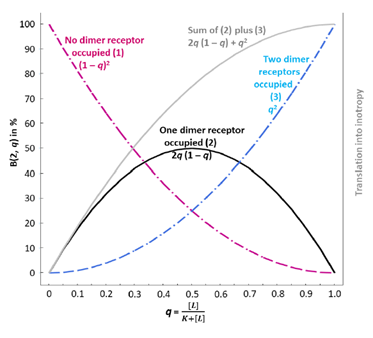
Regarding the agonist occupancy of each individual β1-AR dimer (no protomer occupied, one of the two protomers occupied, both protomers occupied), the fractional agonist receptor occupation, q (running between 0 and 1), is treated as follows according to Feuerstein and Schlicker [65]: Since the total number of receptors per dimer is n = 2, the existence of spare receptors on this functional unit means that the receptor reserve is 50%. Then, the number of occupied receptors is i = 0, 1, or 2 (with i = 1 being the minimal number of β1-ARs of a dimer to be activated for a maximum agonist effect of this dimer). Then, for 0 < i ≤ n, or 0 < 1, 2 ≤ 2, the number i of occupied receptors has a binomial distribution B(n, q) with parameters n = 2 and q [see 65]. How can negative cooperativity be modeled? Obviously, this phenomenon only occurs when both protomers of a dimer are occupied by two agonist molecules. Let us first consider the condition that one of two protomers is occupied. As detailed in Feuerstein and Schlicker [65] the binomial distribution is embodied in the concentration-response curve, i.e. x-axis as q, y-axis: = B(2, q)-response with (B(2, q) = 2q(1 – q). This condition is displayed in FIG. 1A (taken with permission from [65]), solid black curve. In the case of both protomers being occupied the binomial distribution of the (B(2, q)-response corresponds to q2. See FIG. 1A, dashed blue curve. The dashed purple curve, which indicates the probability that no dimer receptor is occupied, does – of course – not translate into inotropy. The black and the dashed blue curves, however, reflect the presence of an agonist and, therefore, translate into inotropy. The gray curve is the sum of the black and the dashed blue curve and reflects a 50% receptor reserve since the activation of one of two dimer receptors yields the same maximum effect (at q = 1) as the activation of two receptors.
However, this q2 does not yet reflect negative cooperativity within the double occupied dimer. Therefore, in order to reflect that two occupied protomers contribute less inotropy from the considered dimer then the condition that only one of two protomers is occupied, the amount of q2 has to be reduced. Feuerstein and Schlicker [65] proposed two models for that:
(1) q2 → q2/2 (halfing of q2) See FIG. 1B (taken with permission from [65] and modified), dashed blue curve (Model 1).
and
(2) q2 → q2·2q(1 – q) (multiplying q2 with the factor 2q(1 – q) which also diminishes q2 since this factor is always less than unity). See FIG. 1B, dashed brown curve (Model 2).
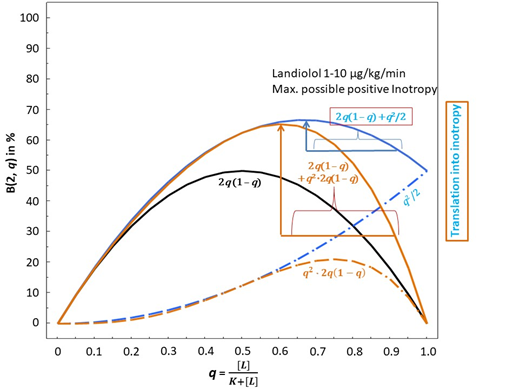
Figure 1B: Binominal distribution and possible translation into inotropy of β1-adrenoceptor ligands at dimer-receptors with consideration of negative cooperativity according to model 1 and 2. The vertical blue or brown arrows represent the maximum possible gain in inotropy induced by Landiolol in model 1 or 2, respectively. This is achieved in the concentration dose range of 1-10 μg/KG/min.
The more complicated approach [2] has the advantage that the inotropy from the considered dimer decreases more when the double occupations of dimers increase at the expense of single occupations.
Note that in both cases the condition that one of two protomers is occupied adds to the condition that both protomers are occupied:
- then yields 2q(1 – q) + q2; see FIG. 1B, solid blue curve.
- yields 2q(1 – q) + q22q(1 – q); see FIG. 1B, solid brown curve.
3. Results
The presence of a pure (neutral) β1-AR antagonist enhances the inotropic action of endogenous agonists, as shown by Feuerstein and Schlicker [65]. This is only true, however, if negative cooperativity prevails, i.e. if binding of an agonist to the first protomer decreases the affinity of the other agonist molecule for the second one (see Methods). Now the β1-AR antagonist effect comes into play. According to basic principles of receptor theory, a pure antagonist does nothing else than to shift the concentration-response curve of an agonist to the left. Usually, in steadily increasing curves, this means that an antagonist reduces the response. However, in our case of dimer occupations subjected to negative cooperativity, the concentration response curves of models (1) and (2) (see Methods and figure 1B) reflect declining parts of the curves with increasing values of q at the abscissa. To shift the concentration-response curve to the left within its declining part means increasing inotropy. This increase in inotropy is also true for the agonist combination of endogenous catecholamines and exogenous dobutamine in the presence of the ultra-short-acting highly selective β1- AR blocker Landiolol. Landiolol, with a KB of 10-7.045757491 M [23], may, dependent on (too high) doses and setting, antagonize positive inotropy and chronotropy [37] or, predominantly, chronotropy [38,42-58]. The contribution of Landiolol to the opposite, the enhancement of inotropy, can be delineated as follows:
The above-mentioned term of fractional agonist receptor occupation (endogenous + exogenous agonist, endogenous agonists NA and A at a normalized concentration of [10-7 M], exogenous agonist dobutamine at a normalized concentration of [10-8.0022] M (see Methods), with a common KA of 10-8 M) is changed by Landiolol with an antagonist dissociation constant of KB = 10-7.045757491 M to
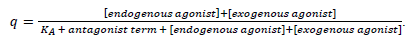
The antagonist term is made up according to equation 1 in Mantovani et al. [66; see also 67] as follows:
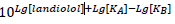
with Lg = log10. Note, that in contrast to the depiction in Feuerstein and Schlicker [65] the dissociation constant of the common agonist is here called KA, not KD, and the antagonist dissociation constant is KB.
To make an example: With the values assumed (see Methods, dobutamine supposed to be a weak, but pure agonist at the β1-AR), the fractional receptor occupation in the presence of [Landiolol] = 10-7.045757491 M, i.e. at a concentration equalling its KB = 10-7.045757491M for instance, is calculated to
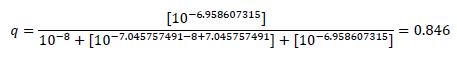
Thus, the presence of the antagonist Landiolol at a concentration equalling its KB slightly reduces the agonist occupation of the β1-AR from 0.917 (see Methods) to 0.846. In other words, at this small concentration, landiolol seemingly doubles KA and shifts the bell-shaped concentration-response relationship slightly to the left (see FIG. 1B, solid blue upwards directed arrow and solid brown upwards directed arrow). The horizontal blue (Model 1) or brown line (Model 2) below the blue or brown arrow in FIG. 1B shows the extent of this left shift. The probabilities that, at different agonist and antagonist concentrations, no protomer, one of two protomers, or both protomers of the dimers are activated can be calculated. Table 1 displays agonist binding probabilities at the dimer receptors in the absence and presence of Landiolol. Clinically realistic concentrations of the endogenous agonists, of dobutamine and of Landiolol are introduced in the described models of probabilities for agonist activations. According to the above considerations we assume that the agonist binding to at least one dimer receptor is the basis of the translation of agonist occupation into inotropy. The following table shows agonist binding probabilities at the dimer receptors in the absence and presence of the β1-AR antagonist Landiolol at different concentrations (expressed as Lg [Landiolol]) at 1, 7, 28 and 70 KB szenarios: It is obvious that in both models positive inotropy is achievable with Landiolol in a range between 1-7 KB whilst 28 KB in Model 1 and 70 KB in both models do not achieve additional inotropy but the contrary, lead to decreased inotropy.
Table 1:
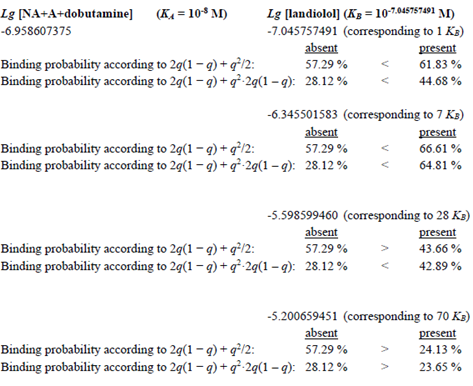
Table 2:
|
Model 1 |
|||||||
|
blood conc nmol/l |
blood conc ng/ml |
multiple KB |
dose µg/KG/min |
Inotropy % |
Inotropy + % |
Inotropy + abs.% |
Inotropy + rel. % |
|
64.5 |
32.9 |
1.0 |
1.4 |
57.29 |
61.83 |
4.54 |
8 |
|
129.0 |
65.7 |
2.0 |
2.9 |
57.29 |
64.54 |
7.25 |
13 |
|
193.4 |
98.6 |
3.0 |
4.3 |
57.29 |
66 |
8.71 |
15 |
|
257.9 |
131.4 |
4.0 |
5.7 |
57.29 |
66.6 |
9.31 |
16 |
|
322.4 |
164.3 |
5.0 |
7.1 |
57.29 |
66.61 |
9.32 |
16 |
|
451.3 |
230.0 |
7.0 |
10.0 |
57.29 |
66.61 |
9.32 |
16 |
|
644.8 |
328.6 |
10.0 |
14.3 |
57.29 |
62.5 |
5.21 |
9 |
|
1805.3 |
920.0 |
28.0 |
40.0 |
57.29 |
43.66 |
-13.63 |
-24 |
|
3223.8 |
1642.9 |
50.0 |
71.4 |
57.29 |
30.76 |
-26.53 |
-46 |
|
8059.5 |
2300.0 |
70.0 |
100.0 |
57.29 |
24.13 |
-33.16 |
-58 |
|
Model 2 |
|||||||
|
blood conc nmol/l |
blood conc ng/ml |
multiple KB |
dose µg/KG/min |
Inotropy % |
Inotropy + % |
Inotropy + abs.% |
Inotropy + rel.% |
|
64.5 |
32.9 |
1.0 |
1.4 |
28.12 |
44.68 |
16.56 |
59 |
|
129.0 |
65.7 |
2.0 |
2.9 |
28.12 |
54.46 |
26.34 |
94 |
|
193.4 |
98.6 |
3.0 |
4.3 |
28.12 |
60.14 |
32.02 |
114 |
|
257.9 |
131.4 |
4.0 |
5.7 |
28.12 |
63.28 |
35.16 |
125 |
|
322.4 |
164.3 |
5.0 |
7.1 |
28.12 |
64.8 |
36.68 |
130 |
|
451.3 |
230.0 |
7.0 |
10.0 |
28.12 |
64.81 |
36.69 |
130 |
|
644.8 |
328.6 |
10.0 |
14.3 |
28.12 |
62.6 |
34.48 |
123 |
|
1805.3 |
920.0 |
28.0 |
40.0 |
28.12 |
42.89 |
14.77 |
53 |
|
3223.8 |
1642.9 |
50.0 |
71.4 |
28.12 |
30.11 |
1.99 |
7 |
|
8059.5 |
2300.0 |
70.0 |
100.0 |
28.12 |
23.65 |
-4.47 |
-16 |
Table 2: Table 2 extends the calculation to further KB values. In addition, it also relates the responses to the corresponding steady state Landiolol blood concentrations achieved by the administration of the corresponding clinical doses. Furthermore, the percentages in inotropic shifts are indicated in absolute values, delta values (+ abs.%) and relative changes (+ rel.%).
From table 2 it is clear that, according to model 1 in relative terms a double digit percentage inotropic increase (up to +16%) is achievable between 2.9 and 10 μg/KG/min. In model 2 a triple digit percentage increase occurs between 4.3 and 14.3 μg/KG/min (up to +130%). In both models it is however obvious that beyond 4.3 μg/KG/min no substantial further gain in inotropy occurs. At the highest recommended permanent-infusion-dose for patients with normal left ventricular function (40 μg/KG/min which corresponds to 28 KB) Landiolol starts to worsen the agonist binding probabilities at the dimer receptors, i.e. positive inotropy starts to turn into negative inotropy in model 1 (-24% ) whilts model 2 still shows a positive shift (+53%). The Landiolol bolus-dose of 100 μg/KG/min which is applied for 1 min in patients with normal left ventricular function leads to a decrease in inotropy of 58 % and 16 % in both models. It has to be mentioned that the corresponding plasma concentration is calculated for a steady state situation which is not the reality for the bolus as the infusion is applied for 1 min only. However, immediately after application, blood concentration levels may achieve 1500-2600 ng/ml, the range for 50 KB in table 2. At this blood concentration model 1 shows a -46 % reduction in inotropy and model 2 shows no reduction but also almost no gain (+7%).
4.Discussion
In view of negative cooperativity, i.e. binding of an agonist to the first protomer decreases the affinity of the agonist for the second one, an extreme example can convincingly explain why a pure (neutral) β1-AR antagonist must enhance inotropy: Let us assume that all dimers are doubly occupied by agonist which may just about be compatible with a basic pumping capacity of the heart, i.e. this most extreme case of negative cooperativity may just about be compatible with survival. Then, the addition of only one β1-AR antagonist molecule will improve the inotropic condition at one single dimer. This is because the single β1-AR antagonist molecule ensures that this dimer is no longer double agonistically occupied: the antagonistic molecule displaces one agonistic molecule. The benefit of antagonistic displacement of agonists will prevail as long as antagonist addition produces more singly activated dimers but few doubly antagonistic ones. This benefit can be assessed using the approach of the present paper. The results show that Landiolol, in presence of other inotropes, can, in certain low dose ranges, recruit positive inotropy. In both models the ideal dose and plasma concentration range seems to be between 2.9 and 4.3 μg/KG/min, although doses as low as 1.4 μg/KG/min can already deliver extra positive inotropy. Interestingly according to the European guidelines for the treatment of acute tachycardic atrial fibrillation, Landiolol is the only beta blocker with an indicated dose range for patients with left ventricular dysfunction [40,41]. This dose range (1-10 μg/KG/min) overlaps in a perfect manner with the dose range we have characterized as being able to recruit positive inotropy. Within this dose range it is possible to define the lowest dose which is able to recruit the maximum possible positive inotropy (7.1 and 10 μg/KG/min in model 1 and 2, respectively). In clinical praxis this dose is of course influenced by several factors such as the degree of left ventricular function, pre- and after load as well as the actual doses of positive inotrope agents in use and the actual heart rate. Thus, it must be emphasized that the ideal positive inotropy dose range varies between individual patients and even within a patient and is always the consequence of a distinct dose finding and dose titration process. It is thus no surprise that Landiolol has already been used successfully in intensive care patients in conjunction with positive inotropic agents [42-53]. The dose range described in these clinical studies was comparable to what we have used as low doses in our model (1-10 μg/KG/min) and was more often between 1-5 μg/KG/min which is the distinct dose range where we observed the lowest possible dose that already achieved substantial possible inotropic response.
5. Conclusion
This article explains that during co-administration of β1-receptor agonists and antagonists, the antagonist may, based on the specific behavior of homodimeric β1-ARs, dose dependently induce a positive inotropic effect in patients with AHF. The approach considers well-established prerequisites, i.e., (i) that the β1-ARs are spare [54-56], (ii) dimer receptors with activation of one receptor dimer are already leading to the maximum effect [60,61], and (iii) that the concentration of the endogenous agonist at the β1-AR are higher than their KA values [KA – instead of KD -: agonist dissociation constant, 57-59]. Our calculation, based on the binomial receptor distribution, shows that, due to the negative cooperativity of the receptor dimers [60], negative inotropy is converted into positive inotropy at moderate to rather low concentrations of the β1-AR antagonist. Both proposed modeling approaches indicate a reduction in positive inotropy again if the concentration of the antagonist becomes too high that it shifts q too far to the left. Then q is in the ascending part of the solid blue or brown curve of Figure 1B. The question can be raised whether this condition with decreasing benefit corresponds to the clinical observation that too high concentrations of β1-adrenoceptor antagonists can worsen heart failure. We have simulated that and can say that such a phenomenon is also described for the clinical situation [68]. To cope with that, the aspect of a short pharmacokinetic half-life in conjunction with a rapid context sensitive half-life is a big advantage in case of Landiolol [37-39]. Former publications on this topic have raised the question whether increased and possibly harmful concentrations of β1-adrenoceptor antagonists can be estimated accurately enough on the basis of the proposed modeling approaches to avoid clinical deteriorations in patients with heart failure [65]. A general answer to this question cannot be drawn as head-to-head studies with β1-blockers in such patients are scarce. Studies have shown that short action duration seems to help when a β1-blocker induces negative inotropy [37,53,68,69] and higher selectivity seems to be a key element [36-39]. Modelling theories such as the current one and other explanations as mentioned above, deliver a molecular theory and a physiological and pathophysiological explanation basis and provide a rational for a concomitant therapy of β1-blocker and positive inotropic agents. In case of Landiolol multiple clinical examples as well as specific dose recommendations in the SmPC and the European guidelines and the short half-life of this substance provide support as far as the specific dosing and handling in such situations is concerned. We therefore believe that this publication along with the already existing clinical evidence may help to erase the skeptic attitude towards the use of β1-AR antagonist in patients with acute left ventricular dysfunction.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
References
- Wachter SB, Gilbert EM. Β-Adrenergic Receptors, from Their Discovery and Characterization through Their Manipulation to Beneficial Clinical Application. Cardiology 122 (2012): 104-112.
- Michel MC, Insel PA. Adrenergic Receptors in Clinical Medicine. The Receptors: The Adrenergic Receptors in Clinical Medicine 506 (2006): 129-147.
- Bristow MR. Treatment of Chronic Heart Failure With β-Adrenergic Receptor Antagonists. Circ Research 109 (2011): 1176-1194.
- Brodde OE, Schüler S, Kretsch R, et al. Regional Distribution of Β-Adrenoceptors in the Human Heart: Boexistence of Functional Β1- and Β2-Adrenoceptors in Both Atria and Ventricles in Severe Congestive Cardiomyopathy. Journal of Cardiovascular Pharmacology 8 (1986): 1235-1242.
- Viskin S, Kitzis I, Lev E, et al. Treatment With β-Adrenergic Blocking Agents After Myocardial Infarction: From Randomized Trials to Clinical Practice. JACC 25 (1995): 1327-1332.
- Kelly RA, Smith TW. Pharmacologic Treatment of Heart Failure. In: Hardman JH, Goodman Gillman A, Limbird LE, editors. Goodman Gillman’s The pharmacological basis of therapeutics,9th edition, New York, London. Mc Graw Hill (1966): 809-838.
- Kotecha D, Holmes J, Krum H, et al. Efficacy of β blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis. Lancet 384 (2014): 2235-2243.
- Mordi IR, Santema BT, Kloosterman M, et al. Prognostic significance of changes in heart rate following uptitration of β-blockers in patients with sub-optimally treated heart failure with reduced ejection fraction in sinus rhythm versus atrial fibrillation. Clinical Research in Cardiology 108 (2019): 797-805.
- Nasr IA, Bouzamondo A, Hulot JS, et al. Prevention of atrial fibrillation onset by β-blocker treatment in heart failure: a meta-analysis. European Heart Journal 28 (2007): 457-462.
- Blumenfeld JD, Sealey JE, Mann SJ, et al. b-Adrenergic Receptor Blockade as a Therapeutic Approach for Suppressing the Renin-Angiotensin-Aldosterone System in Normotensive and Hypertensive Subjects. AJH 12 (1999): 451-459.
- Hara Y, Hamada M, Shigematsu Y, et al. Effect of Β-Blocker on Left Ventricular Function and Natriuretic Peptides in Patients With Chronic Heart Failure Treated With Angiotensin-Converting Enzyme Inhibitor. Jpn Circ J 64 (2000): 365-369.
- Kawai K, Qin F, Shite J, et al. Importance of antioxidant and antiapoptotic effects of β-receptor blockers in heart failure therapy. Am J Physiol Heart Circ Physiol 287 (2004): H1003-H1012.
- Sharma V, McNeill JH. Parallel effects of β-adrenoceptor blockade on cardiac function and fatty acid oxidation in the diabetic heart: Confronting the maze. World Journal of Cardiology 3 (2011): 281-302.
- Freedman NJ, Lefkowitz RJ. Anti–β1-adrenergic receptor antibodies and heart failure: causation, not just correlation. The Journal of Clinical Investigation 113 (2004): 1379-1382.
- Pieske B. Reverse remodeling in heart failure – fact or fiction? European Heart Journal Supplements 6 (Supplement D) (2004): D66-D78.
- WangY, Shi Q, Li M, et al. Intracellular β1-Adrenergic Receptors and Organic Cation Transporter 3 Mediate Phospholamban Phosphorylation to Enhance Cardiac Contractility. Circulation Research 128 (2021): 246-261.
- Duncker DJ, Boontje NM, Merkus D, et al. Prevention of Myofilament Dysfunction by beta-Blocker Therapy in Postinfarct Remodeling. Circ Heart Fail 2 (2009): 233-242.
- Zafrir B, Amir O. Beta Blocker therapy, decompensated heart failure and inotropic interactions: current perspectives. Israel Medical Association Journal Vol. 14 (2012): 184-189.
- Kaye CM, Warrington SJ, Taylor EA, et al. Studies of cardioselectivity And Partial Agonist Activity In β-Adrenoceptor Blockade Comparing Effects On Heart Rate And Peak Expiratory Flow Rate During Exercise. Br. J. clin. Pharmac 5 (1978): 107-120.
- Lonjaret L, Lairez O, Minville V, et al. Optimal perioperative management of arterial blood pressure. Dove Press Journal 7 (2014): 49-59.
- Beattie WS, Wijeysundera DM, Karkouti K, et al. Does Tight Heart Rate Control Improve Β-Blocker Efficacy? An Updated Analysis of the Noncardiac Surgical Randomized Trials. Anesthesia & Analgesia 106 (2008): 1039-1048.
- Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomized controlled trial. Lancet 371 (2008): 1839-1847.
- Nasrollahi-Shirazi S, Sucic S, Yang Q, et al. Comparison of the β-Adrenergic Receptor Antagonists Landiolol and Esmolol: Receptor Selectivity, Partial Agonism, and Pharmacochaperoning Actions. Journal of Pharmacology and Experimental Therapeutics 359 (2016): 73-81.
- Deng CY, Lin SG, Zhang WC, et al. Esmolol Inhibits Na+ Current in Rat Ventricular Myocytes. Methods Find Exp Clin Parmacol 28 (2006): 697-702.
- Fallouh H, Bardswell SC, McLatchie LM, et al. Esmolol cardioplegia: the cellular mechanism of diastolic arrest. Cardiovascular Research 87 (2010): 552-560.
- Tanahashi S, Lida H, Dohi S, et al. Comparative effects of ultra-short-acting β1-blockers on voltage-gated tetrodotoxin-resistant Na+ channels in rat sensory neurons. European Journal of Anaesthesiology 26 (2009): 196-200.
- Shibata S, Okamoto Y, Endo S, et al. Direct Effects of Esmolol and Landiolol on Cardiac Function, Coronary Vasoactivity, and Ventricular Electrophysiology in Guinea-Pig Hearts. J pharmacol Sci 118 (2012): 255-265.
- Akimoto A, Kitagawa T, Kamanaka Y, et al. General Pharmacological Studies of Landiolol Hydrochloride (ONO-1101). Pharmacometrics 54 (1997): 53-67.
- Muraki K, Naxagawa H, Nagano N, et al. Effects of ONO-1 101, a Novel Β-Antagonist, on Action Potential and Membrane Currents in Cardiac Muscle. The Journal of Pharmacology and Experimental Therapeutics 278 (1996): 555-563.
- Wada Y, Aiba T, Tsujita Y, et al. Practical applicability of landiolol, an ultra-short-acting β1-selective blocker, for rapid atrial and ventricular tacharrhythmias with left ventricular dysfunction. Journal of Arrhythmia 320 (2016): 82-88.
- Perrett M, Gohil N, Tica O, et al. Efficacy and safety of intravenous β-blockers in acute atrial fibrillation and flutter is dependent on β-1 selectivity: a systematic review and meta-analysis of randomised trials. ECS Congress (2021): 492.
- Ikeshita K, Nishikawa K, Toriyama S, et al. Landiolol has a less potent negative inotropic effect than esmolol in isolated rabbit hearts. Journal of Anesthesia 22 (2008): 361-366.
- Iizuka T, Kakinuma T, Hamada Y, et al. A novel ultra short acting beta-blocker, landiolol supress the central sympathetic nerve activity directory and exerts a more potent negative chronotropic effect and less effect on blood pressure than esmolol European Journal of Anaesthesiology 21, Supplement 32 (2004): A-524.
- Sasao J, Tarver SD, Kindscher JD, et al. In rabbits, landiolol, a new ultra-short-acting ß-blocker, exerts a more potent negative chronotropic effect and less effect on blood pressure than esmolol. General Anesthesia 48 (2001): 985-989.
- Sugiyama A, Takahara A. Hashimotok: Electrophysiologic, Cardiohemodynamic and f3-Blocking Actions of a New Ultra-Short-Acting f3-Blocker, ONO-IIOI, Assessed by the In Vivo Canine Model in Comparison with Esmolol. Journal of Cardiovascular Phannacology 34 (1999): 70-77.
- Poschenrieder C, Kilger E. Inotropic effects of landiolol versus esmolol in a catecholamine-dependent patient with tachycardia after pericardectomy. Case Reports and Images in Surgery 5 (2022): 1-3.
- Krumpl G, Ul I, Trebs M, et al. Blood Pressure Recovery After Dobutamine Antagonism: Partial With Landiolol, None With Esmolol. Clinical Pharmacology in Drug Development 11 (2022): 309-317.
- Krumpl G, Ulc I, Trebs M, et al. Pharmacokinetics and -dynamics of low, intermediate and high dose landiolol and esmolol during long term infusion in healthy Caucasians. Journal of Cardiovascular Pharmacology 71 (2017): 137-146.
- Krumpl G, Ulc I, Trebs M, et al. Bolus application of landiolol and esmolol: comparison of the pharmacokinetic and pharmacodynamic profiles in a healthy Caucasian group. Eur J Clin Pharmacol 73 (2016): 417-428.
- Landiolol (Rapibloc) SmPC: https://www.medicines.org.uk/emc/product/13830/smpc.
- Hindricks G, Potpara T, Dagres N, et al. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC, European Heart Journal 42 (2021): 373-498.
- Imabayashi T, Murayama H, Kuroki C, et al. Study of hemodynamics in patients treated with landiolol in the ICU. Kagoshima University Hospital, Kagoshima, Japan, Critical Care 13 (2009): 173.
- Morisaki A, Hosono M, Sasaki Y, et al. Very-low-dose continuous drip infusion of landiolol hydrochloride for postoperative atrial tachyarrhythmia in patients with poor left ventricular function. Gen Thorne Cardiovasc Surg 60 (2012): 386-390.
- Kobayashi S, Susa T, Tanaka T, et al. Low-Dose β-Blocker in Combination With Milrinone Safely Improves Cardiac Function and Eliminates Pulsus Alternans in Patients With Acute Decompensated Heart Failure. Circulation Journal 76 (2012): 1646-1653.
- Sakaguchi M, Sasaki Y, Hirai H, et al. Efficacy of Landiolol Hydrochloride for Prevention of Atrial Fibrillation After Heart Valve Surgery. Int Heart Journal 53 (2012): 359-363.
- Hamaguchi S, Nagao M, Takahashi Y, et al. Low Dose Landiolol Combined with Catecholamine Can Decrease Heart Rate without Suppression of Cardiac Contraction after Cardiopulmonary Bypass. Dokkyo Journal of Medical Sciences 41 (2014): 27-33.
- Kobayashi S, Murakami W, Myoren T, et al. A low-Dose (31-Blocker Effectively and Safely Slows the Heart Rate in Patients with Acute Decompensated Heart Failure and Rapid Atrial Fibrillation. Cardiology 127 (2014): 105-113.
- Nitta D, Kinugawa K, Imamura T, et al. An Experience of Landiolol Use for an Advanced Heart Failure Patient With Severe Hypotension. Int Heart Journal Vol 56 (2015): 564-567.
- Yoshida Y, Terajima K, Sato C, et al. Clinical role and efficacy of landiolol in the intensive care unit. Journal of Anesthsia 22 (2008): 64-69.
- Sakai M, Jujo S, Kobayashi J, et al. Use of low-dose β1-blocker for sinus tachycardia in patients with catecholamine support following cardiovascular surgery: a retrospective study. Cardiothorac Surg 14(2019): 145
- Ditali V, Garatti L, Morici N, et al. Effect of landiolol in patients with tachyarrhythmias and acute decompensated heart failure (ADHF): a case series. ESC Heart Fail 9 (2022): 766-770.
- Dabrowski W, Siwicka-Gieroba D, Piasek E, et al. Successful Combination of Landiolol and Levosimendan in Patients with Decompensated Heart Failure. Int Heart J 61(2020): 384-389.
- Anifanti M, Iona I, Tsikritsaki K, et al. Landiolol vs Esmolol on hemodynamic response during weaning of post-operative ICU patients with heart failure. European Respiratory Journal 58 (2021): PA3320.
- Zolk O, Kilter H, Flesch M, et al. Functional coupling of overexpressed beta 1-adrenoceptors in the myocardium of transgenic mice. Biochem Biophys Res Commun 248 (1998): 801-805.
- Kaumann AJ. On spare beta-adrenoceptors of inotropic effect of catecholamines in kitten ventricle. Naunyn Schmiedebergs Arch Pharmacol 305 (1978): 97-102.
- Brown L, Deighton NM, Bals S, et al. Spare receptors for beta-adrenoceptor-mediated positive inotropic effects of catecholamines in the human heart. J Cardiovasc Pharmacol 19 (1992): 222-232.
- Peng Y, Shan J, Qi X, et al. Effects of catecholamine-beta-adrenoceptor-cAMP system on severe patients with heart failure. Chin Med J (Engl) 116 (2003): 1459-1463.
- Morimoto A, Hasegawa H, Cheng HJ, et al. Endogenous β3-adrenoreceptor activation contributes to left ventricular and cardiomyocyte dysfunction in heart failure. Am J Physiol Heart Circ Physiol 286 (2004): 2425-2433.
- Hoffmann C, Leitz MR, Oberdorf-Maass S, et al. Comparative pharma-cology of human Beta-adrenergic receptor subtypes – characterization of stably transfected receptors in CHO cells. Naunyn-Schmiedeberg’s Arch Pharmacol 369 (2004): 151-159.
- Gherbi K, May LT, Baker JG, et al. Negative cooperativity across β1-adrenoceptor homodimers provides insights into the nature of the secondary low-affinity CGP 12177 β1-adrenoceptor binding conformation. FASEB J 29 (2015): 2859-2871.
- Calebiroa D, Riekena F, Wagnera J, et al. Single-molecule analysis of fluorescently labeled G-protein–coupled receptors reveals complexes with distinct dynamics and organization PNAS 110 (2013): 743-748.
- Sanders Williams R, Bishop T. selectivity of dobutamine for adrenergic receptors. In vitro analysis by radioligand binding. J Clin Invest (1981): 1703-1711.
- Habib DM, Padbury JF, Anas NG, et al. Dobutamine pharmacokinetics and pharmacodynamics in pediatric intensive care patients. Crit Care Med 20 (1992): 601-608.
- Ahonen J, Aranko K, Iivanainen A, et al. Pharmacokinetic-Pharmacodynamic Relationship of Dobutamine and Heart Rate, Stroke Volume and Cardiac Output in Healthy Volunteers. Clin Drug Invest 28 (2008): 121-127.
- Feuerstein M, Schlicker E. β1 blockers enhance inotropy of endogenous catecholamines in chronic heart failure. Frontiers in Cardiovascular Medicine 639562 (2021): 1-9.
- Mantovani M, Dooley DJ, Weyerbrock A, et al. Differential inhibitory effects of drugs acting at the noradrenaline ansd 5-hydroxytrytzamine transporters in rat and human neocrotical synaptosomes. Brit J Pharmacol 158 (2009): 1848-1856.
- Cheng YC, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22 (1973): 3099-3108.
- Goto K, Shingu S, Miyakawa H, et al. The effect of landiolol on hemodynamics and left ventricular function in patients with coronary artery disease. Journal of Clin. Anesthesia 19 (2007): 523-529.
- Stix G, Wolzt M, Domanovits H, et al. Open-Label Two-Dose Pilot Study of Landiolol for the Treatment of Atrial Fibrillation/Atrial Flutter in Caucasian Patients. Circ J 84 (2020): 33-42.