The Sleep in the Critically Ill Aged Patients
Article Information
Rodolfo Augusto Alves Pedrão*, Rodrigo Jardim Riella, Silvia Valderramas
Federal University of Paraná, Brazil
*Corresponding author: Rodolfo Augusto Alves Pedrão, Federal University of Paraná, Brazil
Received: 13 July 2022; Accepted: 20 July 2022; Published: 25 July 2022
Citation: Rodolfo Augusto Alves Pedrão, Rodrigo Jardim Riella, Silvia Valderramas. The Sleep in the Critically Ill Aged Patients. Archives of Clinical and Biomedical Research 6 (2022): 631-637.
View / Download Pdf Share at FacebookAbstract
Objective: To assess the characteristics and quality of sleep in critically ill older and younger adults and verify the differences between these groups. Check for associations between sleep and the perception of pain, noise, temperature, environmental luminance and the use of opiods and benzodiazepines.
Method: Cross-sectional observational study, which evaluated lucid critically ill individuals, older and younger adults, with diseases of low or moderate severity. Sleep characteristics were measured using the Bispectral Index; sleep quality was measured using the Richards-Campbell Sleep Questionnaire; pain level was measured by Visual-Analog Scale; we recorded the ambient sound pressure, luminance and temperature, as well as the administered doses of opiods and benzodiazepines.
Results: The medians of total sleep time, deep sleep time, pain intensity, luminance, ambient temperature, continuous sound pressure equivalent and perceived sleep quality were 237 minutes, 0 minutes, 1/10 point, 13.26 Lux, 22.4ºC, 57.27 decibels and 61/100 points, respectively. No older participant achieved deep sleep. In older people, pain and sleep quality are inversely correlated (ρ = -0.48; p<.05); in younger adults, volume and time of deep sleep were inversely correlated with environmental noise (ρ = -0.45; p<0.05 and ρ = -0.44; p<0.05, respectively).
Conclusion: The sleep of adult patients with low and medium severity illnesses admitted to the ICU is of short duration and superficial, especially in the older patients. In these, pain perception is inversely correlated with sleep quality, while, in younger adults, environmental noise is inversely correlated with deep sleep.
Keywords
Intensive care units. Sleep. Sleep deprivation
Intensive care units articles. Sleep articles. Sleep deprivation articles
Article Details
1. Introduction
Sleep is a complex and dynamic process that allows tissue repair and mental reorganization. Many physiological variables have oscillations that follow a circadian rhythm - hemodynamic indices, respiratory function, immunological and coagulation mechanisms [1]. Although sleep plays and importante role in convalescence, patients in the intensive care unit (ICU) commonly have sleep disturbances and disorganized sleep-wake cycle, which compromises recovery and increases the risk of delirium and mortality [2] specially in the aged [3]. Aging is associated with sleep structure degradation, greater number of awakenings, lower regenerative capacity and difficulties in adapting the circadian rhythm to new environments [3]. With the advent of population aging, the global demand of aged people for ICU beds has been progressively growing and a greater understanding of the particular specificities of critically ill aged people is becoming urgent.
In the ICU sleep disorders result from combinations of several factors - environmental noise, exposure to light at night (which suppresses melatonin secretion), uncomfortable environmental temperature, handling care, discomfort caused by mechanical ventilation , side effects of medications [3] and due to the seriousness of the disease that determined the hospitalization [4]. Sleep problems iniciated in the ICU can be associated with persistent psychological disorders after hospital discharge, compromissing quality of life [2]. Although there are studies that describe the sleep of patients admitted to the ICU [5-8], studies that concomitantly consider the multiplicity of potentially intervening environmental and individual factors and the differences in sleep characteristics between aged and non-aged adults in this environment are necessary. In this study, we measured the duration, the depth and the perceived quality of sleep in older and younger adults hospitalized in the ICU. Furthermore, we verified the associations between these variables and pain intensity, noise, luminance, ambient temperature and the use of opioids and benzodiazepines, determining whether there are differences in these parameters between the two age groups.
2. Method
This cross-sectional observational study was conducted in an 8-bed surgical ICU of a public tertiary hospital. It was carried out in compliance with the 1975 Declaration of Helsinki (revised in 2000), having been registered in the Brazilian Registry of Clinical Trials (number of RBR-2ss28r record). All participants consented in writing before starting the monitoring. Data were collected from August 2018 to June 2019. A convenience sample was surveyed and screening for eligibility among patients admitted to the aforementioned ICU was performed every day of the week. Only one patient was monitored each night, as there was only one sleep monitoring device available for the study. All data were collected by the same researcher. Aged (65 years and over) and non-aged adults of both sexes, undergoing treatment for diseases of low or moderate severity, with the prospect of staying the subsequent night in the ICU and who were sufficiently lucid to understand and accept the terms of free and informed consent were included in the study.. Patients undergoing surgical procedures were included in the study the day after the surgical intervention, to minimize the effect of anesthetics on sleep. Patients who presented discomfort with the monitoring equipment, clinical deterioration that compromised the ability to understand and accept the terms of free and informed consent, individuals whose data could not be recorded throughout the night and patients in delirium, identified by screening with the Confusion Assessment Method for Intensive Care Unit (CAM-ICU) [9] instrument were excluded.
Study participants were hospitalized in individual boxes measuring 12 square meters, with wooden and glass partitions. All boxes had a multiparameter monitor and, as needed, infusion pumps and a mechanical ventilator. The audible alarms on the sleep monitoring equipment were all off. There was no active protocol for sleep promotion at the time of data collection, and no participants wore earplugs or eye masks. After recruitment, participants' demographic, anthropometric and clinical data were recorded. The severity of the illness presented by the individual was estimated on the day of monitoring with the Acute Physiology and Chronic Health Evaluation II (APACHE II) index [10]. Participants had a BIS Quatro® sensor (Covidien IIc, Mainsfield, USA) attached to the left frontal region and connected to the BIS Vista® Monitoring System (Covidien IIc, Mainsfield, USA) from 7 pm to 7 am hours of the following morning, which performed the electronic processing of the electroencephalographic record every minute during the observation period and calculated the Bispectral Index (BIS), which allowed the estimation of sleep duration and depth [11]. The parameters recorded by the BIS were: total sleep volume, total sleep time, deep sleep volume and deep sleep time. Sleep duration was expressed in minutes and sleep volume was expressed as a function of the area under the BIS curve versus time (in minutes) [12], adopting BIS values below 80 as the onset of superficial sleep and below 40 as the onset of deep sleep [13, 14].
Immediately after installing the cranial sensor, we estimated the level of pain experienced by the individual, using the Visual-Analog Pain Scale [15]. No participant bathed in bed on the nights of observation. The environmental sound pressure level was recorded from 7:00 pm to 7:00 am the following morning with a professional class 1 monitor with datalogger, model DT-8852 (CEM Instruments, India), submitted to certified calibration and installed 1 meter from the head of the bed of the patient under study. The frequency of sound capture was 2 seconds; the used uptake unit was the decibel (dB); and the compensation circuit was set to slow A-weighting. The equivalent continuous noise levels (LAEq) of the observation periods [6] were calculated. The environmental luminance was also recorded from 7:00 pm to 7:00 am the following morning with a professional digital lux meter with datalogger model DT-8809 (CEM Instruments, India), submitted to certified calibration and installed at 1 meter from the head of the bed of the patient under study. The frequency of ambient luminance capture was 20 seconds; the capture unit used was the Lux. The ambient temperature was recorded with an iButton sensor model DS1921G (Thermochron, Australia) [16], installed 1 meter from the head of the patient's bed. The frequency of capture of ambient temperature was 3 minutes and the unit of capture used was the degree centigrade. Dose-equivalents of morphine and clonazepam (in milligrams/kg/day) administered between 7 am on the day of monitoring and 6:59 am the following day were calculated for each participant. In the mornings following the monitoring nights, the Brazilian Portuguese version of the Richards-Campbell Sleep Questionnaire (RCSQ) [17] was applied to patients. The total score, the average of the five domains that make up the instrument, represented the global perception of sleep. The perceived quality of sleep was graded from 0 to 100 points. The authors declare that there are no conflicts of interests.
2.1 Statistical analysis
The assumption of normality of the variables was verified using the Kolmogorov-Smirnov test and the results were presented as median and 25-75% percentiles, mean and standard deviation or frequency, depending on the type of variable and data distribution. Spearman's rank correlation coefficient was used to determine the degree of association between sleep parameters measured by the BIS, the perceived quality of sleep measured by the RCSQ and pain intensity, LAEq and environmental luminance. The difference between the groups (older and younger adults) was verified using the Mann-Whitney test. The scale of magnitudes proposed by Batterham and Hopkins [18] was used to interpret the correlations, being: < .1 trivial; between .10-.29 small; .30-.49 moderate; .50-.69 high; .70-.90 too high; and > .90, almost perfect. The established level of significance was 5%.
3. Results
The Research Ethics Committee of the institution approved this trial (registration number 83082118.7.0000.0096).
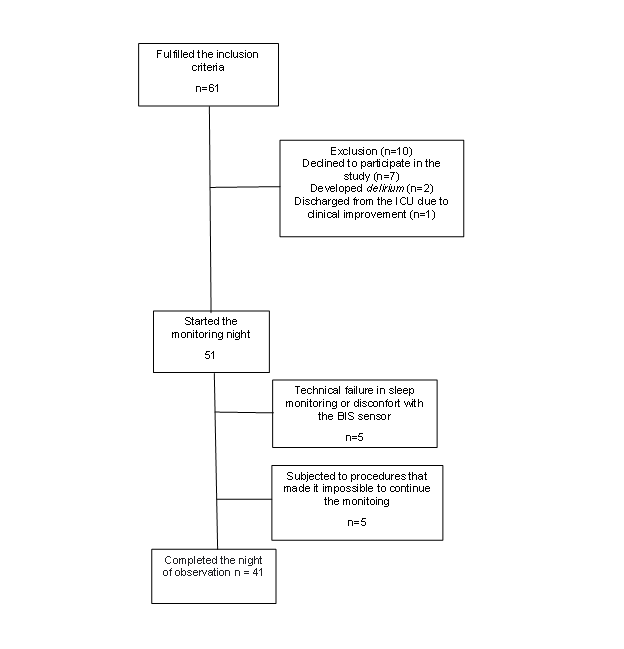
After screening, 61 individuals met the inclusion criteria (Figure 1). However, 7 individuals declined to participate; 1 showed rapid clinical improvement and was discharged from the ICU; 2 had delirium and could not be monitored; 5 abandoned the study after technical failure in sleep monitoring or discomfort with the BIS sensor; and another 5 underwent procedures that made monitoring impossible. Thus, 41 participants completed the monitoring.
Table 1: summarizes the characteristics of the study patients. Aged patients had a more severe clinical picture than non-aged adults (U = 131.5; p<.05). No participant reported hearing deficit
|
Variable |
All participants N = 41 |
Younger adults n = 21 |
Older adults n = 20 |
|
|
Age (years)a |
64 (49.5-71.5) |
51 (33.5-56.5) |
71.5 (68-77.5)** |
|
|
Whiteb |
37 (90.2) |
21 (52.5) |
16 (39) |
|
|
Mixed raceb |
4 (9.8) |
0 |
4 (9.8) |
|
|
Femaleb |
24 (58.5) |
15 (36.6) |
9 (22) |
|
|
APACHE IIa |
12 (8-15.5) |
10 (7-13) |
13 (10.2-16.7)* |
|
|
Current smokerb |
3 (7.3) |
2 (4.8) |
1 (2.4) |
|
|
Current drinkerb |
1 (2.4) |
1 (2.4) |
0 |
|
|
Use of invasive mechanical |
||||
|
ventilationb |
2 (4.8) |
2 (4.8) |
0 |
|
|
Use of noninvasive mechanical |
||||
|
ventilationb |
1 (2.4) |
1 (2.4) |
0 |
|
|
Use of tracheostomyb |
1 (2.4) |
1 (2.4) |
0 |
|
|
Use of opiodsb |
10 (2.4) |
14 (34.1) |
12 ( 29.2) |
|
|
Reason for hospitalization |
||||
|
Gastrointestinal surgeryb |
21 (51.2) |
7 (17) |
14 (28) |
|
|
Thoracic surgeryb |
5 (12.2) |
3 (7.3) |
2 (4.8) |
|
|
Urological surgeryb |
4 (9.7) |
1 (2.4) |
3 (7.3) |
|
|
Acute renal failureb |
1 (2.4) |
1 (2.4) |
0 |
|
|
Sepsisb |
3 (7.3) |
2 (4.8) |
1 (2.4) |
|
|
Pulmonary thromboembolismb |
1 (2.4) |
1 (2.4) |
0 |
|
|
Liver transplantationb |
1 (2.4) |
1 (2.4) |
0 |
|
|
Oral and maxillofacial surgeryb |
2 (4.8) |
2 (4.8) |
0 |
|
|
Orthopedic surgeryb |
1 (2.4) |
1 (2.4) |
0 |
|
|
Leptospirosisb |
1 (2.4) |
1 (2.4) |
0 |
|
|
Myasthenia gravisb |
1 (2.4) |
1 (2.4) |
0 |
|
|
APACHE II: Acute Physiology and Chronic Heatlh Evaluation II; a The results are expressed as median (25th-75th percentile) or b n (%). *p<.05; **p<.01 |
||||
Measurements using the BIS (Table 2) showed that the individuals studied had reduced total sleep time (median of 237 minutes per night of observation), with no differences between older and younger adults. The Mann-Whitney test revealed, however, that deep sleep volume and deep sleep time were significantly higher in the group of younger adults (U=130; p=.01 e U=132.5; p=.012, respectively). No aged participant reached deep sleep during the nights of observation.
Table 2: Sleep parameters evaluated by the Bispectral Index, perceived quality of sleep evaluated by the Richards-Campbell Sleep Questionnaire, pain perception (visual-analogue scale), environmental sound pressure level, environmental luminance, environmental temperature, equivalent dose of morphine and equivalent dose of clonazepan.
|
Variable |
All patients |
Younger adults |
Older adults |
|
|
Sleep parameters (BIS) |
||||
|
Total sleep volume |
2889 (1661-5511) |
3332 (1725-6803) |
2695 (1291-5344) |
|
|
Total sleep time |
237 (127-360) |
246 (156-351) |
215.5 (122-366) |
|
|
Deep sleep volume |
0 (0-9.5) |
0 (0-38.5) |
0 (0-0)* |
|
|
Deep sleep time |
0 (0-3) |
0 (0-5.5) |
0 (0-0)* |
|
|
Perceived quality of sleep (RCSQ) |
51 (34-74) |
60.9 (38.9-74.4) |
38.28 (30.3-76.1) |
|
|
Perceived pain intensity (VAS) |
0.7 (0-3.75) |
1 (0-5) |
0 (0-3) |
|
|
Environmental sound pressure level (LAEq) |
57.27 (55.25-58.91) |
57.55 (55-59.55) |
56.6 (55.4-58.5) |
|
|
Environmental luminance intensity (Lux) |
13.26 (7.2-28.55) |
16.4 (7.8-36.7) |
12.3 (5.6-18.6) |
|
|
Environmental temperature (ºC) |
22.4 (21.2-23.9) |
22.4 (22-23.8) |
21.8 (21-24) |
|
|
Equivalent dose of morphine |
0 (0-.075) |
0 (0-0.015) |
0 (0-0) |
|
|
Equivalent dose of clonazepam |
0 (0-0) |
0 (0-0) |
0 (0-0) |
BIS - bispectral index; RCSQ - Richards-Campbell Sleep Questionnaire; VAS - visual analog scale; LAEq – A-weighted equivalent continuous noise level (in decibels). Values are expressed as median (25th-75th percentil); Sleep time expressed in minutes; total sleep volume expressed in (bispectral index units <80) × time; total deep sleep volume expressed in (bispectral index units <40) × time; equivalent doses of morphine and clonazepam expressed in mg/kg of pacient weight.
*p<.05.
RCSQ showed that the individuals studied perceived their sleep quality as moderate (median 61, with no difference between older and younger adults) (Table 2) and related low levels of pain (intensity 2 in 10), with no significant difference between groups. The uses of opioids and benzodiazepines were very low in the studied sample (Table 2).
We recorded high noise levels and low intensity of ambient luminance on observation nights (median of 57.27 dB LAEq and 13.26 Lux, respectively), with no significant difference between groups (Table 2).
In the group of individuals as a whole, we observed significant inverse correlations of moderate magnitudes between environmental noise and the results of measurements with the BIS of total sleep time, deep sleep volume and deep sleep time (ρ = -.33; p<.05, ρ = -.31; p<.05 e ρ = -.31; p<.05, respectively) (Table 3).
Table 3: Correlation coefficients (Spearmann's rho) between sleep parameters, perceived sleep quality, perceived pain intensity, environmental noise, environmental luminance, environmental temperature, opioids dose and benzodiazepine dose in all patients, older adults and younger adults.
|
Total sleep volume |
Total sleep time |
Deep sleep volume |
Deep sleep time |
Perceived quality of sleep |
|
|
All patients |
|||||
|
Perceived pain |
-0.16 |
-0.09 |
-0.03 |
-0.03 |
-0.13 |
|
Environmental noise |
-0.16 |
-.33* |
-.31* |
-.31* |
0.04 |
|
Environmental luminance |
0.11 |
0.1 |
0.15 |
0.1 |
-0.14 |
|
Environmental temperature |
-0.14 |
-0.004 |
-0.14 |
-0.19 |
0.23 |
|
Opioids dose |
0.02 |
0.02 |
0.03 |
-0.01 |
0.12 |
|
Benzodiazepine dose |
0.11 |
0.07 |
-0.004 |
-0.06 |
0.08 |
|
Younger adults |
|||||
|
Perceived pain |
-0.3 |
-0.3 |
-0.06 |
-0.04 |
0.1 |
|
Environmental noise |
-0.25 |
-0.3 |
-.45* |
-.44* |
0.08 |
|
Environmental luminance |
0.17 |
0.3 |
0.1 |
-0.01 |
-0.4 |
|
Environmental temperature |
-0.39 |
-0.25 |
-0.19 |
-0.32 |
0.04 |
|
Opioids dose |
0.1 |
0.04 |
0.14 |
0.04 |
0.18 |
|
Benzodiazepine dose |
-0.04 |
0.04 |
0.28 |
0.01 |
-0.15 |
|
Older adults |
|||||
|
Perceived pain |
-0.06 |
0.05 |
-0.26 |
-0.26 |
-.48* |
|
Environmental noise |
-0.03 |
-0.31 |
-0.36 |
-0.32 |
0.02 |
|
Environmental luminance |
-.0.03 |
-0.26 |
0.028 |
0.28 |
-0.04 |
|
Environmental temperature |
-0.02 |
0.13 |
-0.29 |
-0.29 |
0.28 |
|
Opioids dose |
0.18 |
0.3 |
-0.12 |
-0.12 |
0.07 |
|
Benzodiazepine dose |
-0.003 |
-0.26 |
0.28 |
0.28 |
-0.04 |
*p<.05.
Analyzing the different age groups separately, we observed that, in non-aged adults, the volume and time of deep sleep are inversely correlated with environmental noise (ρ = -.45; p<.05 and ρ = -.44; p< .05, respectively). In the aged, perceived sleep quality is inversely correlated with perceived pain on the night of observation (ρ = -.48; p<.05) (Table 3).
We did not observe correlations between the use of opioids or benzodiazepines and sleep parameters (Table 3).
4. Discussion
This study demonstrates that in the ICU, sleep in the aged and non-aged adults is short and superficial. None of the aged people reached deep sleep on the night of observation and few non-aged adults slept more deeply, for brief periods. In the aged, pain and sleep quality are inversely correlated, whereas in non-aged adults, environmental noise and deep sleep are inversely correlated. The reduction in total sleep time and sleep superficiality observed in this group of individuals corroborate the findings of other studies [6-7] and the concept that admission to the ICU is associated with deprivation of restful sleep and, potentially, its deleterious consequences [3, 19]. Despite the reduced total sleep time and the near absence of deep sleep, participants reported sleep quality as moderate - median of 61 (out of a possible 100). The authors believe that there may have been an overestimation of the sleep quality perceived by the participants, also observed in other studies using subjective sleep assessment instruments, such as RCSQ [20]. Furthermore, the higher levels of deep sleep volume and deep sleep time presented by younger adults did not translate into a better perception of sleep quality by this group. Correlations between BIS and RCSQ parameters were investigated in another study [11].
Although the study included individuals undergoing major surgery, reported pain intensity was low - median of .7 (out of a possible total of 10). The authors believe that this occurred because, in individuals undergoing surgery, inclusion in the study occurred only on the day after the procedure, which enabled an effective analgesia adjustment. Even so, we observed an inverse correlation between pain intensity and sleep quality in the older adults. The authors believe that these findings reinforce the importance of careful observation of the levels of pain experienced by the aged in the ICU. Despite the use of sedatives being common in the ICU, especially in the aged [3], practically no individual received benzodiazepines on the monitoring day. The authors believe that the lack of prescription of this class of drugs in the monitored individuals was due to the characteristics of the ICU that housed the study (predominantly surgical, without chronically ill patients) and the tendency of the assistant team to avoid, as far as possible, the use of benzodiazepines as part of delirium prevention measures [21].
The ambient sound pressure measured on the nights of observation exceeded the 35 dB recommended by the World Health Organization [22], as routinely reported in studies investigating environmental noise in the ICU [6,5,23] and was negatively correlated with deep sleep in non-aged adults. Such a correlation was not observed in the aged – although none of them reported hearing deficits. Other studies have concluded that, although important, environmental noise is only part of the myriad of factors potentially intervening in sleep [8] and that reducing the level of environmental noise alone reduces the incidence of delirium, but does not translate into necessarily in improving the perception of sleep quality [24]. Even so, sleep quality had a strong inverse correlation with environmental noise in the group of younger adults. The choice of setting the sound level meter to capture A-weighted sounds (to the detriment of C-weighting) was due to the fact that it more accurately records medium-frequency sounds (such as the human voice) and is routinely used in similar research [6, 23]. Although melatonin secretion (and its inhibition by light) is an important part of the physiological mechanisms that influence sleep [3], in this study we did not observe correlations between environmental luminance and sleep parameters. The authors believe that this may have occurred because the median environmental luminance of the observation periods was 13.26 Lux, well below the 20 Lux defined as adequate for the ICU environment [25] and the 100 Lux required to inhibit melatonin secretion [26]. Other studies obtained similar results [6, 23]. Danielson et al [23] even postulated that the luminance of the ICU environment is incapable of providing effective circadian stimulation. In this study, no instrument was used to directly measure the intensity of manipulation of the individual under study by the care team, one of the factors with the potential to compromise sleep in the ICU [3]. The authors believe, however, that the concomitant measurements of the ambient sound pressure level and luminance provided accurate estimates of the intensity of the manipulation suffered by the patient, since any procedure performed at night required that the box lights be turned on and that some noise be produced.
Despite room temperature being considered one of the factors that impact sleep in the ICU environment [3], in this study we did not observe correlations between it and sleep parameters in any of the age groups. The authors believe this is due to the fact that there were no extremes of ambient temperature on the observation nights. In this study, we included individuals on mechanical ventilation (2 of them with a tracheostomy and 1 with a myasthenic crisis, being ventilated through an orotracheal cannula). Even using ventilatory support, all were lucid, comfortable and were not receiving sedatives, as part of the protocol for weaning from mechanical ventilation and humanization of care in the ICU [27].
Although this is the largest study to which the authors are aware of the correlations between multiple environmental factors and the sleep of older adults in the ICU, some limitations should be considered: Although part of the sleep of critically ill patients occurs during the day [28], in this study we only monitored the night period; losses occurred due to sensor displacement (n = 5); even with intra- and inter-individual variability in the sleep of ICU patients [20] and we do not know whether the effect of the first night [29] occurs with the BIS, each participant was monitored for only one night; and these results are valid for lucid patients and those with diseases of low or moderate severity. Even though this selection limited the generalizability of the results, it guaranteed the reliability of the measurements, as the assessment of sleep in clinically unstable or sedated patients is potentially biased [30]. Although the disease severity of the individuals in the sample as a whole was low, the difference observed in the APACHE II score between the two age groups studied may have had a different impact on the results of the measurements.
5. Conclusion
Sleep in older and younger adults in the ICU is short-lived and superficial. In low and medium severity patients admitted to the ICU, self-reported sleep quality has a moderate inverse correlation with the level of pain in older adults and a high inverse correlation with environmental noise in younger adults. The authors believe that it is the duty of the entire multidisciplinary team working in the ICU to implement measures to control pain and environmental noise, seeking to improve the sleep quality of convalescent patients. More studies are needed to explore the correlations between environmental, individual factors and sleep in critically ill aged and non-aged adults.
References
- Tiruvoipati R, Mulder J, Haji K. Improving Sleep in Intensive Care Unit: An Overview of Diagnostic and Therapeutic Options. J Patient Exp 7 (2020): 697–702.
- Altman MT, Knauert MP, Pisani MA. Sleep disturbance after hospitalization and critical illness: A systematic review. Ann Am Thorac Soc 14 (2017): 1457–68.
- Sterniczuk R, Rusak B, Rockwood K. Sleep disturbance in older ICU patients. Clin Interv Aging 9 (2014): 969–77.
- Drouot X, Cabello B, D’Ortho MP, Brochard L. Sleep in the intensive care unit. Sleep Med Rev 12 (2008): 391–403.
- Boyko Y, Jennum P, Nikolic M, Holst R, Oerding H, Toft P. Sleep in intensive care unit: The role of environment. J Crit Care 37 (2017): 99–105.
- Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care 17 (2013): 1-10.
- Elbaz M, Léger D, Sauvet F, Champigneulle B, Rio S, Strauss M, et al. Sound level intensity severely disrupts sleep in ventilated ICU patients throughout a 24-h period: a preliminary 24-h study of sleep stages and associated sound levels. Ann Intensive Care 7 (2017): 1-9.
- Gabor JY, Cooper AB, Crombach SA, Lee B, Kadikar N, Bettger HE, et al. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med 167 (2003): 708–15.
- Gusmao-Flores D, Salluh JI, Dal-Pizzol F, Ritter C, Tomasi CD, Lima MA, et al. The validity and reliability of the Portuguese versions of three tools used to diagnose delirium in critically ill patients. Clin (Sao Paulo) 66 (2011): 1917–22.
- Moreno RP, Nassar Jr AP. Is APACHE II a usefull tool for clinical research? Rev Bras Ter Intensiva 29 (2017): 264-267.
- Pedrão RAA, Riella RJ, Richards K, Valderramas S. Viability and validity of the bispectral index to measure sleep in patients in the intensive care unit. Rev Bras Ter Intensiva 32 (2020): 535–41.
- Bourne RS, Mills GH, Minelli C. Melatonin therapy to improve nocturnal sleep in critically ill patients: Encouraging results from a small randomised controlled trial. Crit Care 12 (2008): 1-9.
- Bilgili B, Montoya JC, Layon AJ, Berger AL, Kirchner HL, Gupta LK, et al. Utilizing Bi-Spectral Index (BIS) for the monitoring of sedated adult ICU patients: a systematic review. Minerva Anestesiol 83 (2017): 288-301.
- Giménez S, Romero S, Alonso JF, Mañanas M, Pujol A, Baxarias P, et al. Monitoring sleep depth: analysis of bispectral index (BIS) based on polysomnographic recordings and sleep deprivation. J Clin Monit Comput 31 (2017): 103–10.
- Audrey G. Gift, Gift AG. Visual analogue scales: measurement of subjective phenomena. Nurs Res 38 (1989): 286–8.
- Hasselberg MJ, McMahon J, Parker K. The validity, reliability, and utility of the iButton® for measurement of body temperature circadian rhythms in sleep/wake research. Sleep Med 14 (2013): 5–11.
- Biazim KS, Souza DA, Carraro Jr H, Richards K, Valderramas S. The Richards-Campbell Sleep Questionnaire and Sleep in the Intensive Care Unit Questionnaire: translation to Portuguese and cross-cultural adaptation for use in Brazil. J Bras Pneumol 46 (2020): 1-9.
- Batterham AM, Hopkins WG. Linear models and effect magnitudes for research, clinical and practical implications. Int J Sports Physiol Perform 1 (2010): 49–57.
- Oldham MA, Lee HB, Desan PH. Circadian rhythm disruption in the critically Ill: An opportunity for improving outcomes. Crit Care Med 44 (2016): 207–17.
- Bourne RS, Minelli C, Mills GH, Kandler R. Clinical review: Sleep measurement in critical care patients: research and clinical implications. Crit Care 11 (2007): 226.
- Airagnes G, Pelissolo A, Lavallée M, Flament M, Limosin F. Benzodiazepine Misuse in the Elderly: Risk Factors, Consequences, and Management. Curr Psychiatry Rep 18 (2016): 1-9.
- Darbyshire JL, Young JD. An investigation of sound levels on intensive care units with reference to the WHO guidelines. Crit Care 17 (2013): 1-8.
- Danielson SJ, Rappaport CA, Loher MK, Gehlbach BK. Looking for light in the din: An examination of the circadian-disrupting properties of a medical intensive care unit. Intensive Crit Care Nurs 1 (2018): 57–63.
- van de Pol I, van Iterson M, Maaskant J. Effect of nocturnal sound reduction on the incidence of delirium in intensive care unit patients: An interrupted time series analysis. Intensive Crit Care Nurs 41 (2017): 18–25.
- Craig T, Mathieu S. CANDLE: The critical analysis of the nocturnal distribution of light exposure – A prospective pilot study quantifying the nocturnal light intensity on a critical care unit. J Intensive Care Soc 19 (2018): 196–200.
- Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. Dose-response relationship for resetting of human circadian clock by light. Nature 379 (1996): 540.
- Wilson ME, Beesley S, Grow A, Rubin E, Hopkins RO, Hajizadeh N, et al. Humanizing the intensive care unit. Crit care 23 (2019): 1-3.
- Billings ME, Watson NF. Circadian Dysrhythmias in the Intensive Care Unit. Crit Care Clin 31 (2015): 393–402.
- Byun JH, Kim KT, Moon H jin, Motamedi GK, Cho YW. The first night effect during polysomnography, and patients’ estimates of sleep quality. Psychiatry Res 274 (2019): 27–9.
- Bridoux A, Thille AW, Quentin S, Lode-Kolz K, Stal V, Diaz V, et al. Sleep in ICU: atypical sleep or atypical electroencephalography? In: Crit Care Med (2014): 312-3.