The Role of Nutrient Supplementation to Prevent Perinatal Depression. A Narrative Review
Article Information
Hend Abdelbary Ibrahim Alya b, Karl J. New c
aDepartment of Family medicine, Primary Health Care corporation, Qatar.
bMSc Clinical Psychiatry, Faculty of Life Science & Education, University of South Wales.
cPhD Clinical Physiology & Nutrition, Faculty of Life Science & Education, University of South Wales.
*Corresponding Author: Hend Abdelbary Ibrahim Aly, Department of Family medicine, Primary Health Care corporation, Qatar.
MSc Clinical Psychiatry, Faculty of Life Science & Education, University of South Wales.
Received: 04 December 2023; Accepted: 13 December 2023; Published: 22 December 2023
Citation: Aly HAI and New KJ. The Role of Nutrient Supplementation to Prevent Perinatal Depression. A Narrative Review. Journal of Psychiatry and Psychiatric Disorders. 7 (2023): 228-247.
View / Download Pdf Share at FacebookAbstract
Background: Micronutrient supplements are widely available and can play a crucial role in the prevention of perinatal depression; however, their benefits are still not well studied.
Objectives: The authors performed this literature review to assess the effectiveness of micronutrients to prevent perinatal depression.
Method: The authors searched electronic databases (PubMed, Scopus, and USW Library) until May 1, 2023. The studies were included if they were evaluating the preventive effect of micronutrients on perinatal depression.
Results: The authors identified 3218 articles, of which 14 were eligible to be included in the review (8 RCTs, 3 Cohort studies, and 3 cross-sectional studies). Results of the review illustrate some degree of effectiveness of vitamin D supplementation, calcium, selenium, manganese, and probiotics to provide preventive effects against perinatal depression, while there is no evidence of a significant preventive effect for PUFAS (fish oil) supplementation.
Conclusion: Despite the popular information. PUFA supplements had no role in preventing perinatal depression. Other nutrients such as vitamin D, calcium, selenium, manganese, and probiotics showed some protective effects, which warranted more research in this area and preferably in women with a history of perinatal depression for a better understanding of their preventive effects.
Keywords
Nutrient; Micronutrients; Postnatal Depression; Perinatal Depression; Antenatal Depression
Nutrient articles; Micronutrients articles; Postnatal Depression articles; Perinatal Depression articles; Antenatal Depression articles
Nutrient articles Nutrient Research articles Nutrient review articles Nutrient PubMed articles Nutrient PubMed Central articles Nutrient 2023 articles Nutrient 2024 articles Nutrient Scopus articles Nutrient impact factor journals Nutrient Scopus journals Nutrient PubMed journals Nutrient medical journals Nutrient free journals Nutrient best journals Nutrient top journals Nutrient free medical journals Nutrient famous journals Nutrient Google Scholar indexed journals Micronutrients articles Micronutrients Research articles Micronutrients review articles Micronutrients PubMed articles Micronutrients PubMed Central articles Micronutrients 2023 articles Micronutrients 2024 articles Micronutrients Scopus articles Micronutrients impact factor journals Micronutrients Scopus journals Micronutrients PubMed journals Micronutrients medical journals Micronutrients free journals Micronutrients best journals Micronutrients top journals Micronutrients free medical journals Micronutrients famous journals Micronutrients Google Scholar indexed journals Postnatal Depression articles Postnatal Depression Research articles Postnatal Depression review articles Postnatal Depression PubMed articles Postnatal Depression PubMed Central articles Postnatal Depression 2023 articles Postnatal Depression 2024 articles Postnatal Depression Scopus articles Postnatal Depression impact factor journals Postnatal Depression Scopus journals Postnatal Depression PubMed journals Postnatal Depression medical journals Postnatal Depression free journals Postnatal Depression best journals Postnatal Depression top journals Postnatal Depression free medical journals Postnatal Depression famous journals Postnatal Depression Google Scholar indexed journals Perinatal Depression articles Perinatal Depression Research articles Perinatal Depression review articles Perinatal Depression PubMed articles Perinatal Depression PubMed Central articles Perinatal Depression 2023 articles Perinatal Depression 2024 articles Perinatal Depression Scopus articles Perinatal Depression impact factor journals Perinatal Depression Scopus journals Perinatal Depression PubMed journals Perinatal Depression medical journals Perinatal Depression free journals Perinatal Depression best journals Perinatal Depression top journals Perinatal Depression free medical journals Perinatal Depression famous journals Perinatal Depression Google Scholar indexed journals Antenatal Depression articles Antenatal Depression Research articles Antenatal Depression review articles Antenatal Depression PubMed articles Antenatal Depression PubMed Central articles Antenatal Depression 2023 articles Antenatal Depression 2024 articles Antenatal Depression Scopus articles Antenatal Depression impact factor journals Antenatal Depression Scopus journals Antenatal Depression PubMed journals Antenatal Depression medical journals Antenatal Depression free journals Antenatal Depression best journals Antenatal Depression top journals Antenatal Depression free medical journals Antenatal Depression famous journals Antenatal Depression Google Scholar indexed journals Postpartum depression articles Postpartum depression Research articles Postpartum depression review articles Postpartum depression PubMed articles Postpartum depression PubMed Central articles Postpartum depression 2023 articles Postpartum depression 2024 articles Postpartum depression Scopus articles Postpartum depression impact factor journals Postpartum depression Scopus journals Postpartum depression PubMed journals Postpartum depression medical journals Postpartum depression free journals Postpartum depression best journals Postpartum depression top journals Postpartum depression free medical journals Postpartum depression famous journals Postpartum depression Google Scholar indexed journals Self-Reporting Questionnaire articles Self-Reporting Questionnaire Research articles Self-Reporting Questionnaire review articles Self-Reporting Questionnaire PubMed articles Self-Reporting Questionnaire PubMed Central articles Self-Reporting Questionnaire 2023 articles Self-Reporting Questionnaire 2024 articles Self-Reporting Questionnaire Scopus articles Self-Reporting Questionnaire impact factor journals Self-Reporting Questionnaire Scopus journals Self-Reporting Questionnaire PubMed journals Self-Reporting Questionnaire medical journals Self-Reporting Questionnaire free journals Self-Reporting Questionnaire best journals Self-Reporting Questionnaire top journals Self-Reporting Questionnaire free medical journals Self-Reporting Questionnaire famous journals Self-Reporting Questionnaire Google Scholar indexed journals Critical Appraisal Skills Program articles Critical Appraisal Skills Program Research articles Critical Appraisal Skills Program review articles Critical Appraisal Skills Program PubMed articles Critical Appraisal Skills Program PubMed Central articles Critical Appraisal Skills Program 2023 articles Critical Appraisal Skills Program 2024 articles Critical Appraisal Skills Program Scopus articles Critical Appraisal Skills Program impact factor journals Critical Appraisal Skills Program Scopus journals Critical Appraisal Skills Program PubMed journals Critical Appraisal Skills Program medical journals Critical Appraisal Skills Program free journals Critical Appraisal Skills Program best journals Critical Appraisal Skills Program top journals Critical Appraisal Skills Program free medical journals Critical Appraisal Skills Program famous journals Critical Appraisal Skills Program Google Scholar indexed journals Randomized control trial articles Randomized control trial Research articles Randomized control trial review articles Randomized control trial PubMed articles Randomized control trial PubMed Central articles Randomized control trial 2023 articles Randomized control trial 2024 articles Randomized control trial Scopus articles Randomized control trial impact factor journals Randomized control trial Scopus journals Randomized control trial PubMed journals Randomized control trial medical journals Randomized control trial free journals Randomized control trial best journals Randomized control trial top journals Randomized control trial free medical journals Randomized control trial famous journals Randomized control trial Google Scholar indexed journals Diagnostic and Statistical Manual of Mental Disorders articles Diagnostic and Statistical Manual of Mental Disorders Research articles Diagnostic and Statistical Manual of Mental Disorders review articles Diagnostic and Statistical Manual of Mental Disorders PubMed articles Diagnostic and Statistical Manual of Mental Disorders PubMed Central articles Diagnostic and Statistical Manual of Mental Disorders 2023 articles Diagnostic and Statistical Manual of Mental Disorders 2024 articles Diagnostic and Statistical Manual of Mental Disorders Scopus articles Diagnostic and Statistical Manual of Mental Disorders impact factor journals Diagnostic and Statistical Manual of Mental Disorders Scopus journals Diagnostic and Statistical Manual of Mental Disorders PubMed journals Diagnostic and Statistical Manual of Mental Disorders medical journals Diagnostic and Statistical Manual of Mental Disorders free journals Diagnostic and Statistical Manual of Mental Disorders best journals Diagnostic and Statistical Manual of Mental Disorders top journals Diagnostic and Statistical Manual of Mental Disorders free medical journals Diagnostic and Statistical Manual of Mental Disorders famous journals Diagnostic and Statistical Manual of Mental Disorders Google Scholar indexed journals
Article Details
List of abbreviations
|
AD |
Antenatal depression |
|
Adj Model |
Adjusted model: statistical analysis adjusted for potential confounding factors. |
|
APrON |
Alberta Pregnancy Outcomes and Nutrition project |
|
CASP |
Critical Appraisal Skills Program |
|
CES-D |
Center for epidemiological studies for depression |
|
DHA |
Docosahexaenoic acid |
|
DHQ |
Diet history questionnaire |
|
DSM |
Diagnostic and Statistical Manual of Mental Disorders |
|
EPA |
Eicosapentaenoic acid |
|
EPDS |
Edinburgh postpartum depression score |
|
FA |
Folic acid |
|
FFQ |
Food frequency questionnaire |
|
FO |
Fish oil |
|
GEE |
Generalized Estimating Equations |
|
ICD |
International classification of diseases |
|
IFA |
Iron and folic acid |
|
K6 |
Kessler Psychological Distress Scale (K6) self-administered questionnaires |
|
LME |
linear mixed effect |
|
LNS |
lipid-based nutrient supplements |
|
MM |
Micronutrient |
|
MMN |
Multiple micronutrient supplements |
|
N/A |
Not applicable |
|
PPD |
Postpartum depression |
|
PRISMA |
Preferred Reporting Items for Systematic reviews and Meta-Analyses |
|
RCT |
Randomized control trial |
|
SRQ |
Self-Reporting Questionnaire |
1. Introduction
Perinatal or maternal depression refers to the occurrence of depressive symptoms during pregnancy and within the first year following childbirth. According to several studies [1, 2, 3], maternal depression has been identified as the primary contributor to disease-related disability in women. According to [4], the rates of antenatal depression (AD) and postpartum depression (PPD) are 18% and 19%, respectively.
The frequency of prenatal depression is similar among different racial groups, levels of parity, age groups, educational backgrounds, and socioeconomic statuses [3]. However, the specific cause of perinatal depression has not been definitively determined [5, 6]. Moreover, the ability to predict the most effective strategies for preventing prenatal depression or identify the specific mothers who will experience depression remains elusive. Nevertheless, a multitude of psychological and social risk factors have previously been discovered. Previous studies have conducted meta-analyses and discovered a total of 15 risk factors. The factors that have been identified as potential contributors to postpartum depression include: (1) belonging to a lower social class; (2) experiencing life stressors during pregnancy; (3) having a complicated pregnancy or birth; (4) having a difficult relationship with family or partner; (5) lacking support from family and friends; (6) having a prior history of psychopathology such as depression or anxiety; (7) facing chronic stressors in the postpartum period, including issues related to child care and dealing with a difficult infant temperament; (8) being unemployed or experiencing instability in employment; (9) experiencing an unplanned pregnancy; and (10) feeling ambivalent about becoming pregnant. Depression during pregnancy has been identified as the most robust predictor [5, 7, 8]. Additional factors that can serve as predictors include a strained maternal bond, a prior experience of sexual abuse, the lack of a trusted confidant, and the practice of bottle feeding. Inflammatory indicators have been the subject of investigation in recent studies exploring biological risk factors [9]. The impact of postpartum depression on the development of newborns and mother-child relationships has been well documented in previous research [10, 11]. An example of this can be seen in a recent meta-analysis conducted by [12], which found a statistically significant correlation between maternal depression and several childhood illnesses. These diseases encompassed internalizing and externalizing psychopathology, as well as negative and positive emotionality. According to [11] meta-analysis, there is evidence of a modest yet statistically significant influence of postpartum depression on the cognitive and emotional development of children. According to [13], several studies indicate that the long-term effects of maternal depression, whether chronic or recurrent, may be more harmful compared to the effects of postpartum depression alone. [14] suggest that there exists a correlation between AD and PPD and various adverse effects on children's cognitive development and behavioral outcomes, as well as their mental and physical health. Despite the existence of various theories, including biological, psychological, and environmental perspectives, the underlying mechanisms responsible for depression remain elusive, and it is probable that multiple factors are involved [15]. Research conducted by [16], [17], and [18] suggests that dietary factors exhibit a favorable impact on depression symptoms experienced during pregnancy. Previous studies [19] have mostly examined the overall patterns and factors influencing maternal depression, while [20] have undertaken a restricted investigation of the association between maternal depression and diet. The purpose of this review is to examine the reciprocal association between depression and nutritional intake in pregnant and postpartum women, aiming to fill the existing gap in information on this topic. According to a study conducted by [21], there is evidence to suggest that nutrition could potentially exert a biological influence on the occurrence of depression. The inclusion of a variety of nutrients is essential for the process of synthesizing and regulating the neurotransmission system, potentially influencing the control of mood (Rechenberg and Humphries, 2013) [22]. There is a limited amount of available information pertaining to therapies that have the potential to prevent or treat perinatal depression. In certain women, the presence of inadequate levels of specific vitamins, minerals, or other essential nutrients may potentially contribute to the occurrence of postpartum depression. Hence, the utilization of dietary supplements to address this deficiency may potentially serve as a preventive measure against postpartum depression. Several potential dietary supplements have been identified for the prevention of postpartum depression. These include omega-3 fatty acids, iron, folate, s-adenosyl-L-methionine, vitamins B12 (cobalamin), B6 (pyridoxine), B2 (riboflavin), vitamin D, and calcium. According to [23]. Therefore, the aim of this literature review is to evaluate the significance of nutrient supplementation in the prevention of prenatal depression.
2. Methods
2.1 General methodology
Ethical approval
The Ethical Committee of the University of South Wales granted low-risk ethical approval in April 2023, by the Faculty of Life Science and Education at the University of South Wales.
2.2 Search strategy
A comprehensive and methodical exploration of electronic databases, including PubMed, Scopus, and USW Library, was conducted up until May 1, 2023. The author employed Mesh terms and free text keywords in their study. Two distinct steps were employed in conducting the database searches: The initial stage encompassed the examination of diet, nutrition, dietary intake, food intake, dietary supplement, micronutrient, and their association with perinatal depression, antenatal depression, prenatal depression, postnatal depression, postpartum depression, maternal depression, and maternal mental health. The second phase of the study involved the integration of dietary terminology with terms related to depression and pregnancy. Specifically, the search terms included variations of dietary terms such as "diet," "nutrition," "food," "dietary intake," "food intake," "dietary supplement," and "micronutrient." Additionally, terms related to pregnancy such as "pregnancy," "pregnant," "mothers," "maternal," "antenatal," "prenatal," "perinatal," "postnatal," and "postpartum" were included. Finally, terms associated with depression such as "depression," "depress," "mood disorder," and "depressive disorder" were incorporated into the search criteria.
2.3 Inclusion criteria
- Peer-reviewed analytical studies.
- Original research published within the last 10 to 20 years in English; and they employed a quantitative research design to examine the relationship between perinatal depression and dietary intake.
- Research assessed depression using a researcher- or clinician-administered or self-report validated depression screening instrument or clinical diagnosis by a specialist, administered to women in the antenatal or postnatal period, in accordance with the international classification of diseases (ICD) or Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria.
- Research evaluated specific dietary patterns or nutrient intake via food or supplements using validated food frequency, food recalls, eating habits and behavior questionnaires administered to women in the antenatal or postnatal period, if they used validated food frequency questionnaires (FFQs) or supplement history questionnaires, assessed outcome during pregnancy or within 12 months postpartum.
2.4 Exclusion criteria
- Research measured food insecurity, hormone levels, chemical analysis of nutrients, or used nutritional biomarkers.
- Studies in which the participants were already diagnosed with depression or had co-morbidities like diabetes, cardiac conditions, etc.
- Letters to editors, abstracts, and conference papers, and studies with non-analytical designs such as case reports and series.
- Studies using non-specific intake questions to measure health behaviors, such as whether a woman took dietary supplements but without measuring the quantity or type of supplement.
The guidelines and checklists from PRISMA were followed as shown in the following figure: PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses), [24].
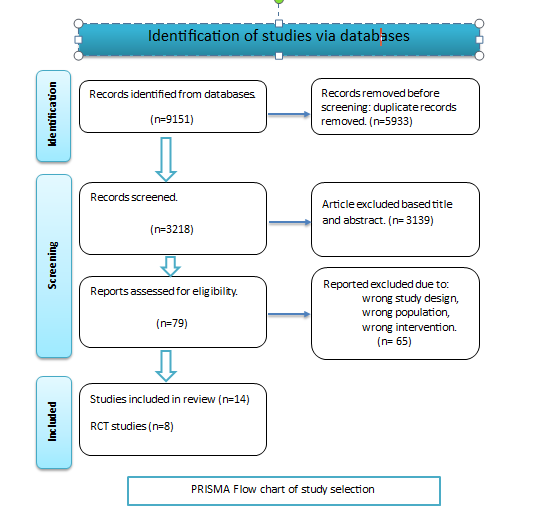
2.5 Article review and data extraction
The characteristics and overall results of all included studies were extracted using a standard data table containing author name, year, country, study type, intervention period, intervention type, control, assessment instruments used, assessment frequency, adjustment model used, and study results. The included studies critically analyzed using the Critical Appraisal Skills Program (CASP) tool to determine the quality and the risk of bias. Data is represented in the appendix table 1.
3. Results
The initial database search yielded a total of 9151 papers that were possibly useful in examining the impact of nutritional supplements on the prevention of prenatal depression. Following the removal of duplicate articles, a total of 3218 unique articles remained for further analysis. After excluding a total of 3,139 articles based on their titles and abstracts, the author proceeded to evaluate the remaining 79 articles for eligibility. Out of these, 14 studies were identified as meeting the eligibility criteria. The author then attempted to exclude studies that utilized chemical biomarkers, as outlined in the study protocol. However, this proved challenging due to the limited availability of research on perinatal depression using micronutrient supplementation. Consequently, the author decided to include a few articles in this review where biochemical assessment was conducted only once or twice during the study, at most. The collection of papers comprises a total of 14 investigations, encompassing 8 randomized control trials, 3 cohort studies, and 3 cross-sectional studies. Each study investigated the impact of a specific vitamin on mitigating the risk of perinatal depression. The preventative benefit of vitamin D supplementation for prenatal depression was revealed in two separate investigations. Using a randomized control trial design, [25] conducted one study in Iran. The second study, carried out in Japan by [26], used a cohort study design. The existing body of research, which includes five studies, encompasses four randomized controlled trials (Sousa and Santos, 2022) [27, 28, 29] and a cohort study conducted by [30]. Collectively, these studies do not provide substantial evidence supporting the notion that fish oil supplementation has a major preventive effect. The study conducted by [28] found that women who had a previous history of depression and ingested fish oil experienced a notable reduction in EDPS. Two cross-sectional studies have been conducted on the topic of calcium and dairy product consumption. According to a study by [31], there is a significant correlation between increased yogurt and calcium consumption and a decrease in the occurrence of depressive symptoms during pregnancy. Conversely, a separate study by [32] did not find any evidence to suggest that dairy supplements taken after childbirth had an impact on postpartum depression (PPD). This review investigates the potential preventative impact of multivitamin supplementation during pregnancy through the analysis of four separate studies. According to a study by [33], folic acid supplementation may have a positive preventive effect for women who are at risk of developing depression. [34], on the other hand, found that adding lipid-based nutrient supplements (LNS) to a mother's diet did not reduce postpartum depression as much as other treatments, like multiple micronutrient supplements (MMN) or iron and folic acid (IFA). [18] conducted a cohort study in 2013 and identified selenium supplementation as having a preventive role in postpartum depression (PPD). Additionally, [35] found that there was an independent inverse association between manganese intake and depressive symptoms during pregnancy.
The likelihood of women in the probiotic therapy group exceeding the threshold for anxiety levels was significantly lower than it was for those in the placebo treatment group, according to the study by [36].
Table: Summary of the included studies.
The characteristics and overall results, table No.1
Adjusted model: statistical analysis adjusted for potential confounding factors., EPDS: Edinburgh postpartum depression score, N/A: not applicable, CES-D: center for epidemiological studies for depression, DHQ: diet history questionnaire, FA: folic acid, FFQ: food frequency questionnaire, FO: fish oil, IFA: Iron folic acid, Kessler Psychological Distress Scale (K6) self-administered questionnaires, MM: micronutrient, MMN: multiple micronutrients, RCT: randomized control trials, SRQ: self-Reporting Questionnaire.
4. Results of individual studies:
1. In Iran, Vaziri conducted a randomized control experiment to evaluate the impact of Vitamin D3 supplementation on prenatal depression ratings. The study population consisted of pregnant women who received prenatal treatment at a teaching hospital located in Shiraz, Iran. The inclusion criteria for this study were as follows: Participants had to be 18 years of age or older, with no previous history of mental illness or internal disorders. Additionally, they were required to have a singleton living fetus without any pregnancy difficulties and a gestational age ranging from 26 to 28 weeks at the time of enrollment. Finally, participants were included if they had a depression score within the range of 0 to 13. The assessment technique utilized in the study was the Edinburgh Postnatal Depression Scale (EPDS). A cohort of 169 individuals was allocated to two distinct groups, namely the placebo group and the vitamin D group, utilizing a block randomization design. The participants in the vitamin D group were administered a daily dosage of 2000 IU of vitamin D3 from the 26th to the 28th week of gestation until the time of delivery. The concentrations of 25-hydroxyvitamin D in the maternal serum were assessed both at the beginning of the study and at the time of childbirth. In addition, assessments of depression scores were conducted on four occasions: throughout the gestational period at 26-28 weeks and 38-40 weeks, as well as at 4 and 8 weeks postpartum.
Vaziri et al. discovered that the two groups exhibited comparable baseline amounts of 25-hydroxyvitamin D. Nevertheless, during the process of birthing, the group that received vitamin D exhibited a notably elevated concentration of 25-hydroxyvitamin D compared to the control group (p < 0.001). Initially, there was no discernible association between the concentration of 25-hydroxyvitamin D and the score for depression (r = 0.13, p = 0.09). No statistically significant difference was seen between the two study groups with respect to the initial depression score. The results of the study indicate that the group receiving vitamin D experienced a significantly higher decrease in depression scores compared to the control group at 38-40 weeks of gestation (p = 0.01), as well as at 4 and 8 weeks postpartum (p < 0.001). The researchers reached the conclusion that the daily consumption of 2000 IU of vitamin D3 during the later stages of pregnancy exhibited efficacy in reducing levels of perinatal depression. and propose conducting additional clinical trials with pregnant women who are at a heightened risk of developing postnatal depression.
2. In their study conducted in 2023, Tsunoda et al. investigated the correlation between maternal vitamin D intake during pregnancy and the occurrence of postpartum depression symptoms. This study investigated the correlation between variables in a substantial cohort of 74,840 pregnant women who were participants in the longitudinal Japan Environment and Children's Study. The assessment of maternal vitamin D consumption during pregnancy, specifically from the time of pregnancy awareness to the later stages of pregnancy, was conducted through the utilization of the Food Frequency Questionnaire. The Edinburgh Postnatal Depression Scale was utilized to evaluate symptoms of postpartum depression one month following childbirth. The logistic regression analysis revealed a decreased likelihood of experiencing postpartum depressive symptoms for all quintiles of vitamin D intake, except for the first quintile. The adjusted odds ratios (with corresponding 95% confidence intervals) for the second, third, fourth, and fifth quintiles were 0.88 (0.82-0.94), 0.83 (0.78-0.89), 0.87 (0.81-0.93), and 0.90 (0.83-0.97), respectively. The results of the post-adjustment trend tests indicate a statistically significant correlation between the consumption of dietary vitamin D and the occurrence of postpartum depressive symptoms (p for trend = 0.004). The findings of their study indicate that there is a correlation between increased vitamin D intake during pregnancy and a decreased likelihood of experiencing postpartum depressive symptoms one month after giving birth. These results suggest that vitamin D may have potential implications for the prevention or reduction of postpartum depression.
3. Hulkkonen et al. (2021) examined in their study the effectiveness of probiotics and/or fish oil (FO) in altering symptoms of depression and anxiety during the prenatal and postnatal periods. The study aimed to identify the trajectories of symptoms and assess the impact of lifestyle factors on these symptoms. A total of 439 overweight women were randomly assigned to different intervention groups (probiotics+FO, probiotics+placebo, FO+placebo, placebo+placebo) during early pregnancy and followed up until six months after giving birth. The participants were evaluated for symptoms of depression and anxiety using the Edinburgh Postnatal Depression Scale (EPDS) and the Anxiety subscale of the Symptoms Checklist (SCL-90) at early and late pregnancy, as well as three, six, and 12 months postpartum. The application of latent growth mixture modeling was employed to effectively model the trajectories of symptoms. The evaluation of dietary quality and physical activity was conducted using recognized indices. The findings indicate that symptom scores were generally at a modest level. A statistically significant intervention effect was observed during pregnancy (p = 0.017). In the group receiving fish oil and probiotics, EPDS scores increased by 1.11 points, while in the group receiving fish oil and placebo, EPDS scores declined by 0.85 points. The group receiving the combination of FO and placebo demonstrated significantly lower scores on the Edinburgh Postnatal Depression Scale (EPDS) compared to the group receiving probiotics and placebo at 12 months postpartum (p = 0.039). There were no observed variations in SCL scores as a result of the intervention. Regardless of the type of intervention, the study discovered three distinct trajectories of depression symptoms and two trajectories of anxious symptoms. There exists a negative correlation between dietary quality and depressive symptoms throughout the early stages of pregnancy as well as six months after childbirth. Additionally, a negative correlation is shown between dietary quality and anxiety symptoms during the early stages of pregnancy. Symptoms were shown to be associated with perinatal events, including colic as reported by the mother. limitations of their study Secondary outcomes pertaining to the primary trial Findings: The results indicate that the intervention had a limited effect on reducing depression symptoms. There was a correlation observed between dietary patterns, obstetric events, and the manifestation of symptoms related to depression and anxiety.
4. In Rio de Janeiro, Brazil, Vaz et al. conducted cohort research as part of which they designed and carried out a randomized, placebo-controlled, double-blind experiment in 2017. A total of sixty pregnant women who were deemed to be at risk for postpartum depression (PPD) were selected and assigned at random to either take fish oil capsules containing 1.8 g (1.08 g of Eicosapentaenoic (EPA) and 0.72 g of Docosapentaenoic (DHA) acids) or a placebo (control). The scoring of the Edinburgh Postnatal Depression Scale (EPDS) was conducted at four different time points: baseline (T0) at 5-13 weeks, during the gestation period at 22-24 weeks (T1) and 30-32 weeks (T2), and finally, 4-6 weeks after childbirth (T3). The administration of supplements commenced during the gestational period between weeks 22 and 24, referred to as T1, and continued for a duration of 16 weeks. The compliance of individuals was assessed by assaying serum fatty acids. The primary goal of the study was to determine the prevalence of EPDS scores greater than or equal to 11. The secondary outcomes included the mean and changes in EPDS scores, length of gestation, and birth weight. The study employed linear mixed-effect (LME) and random-intercept logistic regression models to examine the impact of fish oil supplementation on the prevalence of EPDS scores equal to or greater than 11, as well as the variation in EPDS scores. Results: In the context of intention-to-treat (ITT) analysis, it was shown that women in the fish oil group had elevated serum concentrations of EPA and DHA, as well as a reduced n-6/n-3 ratio, in comparison to the control group at 30-32 weeks of gestation. No significant disparities were observed between the intervention and control groups in terms of the occurrence of EPDS ≥11, EPDS scores across time, or changes in EPDS scores from pregnancy to postpartum in both the intention-to-treat (ITT) and per-protocol analyses. In the fish oil group, women with a prior history of depression had a greater decrease in EPDS scores from the second to the third trimester compared to the control group in the ITT analysis (−1.0 (−3.0-0.0) vs. -0.0 (−1.0-3.0), P = 0.038). The results obtained from the LME model provided confirmation (β = −3.441; 95%CI: -6.532- -0.350, P = 0.029). In conclusion, the administration of a daily dosage of 1.8 g of n-3 PUFAs over a period of 16 weeks did not have a preventive effect on maternal depressive symptoms within a cohort of Brazilian women.
5. The goal of a study by Farshbaf-Khalili et al. (2016) was to evaluate the effects of fish oil supplementation on prenatal and postpartum depression scores. The study design employed in their research was a randomized, triple-blind, placebo-controlled experiment. A total of 150 pregnant women meeting the eligibility criteria, namely having an Edinburgh Postnatal Depression Scale (EPDS) score below 20 and falling between the age range of 18 and 35, were included in the study conducted in Tabriz, Iran, between February 2014 and April 2015. The participants were randomized in a random manner to receive either 1000 mg of fish oil supplements or a placebo, starting at 16 weeks of gestation and continuing until one month after delivery. The EPDS was administered to participants at four time points: baseline, 26-30 weeks, 35-37 weeks, and 30-45 days postpartum. The primary end measures included the average depression score over the time intervals of 26-30 weeks, 35-37 weeks, and the postpartum period. The statistical study employed an intent-to-treat approach. The study had a cohort of 150 female participants, all of whom completed the study without dropping out. There were notable disparities observed between the two cohorts in terms of the average depression score, specifically during the time period of 35-37. The adjusted mean difference was calculated to be -1.4, with a 95% confidence interval ranging from -2.6 to -0.25. The fish oil group exhibited a statistically significant decrease in the mean score of depression during pregnancy and the postpartum period (P < 0.05). No statistically significant changes were seen between the two groups in relation to the initial serum levels of docosahexaenoic acid (DHA) and eicosapentaneoic acid (EPA). Conclusions: The administration of fish oil supplements resulted in a statistically significant reduction in the average score of the Edinburgh Postnatal Depression Scale (EPDS) during the time period of weeks 35 to 37. There is evidence to suggest that women may have potential advantages from the regular consumption of fish oil supplements during pregnancy, particularly in regions where fish consumption is limited. However, additional research is required to validate these findings.
6. In the year 2022, Sousa and Santos conducted a study. A randomized double-blind placebo-controlled trial was undertaken, involving a cohort of sixty pregnant women, with the aim of assessing the impact of antenatal omega-3 supplementation on maternal depressive symptoms during the period spanning from pregnancy to 6 months after childbirth. The study involved participants with gestational ages ranging from 22 to 24 weeks. They were randomly assigned to either the placebo group (olive oil; n = 30) or the omega-3 group (fish oil; n = 30). The participants received supplementation until the time of delivery. The fish oil capsules administered a daily dosage of 1440 mg of docosahexaenoic acid (DHA). The assessment of depressive symptoms took place at several stages of pregnancy, namely: (G1) between 22 and 24 weeks; (G2) between 26 and 28 weeks; (G3) between 30 and 32 weeks; and (G4) between 34 and 36 weeks. Additionally, postpartum evaluations were conducted during the (P1) second week, (P2) first month, (P3) fourth month, and (P4) sixth month, utilizing the Edinburgh Postnatal Depression Scale (EPDS). Generalized Estimating Equations (GEE) were utilized to conduct intention-to-treat and per-protocol analyses. The results of the study indicate that there were no significant variations in the EPDS score between the groups at any point throughout pregnancy or postpartum. This finding was consistent across both the intention-to-treat and per-protocol analyses, with p-values greater than 0.05. Both groups demonstrated a decrease in the EPDS score over the course of the study. Based on an intention-to-treat analysis, it was observed that the placebo group exhibited a decrease in the EPDS score at P1 and P4 as compared to the initial measurement. Conversely, the omega-3 group demonstrated a reduction in the EPDS score at all time points from G4 to P4. In the context of per-protocol analysis, it was observed that the placebo group exhibited a decrease in the EPDS score specifically at P3, whereas the omega-3 group demonstrated a drop in the EPDS score consistently throughout all time points from G2 to P4. Findings: The administration of omega-3 supplements did not result in noteworthy distinctions among the experimental groups. Nevertheless, the omega-3 group exhibited an earlier decrease in the EPDS score, suggesting a potential advantage of prenatal omega-3 supplementation.
7. Hamazaki et al. did a study in 2018 to see if there was a link between eating fish and n-3 polyunsaturated fatty acids (PUFAs) in the diet and the likelihood of experiencing psychological distress during pregnancy for both mothers and fathers, as well as the likelihood of postpartum depression for mothers in Japan. This study utilized a dataset consisting of 104,102 maternal registrations and 52,426 paternal registrations from The Japan Environment and Children's Study. The complete data from questionnaires were analyzed for 75,139, 79,346, and 77,661 women during early pregnancy, mid-pregnancy, late pregnancy, and post-pregnancy, respectively. Additionally, data from 41,506 male partners were included in the analysis. The results of the multivariable logistic regression analysis indicate a decreased likelihood of experiencing psychological distress during early pregnancy in the second and third quintiles of fish intake. Similarly, during mid-late pregnancy, a lower risk of psychological distress was observed in those with fish intake falling within the second to fifth quintiles. There were no observed changes in n-3 PUFA intake during early pregnancy; however, reductions were observed in the second to fourth quintile throughout mid-late pregnancy. The study findings indicate that there were reductions in postpartum depression rates among those with a moderate to high fish diet, namely in the second to fourth quintile. However, these reductions were only detected in the first quintile for n-3 PUFA intake. Regarding fathers’ psychological distress, it was shown that only the fourth percentile for fish intake exhibited a statistically significant association. In summary, the consumption of fish and n-3 polyunsaturated fatty acids (PUFA) exhibited a certain level of correlation with a decreased likelihood of experiencing psychological distress among women in the later stages of pregnancy. However, this link was not as pronounced in their male partners throughout the same period. The correlations exhibited a lesser degree of strength in relation to the consumption of n-3 PUFA compared to the consumption of fish. They recommend additional research, particularly interventional studies, be conducted to validate the results obtained in their study.
8. Miyake et al. (2014) investigate the correlation between the consumption of dairy products and calcium and the prevalence of depression symptoms in pregnant women. Design A cross-sectional study is a research design that involves the collection of data from a population at a certain point in time, with the study being conducted is known as the Kyushu-Okinawa Maternal and Child Health Study (KOMCHS). Sample A group consisting of 1745 pregnant women from Japan. The evaluation of dietary consumption during the previous month was conducted through the utilization of a self-administered food history questionnaire. Scores of 16 or above on the Center for Epidemiologic Studies, The Depression Scale is used to assess and measure the presence of depressive symptoms. Covariates included in the analysis were age, gestation, area of residence, number of children, family structure, history of depression, family history of depression, smoking status, exposure to secondhand smoke at home and at work, job type, household income, education level, and body mass index. In their comprehensive examination of dairy products as a whole, they accounted for fish consumption as a confounding factor. Similarly, when investigating the relationship between calcium intake and other variables, they adjusted for the consumption of saturated fatty acids, eicosapentaenoic acid, docosahexaenoic acid, and vitamin D. The primary measure of interest was the occurrence of depressive symptoms during pregnancy. Results There was a significant association between increased consumption of yogurt and calcium and a reduced occurrence of depressive symptoms during pregnancy. The adjusted odds ratios for extreme quartiles were 0.69 (95% CI 0.48-0.99, P for trend = 0.03) and 0.59 (95% CI 0.40-0.88, P for trend = 0.006), respectively. There was no discernible correlation found between the use of various dairy products, such as milk or cheese, and the manifestation of depression symptoms in pregnant individuals. The findings of the study indicate a potential correlation between increased consumption of yogurt and calcium and a reduced occurrence of depression symptoms in pregnant individuals.
9. Alashmali et al. (2022) assessed the impact of dairy product consumption and nutrient intake following childbirth on the likelihood of postpartum depression (PPD). A study was undertaken utilizing a cross-sectional survey design, wherein participants were requested to complete a food frequency questionnaire (FFQ) in order to evaluate their consumption of dairy products and other essential nutrients. The Edinburgh Postnatal Depression Scale (EPDS) was employed as a tool for the assessment of symptoms related to postpartum depression (PPD). Among the total sample size of 530 participants, a substantial majority of individuals, specifically 395 participants (representing 74.11% of the sample), self-reported experiencing postpartum depression (PPD). The prevalence of postpartum depression (PPD) was shown to be significantly elevated among individuals who consumed dairy products and other nutrients at a Q1 level. Furthermore, a positive correlation was observed between the risk of PPD and the increased intake of these items from Q2 to Q4. Nevertheless, while controlling for any confounding variables, the analysis revealed no statistically significant correlation between the consumption of dairy products and other essential nutrients during the postpartum period and the occurrence of postpartum depression (PPD). The findings suggest that there is little potential for dairy products and nutritional intake to effectively mitigate postpartum depression (PPD). Recommended that additional longitudinal and intervention studies are necessary to provide conclusive findings regarding the potential associations between dairy products, as well as other nutrients (especially anti-depressants), and the risk of postpartum depression (PPD).
10. The study conducted by Nguyen et al. (2017) investigated the impact of pre-conceptional micronutrient supplementation on maternal depressive symptoms (MDS) during pregnancy and the postpartum period. Methods: The data utilized in this study was obtained from a double-blind controlled trial known as PRECONCEPT. The trial had a total of 5011 Vietnamese women who were randomly assigned to one of three groups. The first group received weekly supplements containing various micronutrients (MM), the second group received supplements including iron and folic acid (IFA), and the third group received supplements containing only folic acid (FA). The study focused specifically on the subgroup of women (n = 1813) who continued taking the assigned supplements until conception. The assessment of maternal mental health involved the utilization of two standardized scales: the Center for Epidemiologic Studies Depression Scale (CES-D) was administered prior to conception as a baseline measure, while the Edinburgh Postnatal Depression Scale (EPDS) was employed throughout pregnancy and again at the 3-month postpartum period. Elevated maternal depressive symptoms (MDS) were operationally defined as an Edinburgh Postnatal Depression Scale (EPDS) score equal to or greater than 4.
The group comparisons were conducted utilizing ANOVA or chi-square testing of proportions for both intention-to-treat and per-protocol analyses. It is important to note that in the per-protocol analysis, only women who ingested supplements for at least 26 weeks before conception were included. In addition, they performed stratified analyses using generalized linear models to examine the relationship between preconception CES-D scores and underweight or anemic status. The results indicate that the baseline CES-D scores did not significantly differ among the treatment groups. The prevalence of women reporting high MDS throughout pregnancy varied across trimesters, with proportions of 11.3%, 8.1%, and 4.9% observed during the first, second, and third trimesters, respectively. Additionally, the proportion decreased to 3.6% at 3 months postpartum. The mean scores on the Edinburgh Postnatal Depression Scale (EPDS) throughout the first (1.5 ± 2.7), second (1.1 ± 2.4), and third trimester of pregnancy (0.7 ± 2.0), as well as during the early postpartum period (0.6 ± 1.8), were found to be low and exhibited no significant differences between the treatment groups. Nevertheless, in the subgroup of women with the highest tertile of CES-D scores prior to conception, the average EPDS scores during the first and second trimesters of pregnancy were shown to be lower in the groups receiving MM and IFA supplements compared to the group receiving only FA supplements (P < 0.05). they concluded that the administration of weekly pre-conceptional micronutrient supplements, namely those containing iron, did not provide significant improvements in depression scores when compared to the use of folic acid alone among a diverse population of women. However, it is worth noting that there may be potential benefits for women who were identified as being at risk for depression.
11. In a randomized-controlled trial conducted in rural Malawi, Stewart et al. (2016) aimed to examine the hypothesis that the consumption of a lipid-based nutrient supplement (LNS) rich in fatty acids by women would result in a lower occurrence of depressive symptoms after childbirth compared to those taking iron-folate (IFA) or multiple-micronutrient (MMN) capsules. Female participants were selected from antenatal clinics and assigned randomly to take either lipid-based nutrient supplements (LNS) or multiple micronutrient powders (MMN) throughout their pregnancy and for a duration of 6 months after childbirth. Alternatively, some participants were assigned to receive iron and folic acid (IFA) supplements only during pregnancy. The assessment of maternal depressive symptoms was conducted by employing validated translations of two standardized instruments, namely the Self-Reporting Questionnaire (SRQ) and the Edinburgh Postnatal Depression Scale (EPDS). The measurements were taken during two distinct time periods: antenatally, where just the SRQ was utilized, and at 6 months postpartum, where both the SRQ and EPDS were administered. The analysis was conducted using a modified intention-to-treat approach. A total of 1,391 women were randomly assigned to three groups: low-nutrient supplementation (LNS) with 462 participants, multiple micronutrient supplementation (MMN) with 466 participants, and iron and folic acid supplementation (IFA) with 463 participants. The groups exhibited similarities in certain baseline factors. At the six-month mark after giving birth, a total of 1078 individuals (representing 77.5% of the sample) completed the SRQ assessment. The average scores, along with their standard deviations, were as follows: LNS 1.76 (SD = 2.73), MMN 1.92 (SD = 2.75), and IFA 1.71 (SD = 2.66). The statistical analysis revealed that there was no significant difference between the groups, as shown by a p-value of 0.541. A total of 1,057 participants (76.0%) completed the Edinburgh Postnatal Depression Scale (EPDS). The mean (standard deviation) scores for the EPDS subscales were as follows: LNS 5.77 (5.53), MMN 5.43 (4.97), and IFA 5.52 (5.18). The p-value for the comparison of these scores was 0.676. No statistically significant differences were found between the groups in terms of SRQ or EPDS scores, whether they were analyzed as continuous variables or dichotomized variables, in both unadjusted and adjusted models. In summary, the addition of lipid-based nutrient supplements (LNS) to the maternal diet, as compared to multiple micronutrient supplements (MMN) or iron and folic acid (IFA) supplements, did not result in a reduction in postnatal depressive symptoms in the context of this study.
12. The cross-sectional study conducted by Miyake et al. (2017) in Japan aimed to investigate the potential correlation between the consumption of zinc, magnesium, iron, copper, and manganese and the manifestation of depressive symptoms in pregnant women. A total of 1745 pregnant women were included in the study as subjects. The evaluation of dietary consumption during the previous month was conducted through the utilization of a self-administered food history questionnaire. Depressive symptoms were operationally defined in this study as a score equal to or greater than 16 on the Center for Epidemiologic Studies Depression Scale. Covariate adjustments were performed for various factors including age, gestation, region of residence, number of children, family structure, history of depression, family history of depression, smoking, exposure to secondhand smoke at home and at work, employment status, household income, education level, body mass index, and intake of saturated fatty acids, eicosapentaenoic acid plus docosahexaenoic acid, calcium, vitamin D, and isoflavones. The initial analysis revealed noteworthy negative correlations between the consumption of zinc, magnesium, iron, copper, and manganese and the occurrence of depression symptoms in pregnant individuals. Following the control of confounding variables, it was shown that only manganese intake exhibited an independent and inverse correlation with depression symptoms experienced during pregnancy. The adjusted prevalence ratio between the highest and lowest quartiles was determined to be 0.74, with a 95% confidence range ranging from 0.56 to 0.97. Additionally, a statistically significant trend was seen with a p-value of 0.046. The present investigation, which focused on a sample of Japanese women, revealed a significant correlation between increased manganese consumption and a reduced occurrence of depression symptoms in pregnant individuals.
13. The study conducted by Leung et al. (2013) aimed to examine the relationship between prenatal micronutrient supplementation and the likelihood of experiencing symptoms of postpartum depression. This investigation was carried out using a longitudinal pregnancy cohort derived from the Alberta Pregnancy Outcomes and Nutrition (APrON) project. The participants were selected from a cohort consisting of the initial 600 women enrolled in the Alberta Pregnancy Outcomes and Nutrition (APrON) study. Data on the consumption of additional nutrients and the manifestation of depressive symptoms, as assessed by the Edinburgh Postnatal Depression Scale (EPDS), were gathered during each trimester of pregnancy and at the 12-week mark after childbirth. The findings indicate that out of the 475 individuals who participated in the study and completed the Edinburgh Postnatal Depression Scale (EPDS) at least twice during pregnancy and at 12 weeks postpartum, 416 individuals (88%) scored below the threshold for depression. The results also suggest that prenatal supplemental selenium, with an increase of 10 mcg, was associated with a decrease in the odds ratio (OR) of depression (OR = 0.76, 95% confidence interval [CI] = 0.74 - 0.78, p = 0.0019). Additionally, postnatal social support was found to be protective against depression (OR = 0.87, 95% CI = 0.78 - 0.97, p = 0.0015). Findings: The probability of experiencing postpartum depressive symptoms is influenced by various factors, one of which is the consumption of more selenium.
14. Slykerman et al. (2017) did a study to see how giving Lactobacillus rhamnosus HN001 (HN001) during pregnancy and after birth affected the symptoms of anxiety and depression in the mother during the postpartum period. The secondary result under consideration was the occurrence of eczema in the children at 12 months of age, with the primary outcome being the focus of the study. A randomized, double-blind, placebo-controlled experiment was done to investigate the impact of HN001 on postnatal mood. The study involved a total of 423 women, and the gestational age of the fetus was between 14 and 16 weeks. residing in Auckland , Wellington and New Zealand.
Female participants were assigned randomly to receive either a placebo or HN001 daily, starting from the time of enrollment and continuing for a duration of 6 months postpartum. The assessment of postpartum symptoms of depression and anxiety was conducted using modified versions of the Edinburgh Postnatal Depression Scale and State Trait Anxiety Inventory. After randomization, a total of 212 women were subjected to the administration of HN001, and 211 were in a placebo-controlled group. A total of 380 female participants, accounting for 89.8% of the sample, successfully responded to the questionnaire pertaining to psychological effects. Additionally, 193 In the treatment group, 91.0% of participants exhibited the desired outcome, while in the placebo group, 88.6% of participants showed the same outcome. Mothers who were assigned to the probiotic therapy group reported significantly lower scores on measures of depression (mean = 7.7, SD = 5.4) compared to those in the placebo group (mean = 9.0, SD = 6.0). The effect size for this difference was (-1.2). The study results indicate a statistically significant difference in anxiety levels between the treatment group (HN001) and the placebo group. The 95% confidence interval for the mean difference in anxiety scores is -2.3 to -0.1, and the p-value is 0.037. The effect size for the difference in anxiety scores is -1.0, with a confidence interval of -1.9 to -0.1.
The participants in the treatment group exhibited significantly lower scores (mean difference = -0.2, p = 0.014) compared to those in the placebo group. The prevalence of anxiety with clinical significance as determined by screening measures (score) was found to be considerably lower in the mothers who had HN001 treatment, with an odds ratio of 0.44 (95% confidence interval: 0.26, 0.73) and a p-value of 0.002.
5.Discussion:
This literature review Identified 14 studies (8 RCTs, 3 Cohort studies, and 3 cross-sectional studies). Although there is a paucity of research done to evaluate the role of micronutrient supplements in preventing PPD.
5.1 Vitamin D supplementation and depression:
Vitamin D exerts many effects on cognitive processes within the brain. The direct action of the active form of vitamin D (1,25(OH)2D) is observed in the brain due to the existence of its receptors. The human brain possesses the necessary enzymatic machinery responsible for the hydroxylation of vitamin D, leading to the synthesis of calcitriol. The indirect impacts of vitamin D are associated with its ability to improve muscle function. This intervention results in a rise in physical activity, hence yielding beneficial impacts on one's health. Elevated levels of vitamin D have been observed to result in a reduction in parathyroid hormone secretion. Elevated concentrations of this hormone have the potential to induce cognitive impairment. [37, 38].
Vitamin D has been shown to possess potential anti-inflammatory capabilities within the brain, as it may impede the generation of harmful pro-inflammatory cytokines. There is an established association between vitamin D deficiency and the development of pro-inflammatory diseases. Furthermore, it is plausible that modifications to the cardiovascular system may occur as a consequence of physiological changes that take place during pregnancy. The susceptibility of the maternal brain environment to inflammation may provide a partial explanation for the observed correlation between vitamin D and depression during pregnancy. Since depression is known to have an inflammatory component and vitamin D has potent anti-inflammatory and immunomodulatory effects, inflammation may play a role in the potential association between vitamin D deficiency and depression during pregnancy. According to [39, 40]
Two studies included in this review provide evidence in favor of the utilization of vitamin D supplements for the prevention of prenatal depression. The first study, conducted by [25] in Iran, employed a randomized control trial design. The second study, conducted by [26] in Japan, utilized a cohort study design. This aligns with Multiple studies have demonstrated that a deficit in vitamin D can lead to alterations in neurotransmitters that are associated with depression symptoms [41, 42]. According to the findings of [43], the administration of vitamin D at a dosage range of 1000-3500 IU per day for a duration of 8 weeks to 6 months resulted in a substantial reduction in symptoms associated with postpartum depression. The effect size, as measured by the standardized mean difference (SMD), was determined to be 0.52, with a 95% confidence interval (CI) ranging from 0.84 to 0.20. It is important to note that this effect was observed specifically in mothers who had baseline EPDS scores exceeding 12. According to a study conducted by [44], it was determined that the consumption of more than 2800 IU of vitamin D daily for a minimum duration of 8 weeks is highly likely to be successful.
According to the current study, [25] found that taking 2000 IU of vitamin D3 daily throughout the later stages of pregnancy resulted in significant decreases in perinatal depression ratings. The vitamin D group and the control group exhibited comparable overall features, except for two variables: unforeseen pregnancy and the utilization of supplementary substances outside the study's prescribed protocol. The incidence of unplanned pregnancies was higher in the vitamin D group compared to the control group. Based on prior scholarly investigations, it has been established that unplanned pregnancy serves as a risk factor for the development of perinatal depression. Following the implementation of the intervention, it was observed that the group administered vitamin D exhibited a notable decrease in depression levels, as evidenced by the results obtained from our study. The study conducted by [45] provided evidence for the efficacy of calcium supplementation in the prevention of perinatal depression. The control group was administered supplementary substances. Despite the increased utilization of supplements, specifically calcium, by this particular group, their depression scores were found to be significantly higher compared to the group that consumed vitamin D.
Vaziri et al. (2016) observed variations in the administration of vitamin D and calcium supplements among the cohorts under investigation. In their study, Tusnada et al. (2023) observed a positive correlation between vitamin D intake and calcium intake among different groups. Notably, the group with the highest vitamin D intake also exhibited the highest calcium intake. Furthermore, this particular group has shown a lower incidence of postpartum depression symptoms. Hence, further investigation was necessary to assess the sole consumption of Calcium.
5.2 Calcium and dairy product consumption:
Two cross-sectional studies have been undertaken pertaining to the consumption of calcium and dairy products. [31] discovered a positive correlation between increased intake of yogurt and calcium and a decreased occurrence of depressive symptoms in pregnant individuals. Conversely, [32] did not observe any impact of postpartum dairy supplements on postpartum depression (PPD). While both trials share a cross-sectional design, there is a notable difference in the time of supplementation. The observed effect of the calcium supplement was comparable to the findings of [46] study, which demonstrated a linear correlation between dietary calcium consumption and the manifestation of depressive symptoms (nonlinear p = 0.148). With the exception of interactions among different races, none of the interactions observed in the study were found to be statistically significant (p for interaction = 0.001). It is conceivable that an increased consumption of calcium could potentially mitigate variations in extracellular calcium levels and hinder inhibitory transmission, thereby leading to a reduced incidence of depressive symptoms. According to the study conducted by [47],
There exist several putative processes that could elucidate the inverse correlation observed between calcium consumption and depression. According to [48], calcium has a regulatory role in the hypothalamic-pituitary-adrenal (HPA) system, which is widely recognized as the principal stress response system in the human body. Corticotropin-releasing hormone (CRH) starts the release of adrenocorticotropic hormone (ACTH) from the hypothalamus. CRH also controls the release of adrenocorticosteroid [49] (Parrott, 2009). Consequently, in the event of dysregulation between the corticotropin-releasing hormone (CRH) and hypothalamic-pituitary-adrenal (HPA) systems, the functioning of stress-related hormones, specifically cortisol, may be impacted, thereby influencing the manifestation of depressive symptoms [50].
Furthermore, the influx of extracellular Calcium plays a vital role in various neuronal functions. In their study, [51] observed that alterations in extracellular Ca2+ levels could potentially contribute to the control of emotions. This effect is likely attributed to the direct impact of Ca2+ on the stabilization of the plasma membrane. Additionally, the authors noted that brain plasticity may possibly be influenced by methyl-D-aspartate. According to the findings of [52], the involvement of Ca/calmodulin-dependent protein kinase II in the synthesis of metabotropic glutamate receptors within group I of the hippocampus in rodents has been seen, resulting in the induction of long-term depression. Calcium (Ca) activates the enzyme tryptophan hydroxylase in the biosynthetic pathways responsible for the manufacture of serotonin. Additionally, the concentration of Ca in the cytosol is crucial for the coupling of stimuli and responses in many tissues. The dysregulation of cellular processes can have diverse implications for cellular function, potentially leading to alterations in mood [53].
The existing body of epidemiological research presents divergent findings about the potential association between dairy consumption and depression. Several studies have yielded divergent findings about the association between dairy consumption and depression. [54] reported no significant correlation, whereas [55] and [56] identified robust connections between specific dairy products, such as yogurt, milk, and cheese, and depressive symptoms. Furthermore, it has been observed that dairy products exhibit variances in their nutritional composition, including differences in fat content [57]. The current understanding of the role of dairy fats and fermented foods in relation to depression remains inconclusive, despite growing attention from the public health community to their potential health effects.
5.3 Poly unsaturated fatty acids (PUFAs) intake:
N-3 polyunsaturated fatty acids (n-3 PUFAs) are present in cellular membranes and serve a significant function in numerous physiological processes [58, 59, 60]. The factors encompassed in this category encompass the overall metabolic processes of the brain as well as the specific neuronal mechanisms that are especially associated with symptoms of depression.
The suggested mechanisms through which n-3 PUFAs exert their antidepressant effects encompass several distinct paths. [61] have demonstrated the presence of beneficial impacts on monoamine neurotransmission, neurogenesis, and inflammatory responses. The study conducted by [61] demonstrated that the administration of eicosapentaenoic acid (EPA) supplements led to a noteworthy decrease in depression scores exclusively among individuals exhibiting elevated levels of inflammation. This finding provides further evidence supporting the role of n-3 polyunsaturated fatty acids (PUFAs) in the modulation of inflammatory processes. The findings from animal research also suggest a potential involvement of n-3 PUFAs in the functioning of the hypothalamic-pituitary-adrenal (HPA) axis. According to [61], there exists a correlation between hypercortisolemia and diminished levels of the omega-3 fatty acid docosahexaenoic acid (DHA), and conversely, Furthermore, there exists a correlation between reduced levels of n-3 PUFA and mood disorders such as depression [62].
This review acknowledges five studies, consisting of four randomized controlled trials (Sousa and Santos, 2022) [27, 28, 29] and a cohort study conducted by [30]. These studies collectively do not provide substantial evidence supporting the preventive efficacy of fish oil supplementation. This finding contrasts with the systematic reviews conducted by [62], which suggested that polyunsaturated fatty acids (PUFAs) may be effective in mitigating symptoms of perinatal depression.
According to the findings of [28], there was no statistically significant impact observed on the average depression scores or the occurrence of severe depressive symptoms in pregnant women in the initial period following childbirth. The delayed adoption of supplements has been identified as the cause of this phenomenon. The assertion made in this statement was refuted by the findings of a randomized controlled trial (RCT) that involved the administration of 220 mg of docosahexaenoic acid (DHA) per day to one group (n = 42) and a combination of DHA and arachidonic acid (20:4n-6) at the same dosage to another group (n = 41). This supplementation was initiated from the 16th week of gestation until the 12th week after childbirth. However, the study did not yield any statistically significant outcomes in terms of preventing symptoms of postpartum depression. According to the study conducted by [64],
The study conducted by [28] found that women with a prior history of depression who consumed Fish oil experienced a notable reduction in EDPS. However, another randomized controlled trial (RCT) investigating the impact of omega-3 supplementation on the prevention of prenatal and postnatal depression in at-risk females did not yield a significant difference in depression scores. In this RCT, participants were administered either EPA- or DHA-rich fish oil supplements or a placebo. According to the study conducted by [65].
In a study conducted by [29], it was observed that the administration of fish oil supplements throughout the 35-37-wweek period of pregnancy resulted in a significant reduction in the average score of depression. This intervention played a crucial role in reducing the differences between the various groups involved in the study. While the fish oil supplements demonstrated a decrease in the average (standard deviation) depression score between 26 and 30 weeks, as well as 30-45 days following childbirth in the fish oil group, there were no statistically significant variances seen between the two groups.
5.4 Multivitamin supplements
Four studies examined the preventive effect of multivitamin use during pregnancy: The first study conducted by [33] suggests that the supplementation of folic acid may have a beneficial preventive effect on women who are at risk of developing depression. In contrast, the study conducted by [34] indicates that fortifying the maternal diet with LNS, as opposed to MMN or IFA, does not reduce postnatal depression. Additionally, a cohort study conducted by [18] recognizes the potential preventive role of selenium supplementation in postpartum depression. Lastly, [35] found that there is an independent inverse association between manganese intake and depressive symptoms during pregnancy.
The preventive role of selenium was identified through a systematic evaluation of 20 papers, of which 15 were included in the meta-analysis [66]. There were no significant differences observed in serum selenium levels between individuals diagnosed with depression and those who were considered healthy (weighted mean difference: 2.12 mg/L; 95% confidence interval: 0.11, 4.36; I2 = 98.0%; p < 0.001). Furthermore, the study found no significant association between serum selenium levels and depression scores (r = 0.12; 95% CI: 0.33, 0.08; I2 = 73.5%, P = 0.010). However, there was a notable negative correlation observed between high selenium consumption and the likelihood of experiencing postpartum depression (odds ratio: 0.97; 95% confidence interval: 0.95, 0.99; I2 = 0%, P = 0.507). Furthermore, the inclusion of selenium supplements resulted in a significant decrease in depressive symptoms (weighted mean difference: 0.37; 95% confidence interval: 0.56, 0.18; I2 = 0%, P = 0.959). Collectively, these findings indicate that selenium may possess a safeguarding influence against postpartum depression and might be regarded as a good supplementary treatment for individuals experiencing depression.
5.5 Probiotic supplementation:
According to the study conducted by [36], it was observed that the likelihood of women in the probiotic therapy group surpassing the threshold for anxiety levels was much lower compared to those in the placebo control group. This finding is similar to a meta-analysis that showed a statistically significant reduction in the depression scale score (mean difference [MD] for depressive disorder = -0.30, 95% confidence interval [CI] = -0.51 to -0.09, p = 0.005) among the participants following probiotic intervention. The administration of probiotics has shown a significant impact on both individuals without any diagnosed health conditions (mean difference [MD] = -0.25, 95% confidence interval [CI] -0.47 to -0.03, p = 0.03) as well as patients diagnosed with major depressive disorder (MDD) (MD = -0.73, 95% CI -1.37 to -0.09, p = 0.03). The results of the study indicate that the use of probiotics had a significant impact on individuals under the age of 60 (mean difference = -0.43, 95% confidence interval [-0.72, -0.13], p = 0.005). However, no significant effect of probiotics was observed in individuals aged 65 and above (mean difference = -0.18, 95% confidence interval [-0.47, 0.11], p = 0.22). According to [67].
Strengths and limitations
The authors employed comprehensive selection criteria and a meticulous search technique, ensuring the inclusion of diverse research papers that encompassed a broad spectrum of measurements related to micronutrient intake. In order to have a thorough picture of the impact of perinatal depression, the author incorporated pre-conceptional, prenatal, and postnatal studies into their research.
The present review is subject to certain limitations, namely the scarcity of studies investigating the preventive efficacy of micronutrients and the constraints imposed by the study designs employed.
The utilization of a cross-sectional design in this study prevents the author from establishing causal relationships. However, it is important to note that longitudinal research has its own limitations, such as the impact of multicollinearity. This issue is commonly encountered in nutrition studies, particularly those investigating nutrient intake from supplements. The presence of multiple nutrients in supplements, often in similar quantities and combinations, is a consequence of nutrient recommendations provided by health agencies. The studies used in this analysis did not possess the means to measure blood levels of the nutrients throughout the duration of the investigation. As a result, the impact of supplementation on biological levels remains uncertain.
Randomized controlled trials (RCTs) are subject to some limitations that warrant consideration. Firstly, the selection of patients in RCTs may not accurately reflect the characteristics of the broader population under investigation. This potential lack of representativeness might limit the generalizability of the trial's findings. Secondly, RCTs may encounter a notable proportion of missing outcome data, which can compromise the validity and reliability of the study results. Lastly, there is a possibility of misinterpretation or misunderstanding of questionnaires utilized in RCTs, potentially leading to inaccurate or incomplete data collection. These limitations should be acknowledged and addressed when interpreting the outcomes of RCTs. The limitations of measurement accuracy the validation of the FFQ for application among pregnant women has not been conducted. In relation to the CES-D, it is worth noting that physical symptoms, including exhaustion and physical discomfort, are frequently experienced by pregnant individuals. This overlap of symptoms may have led to an overestimation of the prevalence of depressive symptoms.
An additional issue that warrants investigation is the susceptibility of all randomized controlled trials (RCTs) to the placebo effect. In the context of mood disorders, individuals who engage in a trial that involves heightened interaction with researchers or healthcare professionals may experience a concealment of depressive symptoms. This phenomenon can be attributed to the therapeutic setting created by such increased contact, which in turn triggers placebo effects in both groups of participants. Consequently, this may potentially result in the spontaneous alleviation of depressive symptoms. According [68-71].
6. Conclusion:
This literature review revealed the positive effect of calcium, vitamin D, selenium, manganese, and probiotics in preventing perinatal depression; however, additional research is needed, preferably randomized controlled trials on women with a history of perinatal depression, along with the use of chemical biomarkers, to fully evaluate the relationship between micronutrients supplement and perinatal depression.
Conflicts of interest
The authors declared no conflict of interest.
References
- Davé S, Petersen I, Sherr L, et al. Incidence of maternal and paternal depression in primary care: a cohort study using a primary care database.Arch Pediatr Adolesc Med 164 (2010): 1038-1044.
- Wang J, Williams J, Lavorato D, et al. The incidence of major depression in Canada: The National Population Health Survey.J Affect Disorders 123 (2010): 158-163.
- Gavin NI, Gaynes BN, Lohr KN, et al. Perinatal Depression. Obstet Gynecol 106 (2005): 1071-1083.
- O’Hara MW and Wisner KL. Perinatal mental illness: Definition, description and aetiology. Best Pract Res Clin Obstet Gynecol 28 (2014): 3-12.
- Beck CT. Predictors of postpartum depression: an update.Nursing Res 50 (2005): 275-85.
- Bobo WV and Yawn BP. Concise Review for Physicians and Other Clinicians: Postpartum Depression.Mayo Clin Pro 89 (2014): 835-844.
- Williamson V and McCutcheon H. Postnatal depression: a review of current literature. Australian Midwifery 17 (2004): 11-
- Seguin L, Potvin L, St-Denis M, et al. Depressive Symptoms in the Late Postpartum Among Low Socioeconomic Status Women. Birth 26 (1999): 157-163.
- Osborne LM and Monk C. Perinatal depression—The fourth inflammatory morbidity of pregnancy? Psychoneuroendocrinol 38 (2010): 1929-1952.
- Murray L and Cooper PJ. Effects of postnatal depression on infant development. Arch Disease Childhood 77 (1997): 99-101.
- Beck CT. The effects of postpartum depression on child development: A meta-analysis.Arch Psych Nursing 12 (1998): 12-20.
- Goodman SH, Rouse MH, Connell AM, et al. Maternal Depression and Child Psychopathology: A Meta-Analytic Review. Clin Child Fam Psychol Rev 14 (2011): 1-27.
- Grace SL, Evindar A and Stewart DE. The effect of postpartum depression on child cognitive development and behavior: A review and critical analysis of the literature. Arch Women’s Mental Health 6(2003): 263-274.
- Letourneau NL, Dennis C-L, Cosic N, et al. The effect of perinatal depression treatment for mothers on parenting and child development: A systematic review.Depress Anxiety 34 (2017): 928-966.
- Krishnan V and Nestler EJ. Linking Molecules to Mood: New Insight Into the Biology of Depression.Am J Psych 167 (2010): 1305-1320.
- Leung BMY and Kaplan BJ. Perinatal Depression: Prevalence, Risks, and the Nutrition Link—A Review of the Literature.J Am Diet Assoc 109 (2009): 1566-1575.
- Leung BM, Kaplan BJ, Field CJ, et al. Prenatal micronutrient supplementation and postpartum depressive symptoms in a pregnancy cohort. BMC Pregnancy and Childbirth 13 (2013).
- Sparling TM, Henschke N, Nesbitt RC, et al. The role of diet and nutritional supplementation in perinatal depression: a systematic review. Maternal& Child Nutrition 13 (2016).
- Baskin R, Hill B, Jacka FN, et al. The association between diet quality and mental health during the perinatal period’ A systematic review. Appetite 91 (2015): 41-47.
- Trujillo J, Vieira MC, Lepsch J, et al. A systematic review of the associations between maternal nutritional biomarkers and depression and/or anxiety during pregnancy and postpartum. J Affect Disorders 232 (2018): 185-203.
- Kaplan BJ, Crawford SG, Field CJ, et al. Vitamins, minerals, and mood. Psychol Bulletin 133 (2017): 747-760.
- Bodnar LM and Wisner KL. Nutrition and Depression: Implications for Improving Mental Health Among Childbearing-Aged Women.Biol Psych 58 (2005): 679-685.
- Miller BJ, Murray L, Beckmann MM, et al. Dietary supplements for preventing postnatal depression.Cochrane Database of Sys Rev 2013 (2013).
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews.PLOS Med 18 (2021): e1003583.
- Vaziri F, Nasiri S, Tavana Z, et al. A randomized controlled trial of vitamin D supplementation on perinatal depression: in Iranian pregnant mothers. BMC Pregnancy Childbirth 16 (2016).
- Tsunoda K, Kei Hamazaki, Matsumura K, et al. Dietary Intake of Vitamin D during Pregnancy and the Risk of Postpartum Depressive Symptoms: The Japan Environment and Children’s Study 69 (2023): 14-20.
- Hulkkonen P, Kataja E-L, Vahlberg T, et al. The efficacy of probiotics and/or n-3 long-chain polyunsaturated fatty acids intervention on maternal prenatal and postnatal depressive and anxiety symptoms among overweight and obese women. J Affect Disorders 289 (2021): 21-30.
- Vaz J, dos S, Farias DR, et al. Omega-3 supplementation from pregnancy to postpartum to prevent depressive symptoms: a randomized placebo-controlled trial. BMC Pregnancy Childbirth 17 (2017).
- Farshbaf-Khalili A, Mohammad-Alizadeh S, Farshbaf-Khalili A, et al. Fish-Oil Supplementation and Maternal Mental Health: A Triple-Blind, Randomized Controlled Trial. Iranian Red Cresc Med J 19 (2016).
- Kei Hamazaki, Ayako Takamori, Akiko Tsuchida, et al. Dietary intake of fish and n-3 polyunsaturated fatty acids and risks of perinatal depression: The Japan Environment and Children's Study (JECS). J Psych Res 98 (2018): 9-16.
- Miyake Y, Tanaka K, Okubo H, et al. Intake of dairy products and calcium and prevalence of depressive symptoms during pregnancy in Japan: a cross-sectional study. BJOG: An Inter J Obstet Gynaecol 122 (2014): 336-343.
- Alashmali S, Almasaudi AS, Zedan HS, et al. The Effect of Dairy Products and Nutrient Intake after Childbirth on the Risk of Postpartum Depression. Inter J Environ Res Public Health 19 (2013): 16624.
- Nguyen PH, DiGirolamo AM, Gonzalez-Casanova I, et al. Impact of preconceptional micronutrient supplementation on maternal mental health during pregnancy and postpartum: results from a randomized controlled trial in Vietnam. BMC Women’s Health 17 (2017).
- Stewart RC, Ashorn P, Umar E, et al. The impact of maternal diet fortification with lipid-based nutrient supplements on postpartum depression in rural Malawi: a randomised-controlled trial. Maternal Child Nutrition 13 (2016): e12299.
- Miyake Y, Tanaka K, Okubo H, et al. Manganese intake is inversely associated with depressive symptoms during pregnancy in Japan: Baseline data from the Kyushu Okinawa Maternal and Child Health Study. J Affect Disorders 211 (2017): 124-129.
- Slykerman RF, Hood F, Wickens K, et al. Effect of Lactobacillus rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomized Double-blind Placebo-controlled Trial. E BioMedicine 24 (2017): 159-165.
- Kesby JP, Eyles DW, Burne THJ, et al. The effects of vitamin D on brain development and adult brain function.Molecular and Cellular. Endocrinol 347 (2021): 121-127.
- Eyles DW, Burne THJ and McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease.Front Neuroendocrinol 34 (2013): 47-64.
- Accortt EE, Schetter CD, Peters RM, et al. Lower prenatal vitamin D status and postpartum depressive symptomatology in African American women: Preliminary evidence for moderation by inflammatory cytokines. Arch Women’s Mental Health 19 (2015): 373-383.
- Guillot X, Semerano L, Saidenberg-Kermanac’h N, et al. Vitamin D and inflammation. Joint Bone Spine 77 (2010): 552-557.
- Upadhyaya S, Ståhlberg T, Silwal S, et al. Maternal Vitamin D Levels during Pregnancy and Offspring Psychiatric Outcomes: A Systematic Review. Int J Mol Sci 24 (2022): 63.
- Eyles DW. Vitamin D: Brain and Behavior. JBMR Plus 5 (2020).
- Tsai Z, Shah N, Tahir U, et al. Dietary interventions for perinatal depression and anxiety: a systematic review and meta-analysis of randomized controlled trials. The Am J Clin Nutrition 117 (2022): 1130-1142.
- Lentjes MA. The balance between food and dietary supplements in the general population. The Proc Nutrition Soci 78 (2022): 97-109.
- Harrison-Hohner J, Coste S, Dorato V, et al. Prenatal calcium supplementation and postpartum depression: an ancillary study to a randomized trial of calcium for prevention of preeclampsia.Arch Women’s Mental Health 3 (2021): 141-146.
- Shen X, Gu X, Liu Y-Y, et al. Association between dietary calcium and depression among American adults: National health and nutrition examination survey. Front Nutrition 10 (2023).
- Wang F, Smith NA, Xu Q, et al. Astrocytes Modulate Neural Network Activity by Ca2+-Dependent Uptake of Extracellular K+. Sci Signal 5 (2013): 26-26.
- Sartori SB, Whittle N, Hetzenauer A, et al. Magnesium deficiency induces anxiety and HPA axis dysregulation: Modulation by therapeutic drug treatment.Neuropharmacol 62 (2010): 304-312.
- Silva AP, Schoeffter P, Weckbecker G, et al. Regulation of CRH-induced secretion of ACTH and corticosterone by SOM230 in rats.European J Endocrinol 153 (2005): 7-10.
- Xu L, Zhang S, Chen W, et al. Trace elements differences in the depression sensitive and resilient rat models. Biochem Biophys Res Commun 529 (2020): 204-209.
- Wu CC and Bohr DF. Mechanisms of calcium relaxation of vascular smooth muscle.The Am J Physiol 261 (1991): 1411-1416.
- Mockett BG, Guevremont D, Wutte M, et al. Calcium/Calmodulin-Dependent Protein Kinase II Mediates Group I Metabotropic Glutamate Receptor-Dependent Protein Synthesis and Long-Term Depression in Rat Hippocampus.J Neurosci 31 (2011): 7380-7391.
- Knapp S, Mandell AJ and Bullard WP. Calcium activation of brain tryptophan hydroxylase.Life Sci 16 (1975): 1583-1593.
- Tsai AC, Chang T-L and Chi S-H. Frequent consumption of vegetables predicts lower risk of depression in older Taiwanese - results of a prospective population-based study.Public Health Nutrition 15 (2012): 1087-1092.
- Pasco JA, Williams LJ, Brennan-Olsen SL, et al. Milk Consumption and the Risk for Incident Major Depressive Disorder. Psycho Psychos 84 (2015): 384-386.
- Yu B, Zhu Q, Meng G, et al. Habitual yoghurt consumption and depressive symptoms in a general population study of 19,596 adults. European J Nutrition 57 (2017): 2621-2628.
- Perez-Cornago A, Sanchez-Villegas A, Bes-Rastrollo M, et al. Intake of High-Fat Yogurt, but Not of Low-Fat Yogurt or Prebiotics, Is Related to Lower Risk of Depression in Women of the SUN Cohort Stud. The J Nutrition 146 (2016): 1731-1739.
- Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci 7 (2015): 52.
- Perica MM and Delaš I. Essential Fatty Acids and Psychiatric Disorders.Nutrition Clin Pract 26 (2011): 409-425.
- Grosso G, Galvano F, Marventano S, et al. Omega-3 Fatty Acids and Depression: Scientific Evidence and Biological Mechanisms.Oxidative Med Cell Long 2014 (2014): 1-16.
- Song C, Shieh C-H, Wu Y-S, et al. The role of omega-3 polyunsaturated fatty acids eicosatetraenoic and docosahexaenoic acids in the treatment of major depression and Alzheimer’s disease: Acting separately or synergistically?Progress in Lipid Res 62 (2016): 41-54.
- Fernandes M, Mutch D and Leri F. The Relationship between Fatty Acids and Different Depression-Related Brain Regions, and Their Potential Role as Biomarkers of Response to Antidepressants.Nutrients 9 (2017): 298.
- Suradom C, Suttajit S, Oon-arom A, et al. Omega-3 polyunsaturated fatty acid (n-3 PUFA) supplementation for prevention and treatment of perinatal depression: a systematic review and meta-analysis of randomized-controlled trials. Nordic J Psych (2022): 1-8.
- Doornbos B, Goor van DA, Janneke Dijck-Brouwer, et al. Supplementation of a low dose of DHA or DHA+AA does not prevent peripartum depressive symptoms in a small population-based sample. Prog Neuropsychopharmacol Biol Psychiatry 33 (2009): 49-52.
- Golding J, Steer C, Emmett P, et al. High Levels of Depressive Symptoms in Pregnancy with Low Omega-3 Fatty Acid Intake from Fish.Epidemiol 20 (2009): .598-603.
- Sajjadi SS, Foshati S, Haddadian-Khouzani S, et al. The role of selenium in depression: a systematic review and meta-analysis of human observational and interventional studies. Sci Reports 12 (2022).
- Huang R, Wang K and Hu J. Effect of Probiotics on Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 8 (2016): 483.
- Walsh BT, Seidman SN, Sysko R, et al. Placebo Response in Studies of Major Depression.JAMA 287 (2002): 1840.
- Baker JD. The Purpose, Process, and Methods of Writing a Literature Review. AORN J 103 (2016): 265-269.
- Burt VK & Stein K. Epidemiology of depression throughout the female life cycle. J Clin Psychiatry 63 (2002): 9-15.
- Green BN, Johnson CD and Adams A. Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. J Chiropractic Med 5 (2006): 101-117.
