The Role of Neurotrophins in Hearing Loss and their Implications in Developing Innovative Therapies
Article Information
Pasquale Cocchiaro#, Cristina Giorgio#, Rubina Novelli, Andrea Aramini, Marcello Allegretti, Laura Brandolini*
Research and Early Development, Dompé Farmaceutici SpA, Via Tommaso De Amicis, 80131 Naples, Italy
#These authors contributed equally to this work.
*Corresponding author: Laura Brandolini, Research and Early Development, Dompé Farmaceutici SpA, Via Tommaso De Amicis, 80131 Naples, Italy.
Received: 30 March 2022; Accepted: 12 April 2022; Published: 20 June 2022
Citation: Pasquale Cocchiaro, Cristina Giorgio, Rubina Novelli, Andrea Aramini, Marcello Allegretti, Laura Brandolini. The Role of Neurotrophins in Hearing Loss and their Implications in Developing Innovative Therapies. Journal of Biotechnology and Biomedicine 5 (2022): 117-136.
View / Download Pdf Share at FacebookAbstract
Neurotrophins (NTs) are pleiotropic molecules that can exert a variety of function in both the central and peripheral nervous systems, modulating survival, development and function of neurons. Due to their crucial involvement in the development and innervation of the inner ear, NTs have been considered as potential therapeutic approaches for the treatment of hearing loss. Positive results obtained in various preclinical models of hearing loss opened the way for the clinical use of NTs to counteract synaptopathy, improve cochlear implant performance or prevent long-term neural loss after noise exposure. However, although promising results have been obtained also in clinical trials, NT treatments for hearing loss have not yet achieved the clinical practice. Here, we will review the repair and regeneration potential of inner ear cells and discuss how NTs can contribute to these processes and can thus be used for the treatment of hearing loss. In this context, we will examine the limitations of current NT treatments and the status of development of novel NT-based potential therapeutic approaches for hearing diseases.
Keywords
Hearing loss; Neurotrophins; Cochlear regeneration; Hair cell
Hearing loss articles Hearing loss Research articles Hearing loss review articles Hearing loss PubMed articles Hearing loss PubMed Central articles Hearing loss 2023 articles Hearing loss 2024 articles Hearing loss Scopus articles Hearing loss impact factor journals Hearing loss Scopus journals Hearing loss PubMed journals Hearing loss medical journals Hearing loss free journals Hearing loss best journals Hearing loss top journals Hearing loss free medical journals Hearing loss famous journals Hearing loss Google Scholar indexed journals Neurotrophins articles Neurotrophins Research articles Neurotrophins review articles Neurotrophins PubMed articles Neurotrophins PubMed Central articles Neurotrophins 2023 articles Neurotrophins 2024 articles Neurotrophins Scopus articles Neurotrophins impact factor journals Neurotrophins Scopus journals Neurotrophins PubMed journals Neurotrophins medical journals Neurotrophins free journals Neurotrophins best journals Neurotrophins top journals Neurotrophins free medical journals Neurotrophins famous journals Neurotrophins Google Scholar indexed journals Cochlear regeneration articles Cochlear regeneration Research articles Cochlear regeneration review articles Cochlear regeneration PubMed articles Cochlear regeneration PubMed Central articles Cochlear regeneration 2023 articles Cochlear regeneration 2024 articles Cochlear regeneration Scopus articles Cochlear regeneration impact factor journals Cochlear regeneration Scopus journals Cochlear regeneration PubMed journals Cochlear regeneration medical journals Cochlear regeneration free journals Cochlear regeneration best journals Cochlear regeneration top journals Cochlear regeneration free medical journals Cochlear regeneration famous journals Cochlear regeneration Google Scholar indexed journals Hair cell articles Hair cell Research articles Hair cell review articles Hair cell PubMed articles Hair cell PubMed Central articles Hair cell 2023 articles Hair cell 2024 articles Hair cell Scopus articles Hair cell impact factor journals Hair cell Scopus journals Hair cell PubMed journals Hair cell medical journals Hair cell free journals Hair cell best journals Hair cell top journals Hair cell free medical journals Hair cell famous journals Hair cell Google Scholar indexed journals anatomy articles anatomy Research articles anatomy review articles anatomy PubMed articles anatomy PubMed Central articles anatomy 2023 articles anatomy 2024 articles anatomy Scopus articles anatomy impact factor journals anatomy Scopus journals anatomy PubMed journals anatomy medical journals anatomy free journals anatomy best journals anatomy top journals anatomy free medical journals anatomy famous journals anatomy Google Scholar indexed journals injection articles injection Research articles injection review articles injection PubMed articles injection PubMed Central articles injection 2023 articles injection 2024 articles injection Scopus articles injection impact factor journals injection Scopus journals injection PubMed journals injection medical journals injection free journals injection best journals injection top journals injection free medical journals injection famous journals injection Google Scholar indexed journals aminoglycoside articles aminoglycoside Research articles aminoglycoside review articles aminoglycoside PubMed articles aminoglycoside PubMed Central articles aminoglycoside 2023 articles aminoglycoside 2024 articles aminoglycoside Scopus articles aminoglycoside impact factor journals aminoglycoside Scopus journals aminoglycoside PubMed journals aminoglycoside medical journals aminoglycoside free journals aminoglycoside best journals aminoglycoside top journals aminoglycoside free medical journals aminoglycoside famous journals aminoglycoside Google Scholar indexed journals antibiotics articles antibiotics Research articles antibiotics review articles antibiotics PubMed articles antibiotics PubMed Central articles antibiotics 2023 articles antibiotics 2024 articles antibiotics Scopus articles antibiotics impact factor journals antibiotics Scopus journals antibiotics PubMed journals antibiotics medical journals antibiotics free journals antibiotics best journals antibiotics top journals antibiotics free medical journals antibiotics famous journals antibiotics Google Scholar indexed journals erythromycin articles erythromycin Research articles erythromycin review articles erythromycin PubMed articles erythromycin PubMed Central articles erythromycin 2023 articles erythromycin 2024 articles erythromycin Scopus articles erythromycin impact factor journals erythromycin Scopus journals erythromycin PubMed journals erythromycin medical journals erythromycin free journals erythromycin best journals erythromycin top journals erythromycin free medical journals erythromycin famous journals erythromycin Google Scholar indexed journals Haemophilus influenzae articles Haemophilus influenzae Research articles Haemophilus influenzae review articles Haemophilus influenzae PubMed articles Haemophilus influenzae PubMed Central articles Haemophilus influenzae 2023 articles Haemophilus influenzae 2024 articles Haemophilus influenzae Scopus articles Haemophilus influenzae impact factor journals Haemophilus influenzae Scopus journals Haemophilus influenzae PubMed journals Haemophilus influenzae medical journals Haemophilus influenzae free journals Haemophilus influenzae best journals Haemophilus influenzae top journals Haemophilus influenzae free medical journals Haemophilus influenzae famous journals Haemophilus influenzae Google Scholar indexed journals
Article Details
1. Introduction
Today, hearing loss affects over 460 million people worldwide, and numbers are expected to increase, reaching over 900 million people by 2050 [1]. The causes that can lead to hearing loss are several, and different are the phenotypes of the disease, as well as the severity grades of the pathology, depending on the specific component or structure of the auditory system that has been damaged. Despite the significant efforts and investments that have been recently made to uncover the principal mediators and mechanisms involved in this disease, still no drug-based therapy has been approved by the Food and Drug Administration, and treatment options for hearing loss are limited to devices and cochlear implants [2]. Among the factors that have been investigated as potential treatments aimed at ameliorating or even restoring the underlying pathology, neurotrophins (NTs) have emerged as promising approaches, progressively and increasingly attracting the interest of researchers and clinicians. In this review, we will provide an overview of the auditory system and the possible causes of hearing loss, thus discussing the repair and regeneration potential of inner ear cells, focusing on the potential use of NTs for the treatment of hearing loss and the current status of development of NT-based therapeutic approaches.
2. Auditory system: anatomy and function
The auditory system is one of the mechanically most sensitive organs of the human body [3], and it is composed by the outer, middle, and inner ear (Figure 1), the auditory nerve and the central auditory pathways. The outer ear has a complex structure and shape and consists of the auricle (or pinna) and the ear canal. It collects sound waves and guides them to the tympanic membrane in the middle ear [4], which is composed also by 3 ossicles (malleus, incus and stapes) with the associated muscles, tendons, and ligaments, as well as the Eustachian tube. From the tympanic membrane, sound vibrations are conveyed to the cochlea in the inner ear via the auditory ossicles [5]. The cochlea is a spiral fluid-filled tube that is part of the peripheral nervous system; it contains the organ of Corti, a specialized sensory epithelium, in which Hair Cells (HCs) allow for the transduction of sound vibrations into neural signals. Cochlea’s functions crucially depend on the integrity of the HCs, their postsynaptic partners and the Spiral Ganglion Neurons (SGNs), which convey the information to the auditory brainstem of the central nervous system [6].
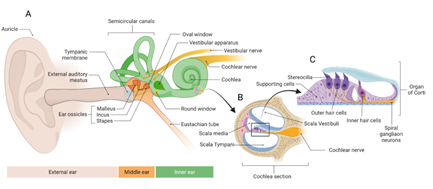
Figure 1: Auditory system anatomy.
Overview of the ear (external middle and inner ear). B. Section of a turn of the cochlea. C. Organ of Corti which contains the hair cells.
3. Causes of hearing loss
Degeneration of cells in the cochlea and loss of their connection with the auditory brainstem of the central nervous system lead to hearing loss. The damage and permanent loss of HCs in the cochlea are typical features of sensorineural hearing loss, which is the most common form of deafness [7] and most frequently occurs because of aging, pathogen infections, ototoxic drug or noise exposure [7,8].
3.1 Aging
Age-Related Hearing Loss (ARHL), also known as presbycusis, is the most common cause of hearing loss and one of the most prevalent sensory deficits affecting elderly worldwide [9]. Sixty millions of Americans between the ages of 50 and 65 are expected to be affected by ARHL by 2025, with an increase of prevalence from 9.3% in 2007 to 19% in 2025 [10]. Although often underestimated, the impact of ARHL on patients’ quality of life is enormous with repercussions on physical, psychological and social levels; in some cases, it might be also a predictor of dementia [11-13]. ARHL is a polygenic/multifactorial disorder, the onset of which is mainly caused by age-related cumulative effects of extrinsic damages, such as noise exposure or ototoxic medications, intrinsic systemic disorders (e.g. diabetes, hypertension) and genetic predisposition [10, 14]. Although the cochlea is severely affected by the aging process, histopathologic findings have shown that ARHL can be caused by the alteration of different auditory structures, each of which can result in different phenotypes: the degeneration of cochlear inner and outer HCs (sensory) leads to high-frequency hearing loss; strial vascularis atrophy (strial or metabolic) causes diminished pure tone thresholds in all frequencies; and the degeneration of the auditory nerve (neural) reduces word discrimination scores and relatively stable pure tone thresholds [15-17]. In most cases, a mixture of these and other indeterminate pathological changes have been reported [16,18], indicating that the mechanisms underlying ARHL are multiple and still not completely understood. In this context, also alterations in genes involved in cochlear function can influence susceptibility to ARHL; however, to date, ARHL genome wide association studies have identified only few genes that are linked to hearing loss [19, 20]. Hopefully, whole-genome sequencing approaches would identify genetic variants involved in ARHL, and thus lead to the development of novel pharmaceutical intervention and to a better defined patient stratification.
3.2 Infectious pathogens
Hearing loss can be caused also by infections of pathogens such as bacteria, viruses, protozoons, or mycetes [21, 22]. Infections by Treponemas pallidum and Borrelia burgdorferi, which are the causative pathogens of syphilis and Lyme disease, respectively, can cause severe labyrinthitis of the inner ear. Bacteria that cause meningitis, including meningococcus, pneumococcus, and Haemophilus influenzae, can directly infect labyrinth and cochlea, leading to hearing loss. The bacterium Toxoplasma gondii, which causes toxoplasmosis, is known to damage the auditory system via calcifications in cochlea and spiral ligaments [23]. Viruses, on the other hand, might increase the susceptibility to bacterial infection and induce auditory system damage at various levels, altering patient immune-response or directly damaging inner ear structures. About 20–65% and 50% of individuals with congenital Cytomegalovirus and Rubella viral infections, respectively, experience hearing loss, labyrinthitis and death of sensorial cells in the organ of Corti [24], while Morbillivirus and viral meningitis may also lead to severe sensorineural hearing loss. The prevalence of hearing loss in patients with HIV is 14% to 49%, which can be exacerbated by ototoxic drug for HIV treatment [25, 26]. Recent studies revealed that Sensori Neural Hearing Loss (SNHL) might be a consequence of also COVID-19 infection; however, this finding need to be further elucidated [27].
3.3 Ototoxicity
The damage to the inner ear caused by several drugs is defined as ototoxicity [28-31]. Depending on the part of the inner ear that is affected, ototoxicity can involve non-sensory cochlear or vestibular cells, which are crucial for sensitive hair cell function [32]. Among the ototoxic drugs, antibiotics have been associated to permanent damage to sensory cells and neurons, and thus to irreversible hearing loss [33]. Among the aminoglycoside antibiotics, amikacin, neomycin, anamycin, eptomycin, and kanamycin induce cochlear toxicity, while streptomycin and gentamicin primarily cause vestibular deficits [33]. On the other hand, non-aminoglycoside antibiotics as erythromycin [34], vancomycin [35], azithromycin, and clarithromycin [36, 37], can lead to sensorineural hearing loss, dizziness, and tinnitus, particularly in neonates [31]. Besides antibiotics, irreversible and progressive death of cochlear outer hair cells can be also induced by platinum-based drugs, such as cisplatin [38-41]. Cisplatin is an anticancer drug used to treat several tumors, and its ototoxic effects are dose cumulative [42] and related to the number of cycles and route of administration [43]. In pediatric patients, Cisplatin-Induced Hearing Loss (CIHL) develops early during therapy, with clinical consequences that include impairment of speech and language acquisition, as well as cognitive and psychosocial development, leading to isolation and depression [44-47]. Although clinical practice guidelines are available for ototoxicity surveillance, none focuses on interventions to reduce ototoxicity [48]. Treatments able to reduce cisplatin-induced ototoxicity without decreasing survival rate are an urgent and unmet need for cancer patients [48].
3.4 Noise exposure
Noise exposure is responsible for about 10% of hearing loss in adults and it is frequently observed in military service members, which are often overexposed to high intensity noises that frequently and irreversibly damage cellular structures at multiple locations in the auditory system [49, 50]. Although noise exposure is primarily associated with damage and death of cochlear hair cells, prolonged noise exposure has been reported to cause also myelin alterations along the cochlear nerve in both preclinical models and humans [51,52], as well as cochlear synaptopathy, which is an irreversible damage to the synapses between the inner hair cells and auditory nerve fibers within the cochlea [53,54]. In this context, remyelinating compounds [55] could be used to avoid histological cochlear and auditory nerve alterations and to prevent hearing loss induced by acoustic trauma, along with anti-inflammatory drugs [56] or antioxidants, such as ascorbic acid, resveratrol, and glutathione, which may reduce noise-induced oxidative stress, which has been shown to lead to cochlear damage and hearing loss after acoustic trauma [57]. Thus, for all factors described above involved in onset and progression of acquired hearing loss, prevention, early diagnosis, greater insight of pathophysiology and development of proper treatments are fundamental steps to be implemented for the appropriate management and monitoring of patients with hearing loss.
4. Regeneration of the Inner Ear: underlying mechanisms and cell therapies
In recent years, biomedical research in inner ear regeneration has been focused on studying the protection, regeneration, and functional recovery of mammalian auditory HCs and neurons. Unlike birds [58] and fish [59] in fact, where the spontaneous ability of HCs to regenerate after damage has been widely demonstrated, in mammals the regeneration process of HCs is still controversial. The first piece of evidence of HC regeneration in humans has been reported about 40 years ago, when a 37-year-old man developed bilateral deafness after gentamicin administration, but then, progressively showed an improvement in hearing at 3 weeks and 8 months from treatment discontinuation [60]. In a larger case study, Fee and colleagues reported a series of 138 patients, of which approximately 50% recovered hearing and vestibular function between 1 week and 9 months after ototoxic treatment with tobramycin and gentamicin [61]. In the last decade, numerous studies have investigating the mechanisms and cellular components that could be involved in mammalian HC regeneration. Among them, a recent one has demonstrated that c-myc and Notch pathways co-activation can induce renewed proliferation of inner ear cells and the regeneration of HCs in adult mice [62]. Another study in newborn mice, on the other hand, showed that the suppression of Notch signaling in the cochlea triggered the trans-differentiation of supporting cells into HCs [63,64]; in line with this, a combination of four transcription factors (Six1, Atoh1, Pou4f3, and Gfi1) has been identified as able to convert mouse embryonic fibroblasts and postnatal supporting cells into HCs [65]. Moreover, a population of supporting cells was found capable to proliferate and differentiate into new HCs in neonatal mammalian cochlea [66-68]. Although supporting cells, under specific stimuli, may thus regenerate lost HCs, the whole process still remains difficult to observe in mature mammalian cochlea. Therefore, further understanding of the molecular mechanisms underlying HCs regeneration might represent an opportunity to implement new strategies to restore the lost connections between HCs and neurons in hearing loss.
Another approach that has been investigated for the regeneration of the remaining cells of the inner ear is the replacement of lost and injured cells with new transplanted progenitor cells. Studies have shown that stem cells can engraft in the inner ear [69] and may differentiate either into inner ear cells or into hair cells, thus contributing to the regeneration of the sensory epithelium [70]. Probably, the most suitable and renewable source for the generation of sensory hair cells are pluripotent stem cells, such as Embryonic Stem Cells (ESCs) and Induced Pluripotent Stem Cells (iPSCs). These cells can in fact functionally and morphologically differentiate into hair cells and promote the organization of the cells into hair-like cells with stereociliary bundles [71]. Alternatively, the administration of Mesenchymal Stem Cells (MSCs) has been found safe in a human study [72] and efficacious in repairing spiral ganglion in cochlear cultures from neonatal rats injured by gentamicin [73] and in rats with cochleae damaged by noise or ototoxic drugs [74]. Interestingly, in this latter study, the migration of MSCs into the cochlea was associated with the expression of the brain-derived neurotrophic factor (BDNF) [74], suggesting that neurotrophins can be involved and play an important role in mediating inner ear regeneration.
5. Neurotrophins in the ear: expression and functions
Neurotrophins (NTs) are a family of growth factors that are crucially involved in the regulation of neural survival, development, differentiation, and plasticity [75]. Produced by the cells that are the targets of innervation, NTs are synthetized as precursors (proneurotrophins) and subsequently cleaved intracellularly and extracellularly to obtain the mature form [73]. In mammals, four NTs have been characterized: the Nerve Growth Factor (NGF) [76], the Brain-Derived Neurotrophic Factor (BDNF), the Neurotrophin-3 (NT-3), and the neurotrophin-4/5 (NT-4/5). They exert different functions via two classes of receptor: the Tropomyosin Receptor Kinase (Trk), which belongs to the family of receptor tyrosine kinases and includes TrkA, TrkB and TrkC subtypes, and the p75 neurotrophin receptor (p75NTR) [77]. Upon ligand binding on their extracellular domain, Trk receptors dimerize and activate, leading to receptor autophosphorylation and subsequent activation of several signaling cascades [78]. The high structural homology, both among the 3 subtypes of Trk and among the NTs, allows for a cross-activation between factors and receptors, but usually, the binding between mature NTs and Trk subtypes occurs with different specificities. NGF, for example, preferentially binds TrkA, BDNF and NT4/5 have higher specificity for TrkB, while NT3 primarily binds TrkC [78]. The p75NTR, on the other hand, binds all mature NTs with similar low affinity, while it binds with high affinity the proneurotrophins [78]. Depending on the class of receptor activated, NTs can trigger two different cellular pathways: the interaction of mature NTs with Trk receptors leads to survival signals, whereas the binding to p75NTR leads to apoptosis [79]. BDNF and NT-3, with their high-affinity receptors TrkB and TrkC, respectively, have been identified as key factors for the innervation, development and maintenance of the ear [80]. BDNF is expressed by HCs of all sensory epithelia in late embryonic life, while NT-3 is mainly expressed by the supporting cells of the cochlea, saccule and utricle [80]. In vivo studies on mutant BDNF or NT-3, or their specific receptors, have demonstrated that the spatio-temporal expression pattern of these two NTs is fundamental to provide trophic support for inner ear sensory neuron afferents, and thus to guarantee the correct development of the inner ear [81-83]. Depletion of both NT-3 and BDNF or of their receptors during development determines the complete loss of SGNs [84], indicating that the remaining NTs cannot suffice to support SGN survival in the cochlea [85]. Interestingly, the presence of either NT-3 or BDNF is sufficient to support neuronal survival in ear development, suggesting a comparable role of these two NTs in supporting neuronal survival in ear development [80]. While also NT-4/5 has been shown to exert pro-survival effects on SGNs during development [86], available data suggest that NGF is not crucially involved in the development of the auditory system [87].
5.1. Neurotrophins: Potential therapeutic approaches for hearing loss
Due to their involvement in several neuronal processes, including neuron development and function [88], NTs have been considered as potential therapeutic approaches for the treatment of hearing loss. To test the potential therapeutic effects of NTs, several studies have investigated the exogenous NT delivery in different animal models (Table 1), showing that NTs can be effective in preserving the function and morphology of the auditory nerve from both noise exposure-induced hearing loss and drug-induced ototoxicity and can increase cochlear neuron survival [89-91]. In guinea pigs deafened by loud noises, a single dose application of NT-3 or BDNF to the round window restored hearing function to the damaged cochlea and reduced internal HC synaptopathy [92]. Confirming these data, local delivery of NT-3 to the inner ear in noise-exposed mice induced neurite growth from available auditory neurons and regenerated HC synapses, partially reversing cochlear synapthopathy [93]. BDNF strongly reduced auditory thresholds and repaired the damaged connections between inner HCs and auditory nerve fibers also when administered to guinea pigs deafened by kanamycin [94,95], and similarly, in a guinea pig model of cisplatin-induced ototoxicity, BDNF treatment led to an improvement in hearing [96]. Exogenous administration of BDNF has led to an enhancement of SGC survival in different species, such as cats [97,98], rats [99] and guinea pigs [100]; in particular in deafened guinea pigs, short-term treatment with BDNF has been shown to prevent long term auditory nerve degeneration and to recover SGC function, ensuring a successful cochlear implant [90]. Notably, also in rat models, the administration of both BDNF and NT-3 enhanced spiral ganglion neurites length and number toward cochlear implants [101], suggesting a new potential therapeutic approach for cochlear implantation. The higher success rate of this approach in patients has been in fact associated with the number of surviving SGNs or HCs, suggesting that neuronal survival might represent a limiting factor for implant success [102] and thus, that the combination of cochlear implant with NTs may represent an advantage for the success of cochlear implant by stimulating innervation toward the implant. Despite its role in the development of the auditory system is still undefined, exogenous NGF administration exerted protective and beneficial effects both in vitro and in vivo. In vitro, NGF treatment induced dose dependent growth of Statoacoustic Ganglion (SAG)-derived Neural Progenitor (NP) and stimulated their neuronal/glutamatergic differentiation, while in vivo, it also dramatically enhanced SAG-NP survival rate after implantation into adult mammalian inner ear. Exogenous administration of NGF enhanced Dorsal Root Ganglia (DRG) survival and stimulated their extensive neurite projections following transplantation into adult rat cochlea [103,104]. Moreover, NGF treatment preserved SGNs and outer hair cells in the cochlea of mice with early onset of hearing loss [105], while it exerts otoprotective effects in neomycin-induced auditory neural degeneration in guinea pigs [106]. Overall, these results provide a solid proof-of-concept for the use of NTs in patients to counteract synaptopathy, improve cochlear implant performance or prevent long-term neural loss after noise exposure [90, 91, 94, 107, 108]. Clinical and audiometric improvement has been found in patients affected by SNHL and tinnitus after autologous stimulation of NGF in nasal fluid [109]. Moreover, a recruiting, randomized, double-blind, placebo-controlled Phase 1/2 clinical trial reported that a single intratympanic injection of BDNF was well tolerated in subjects with speech-in-noise hearing loss, and the proportion of subjects with a clinically meaningful improvement was higher in the BDNF-treated group than in the placebo (NCT04129775). Despite these promising results however, significant safety and clinical data are still needed to grant the pharmacological use of NTs in clinical practice to treat hearing loss.
|
Neurotrophin |
Species |
Method of hearing loss |
Treatment |
Outcome |
References |
|
NGF (200 μg/ml) |
Guinea pig |
30% of neomycin solution by osmotic pump |
2 weeks, infusion by osmotic pump |
Protection of the auditory nerve |
Schindler RA et al., 1995 [127] |
|
NGF (200 μg/ml) |
Guinea pig |
30% of neomycin solution by osmotic pump |
2 weeks, infusion by osmotic pump |
Spiral ganglion survival |
Shah SB et al., 1995 [106] |
|
NT-3 and BDNF (1 mg/mL-1) |
Guinea pig |
Kanamycyn (400 mg Kg -1) followed 2 h later by ethacrynic acid (40 mg Kg-1) |
8 weeks, infusion by osmotic pump |
Prevention of the degeneration of auditory hair cells |
Staecker H et al., 1996 [128] |
|
NT-3 (62 μg/ml) |
Guinea pig |
Oxytetracyclin (10 mg/kg) |
2 weeks, infusion by osmotic pump |
Protecting spiral ganglion neurons |
Ernfors P al., 1996 [129] |
|
BDNF (62.5 μg/mL) |
Guinea pig |
Ketamine (40 mg/kg) and xylazil (4 mg/kg) |
28 days, infusion by osmotic pump |
Prevention of the degeneration of auditory neurons |
Gillespie LN et al., 2003 [130] |
|
BDNF, NT-3, NT-4/5, NGF (10 mg of neurotrophin delivered into the scala tympani) |
Guinea pig |
Ketamine (40 mg/kg) and xylazil (4 mg/kg) |
28 days, infusion by osmotic pump |
Auditory neuron survival |
Gillespie LN et al., 2004 [131] |
|
NT-3 (50 µg/ml) |
Guinea pig |
Kanamycin (400 mg/kg) and frusemide (100 mg/kg) |
28 days, infusion by osmotic pump |
Trophic effects on SGN cell bodies |
Richardson RT et al.,2005 [132] |
|
BDNF (62.5 μg/mL) |
Guinea pig |
Kanamycin (400 mg/kg) and frusemide (100 mg/kg), |
28 days, infusion by osmotic pump with chronic electrical stimulation |
Trophic or survival advantage in the base of the cochlea |
Shepherd RK et al., 2005 [133] |
|
BDNF and NT-3 (50 µg/ml) |
Guinea pig |
Kanamycin (400 mg/kg) and frusemide (100 mg/kg), |
28 days, infusion by osmotic pump |
Re-sprouting of peripheral processes |
Wise AK et al., 2005 [134] |
|
BDNF (5.4μg/ml) |
Wistar rat |
Furosemide (175mg/kg) followed by gentamicin (350mg/kg) |
28 days, infusion by osmotic pump |
SGN rescue |
McGuinness SL, Shepherd RK, 2005 [99] |
|
BDNF (100 µg/ml) |
Guinea pig |
Kanamycin (400 mg/kg) and frusemide (100 mg/kg) |
4 weeks, infusion by osmotic pump |
SGC protection and rescue |
Agterberg MJH et al., 2008 [95] |
|
BDNF (94µg/ml) |
Cat |
Neomycin sulfate (60 mg/kg) |
10 weeks, infusion by osmotic pump |
SG survival neurons |
Leake PA et al., 2010 [97] |
|
BDNF (62.5 μg/mL) |
Guinea pig |
Kanamycin (400 mg/kg) and frusemide (100 mg/kg), |
4 weeks, infusion by osmotic pump |
SGN survival HC protection |
Sly DJ et al., 2011 [94] |
|
BDNF (100 µg/ml) |
Guinea pig |
Kanamycin (400 mg/kg) and frusemide (100 mg/kg), |
4 weeks, infusion by osmotic pump |
long-term SGC survival and protection |
Ramekers D et al., 2015 [90] |
|
NT-3 (1µg/µl) or BDNF (1µg/µl) |
Guinea pig |
2 hours to 4 to 8 kHz noise at either 95 or 105 dB SPL |
A 4µl test bolus was delivered to the round window |
Rescue auditory function and inner HC synaptopathy reduction |
Sly DJ et al., 2016 [92] |
|
NT-3 (300 ng/μl /30 ng/μl ) |
CBA/CaJ mice |
Noise (8–16 kHz) for 2 hours at 98 dB sound pressure level (SPL) |
Local delivery of NT-3 to the round window niche |
Cochlear synaptic regeneration |
Suzuki J et al., 2016 [93] |
|
mNGF (3.6×10-3 μg/g body mass) |
A/J mice |
Age-related |
Intramuscular injection in the hips once every other day.from postnatal day 7 |
SGN and OHC protection |
Gao L et al., 2017 [105] |
|
BDNF (50 ng/mL) |
Guinea pig |
Kanamycin (400 mg/kg) two hours later 40 mg/kg ethacrynic |
mini-osmotic pump for 14 days delivery |
SGN protection |
Scheper V et al., 2020 [100] |
|
BDNF (0.05 µg) |
Guinea pig |
Cisplatin 4 mg/kg per 3 doses on alternate days for a total of 12 mg/kg. |
BDNF injected into the round window |
Hearing restoration |
Blakley BW, Seaman, M. & Alenezi, A, 2020 [96] |
Table 1: Neurotrophins treatment in preclinical studies (in chronological order).
6. Novel neurotrophin-based approaches for the treatment of hearing loss
One of the most significant challenges for the treatment of hearing loss is to effectively deliver the drug, as the anatomy of the ear makes it difficult to achieve the uniform delivery of the drug and a residence time that could allow reaching therapeutically meaningful concentrations. As systemic administration has been limited so far by the onset of side effects, in the last years different approaches have been investigated to optimize NT delivery to ultimately protect the residual cochlear function [110-112]. Among the available administration routes, the intratympanic injection has been widely used, but the leakage of liquid formulations through the Eustachian tube represents the major limitation of this approach. On the other hand, the use of specific biomaterials [113] and advanced microneedles [112] has highly improved the rate of drug delivery from middle to inner ear, preventing the drawbacks of liquid formulations. Among the biomaterials, hydrophilic polymers in particular, such as hydrogels, have attracted the interest of drug researchers because of their chemical functionality, biocompatibility, physical properties and drug loading and degradation capabilities [114,115]. Indeed, their high viscosity and viscoelastic properties allow hydrogel delivery systems to locally retain drugs in a solidified status, preventing NTs from rapidly flowing through the Eustachian tube in the middle ear. This increases NT drugs residence time and extend their diffusion across the Round Window Membrane (RWM), thus achieving a more uniform delivery to the inner ear and allowing to reach therapeutically meaningful concentrations [116]. In line with these data, the application of hydrogels loaded with NTs onto the RWM of deafened guinea pigs resulted in SGC survival [117] and slowed neuron loss [118]. Another possible approach for the targeted delivery of drugs is the nanoparticle (NP). NPs are less-than-100-nm diameter solid particles that are synthesized from compounds such as Polylactide-Co-Glycolide (PLGA) and a diblock copolymer containing Poly-L-Lactide And Polyethylene Glycol (PLLA–mPEG). As alternative nonbiological carriers, NPs carry small hydrophobic drugs, hydrophilic substances and biomolecules, such as peptides and proteins [119], that are delivered to specifically targeted cells or organs [120]. With the aim of increase the half-life of drugs and achieve their sustained or targeted release, NPs have been thus also tested for the delivery of NTs through the RWM. NGF functionalized NPs have been investigated in particular for their ability to target cells of the inner ear in organotypic explant cultures of the mouse inner ear and PC-12 rat pheochromocytoma cells. Notably, these NPs did not show any signs of toxicity, thus implying the potential application of NT-loaded NPs as therapeutic approaches for the stimulation of nerve and hair cell regeneration or repair [121].
Since the neurotrophic and neuroprotective role of NTs is defined and well established, clinical research has been recently focused on the generation of small molecules and monoclonal antibodies that mimic NTs’ function and can target Trk receptors to prevent neural cell death and support neural survival. Among the available NT mimetics, 1Aa and Ris-1Aa and 7,8-Dihydroxyfla-vone (DHF) are a small molecule analogue of NT-3 and a selective agonist of TrkB, respectively, that have shown to stimulate SGNs and promote regeneration of synapses between SGNs and inner hear cells in in vitro studies [122,123]. DHF has been reported to be effective also in protecting and restoring both synapses and neural function in noise-exposed mouse ears [124]. However, these results have not been confirmed using another small molecule TrkB agonist (7,8,30-trihydroxyflavone) that did not affect survival of SGCs in deafened guinea pigs [125]. In a recent report, DHF has been compared to a new class of monoclonal antibody agonists of TrkB or TrkC, known as “M-antibodies” for their effects on rat cochlea ex vivo models. Four of these M-antibodies activate TrkB (M3, M4, M5 and M6) and three of them activate TrkC (M1, M2 and M7). Among all, M3 showed the greatest activity on SGN survival, neurite extension and synapse restoration, suggesting that this antibody can be potentially used for further investigations for the treatment of hearing loss [126]. However, additional studies are required for the development of these agonists, including an evaluation of biostability, bioavailability, safety and efficacy in animal models to further investigate their potential to treat hearing loss.
7. Conclusions and future perspectives
Although in the last decade considerable progress has been made in the understanding of the causes and molecular mechanisms underlying hearing loss, the need for new therapeutic approaches for the treatment of this disorder remains high. The crucial and multifaced role of NTs in regulating the development of the mammalian nervous system and the functions of both neuronal and non-neuronal cells also after the developmental stage, have suggested the potential application of NTs as therapeutic approaches for the treatment of hearing loss disorders. Supporting this concept, the treatment with NTs, and in particular with NGF and BDNF, has shown the ability to induce the regeneration of hair cells, spiral ganglion neurons and stria vascularis, ultimately improving hearing functions in various models of ear diseases. Despite the promising preclinical results and the potential application for the rescue of the SGN degeneration and for patients undergoing cochlear implantation, however, NT treatments have not yet achieved clinical translation. Since this can be attributed mainly to the limitations due to ear delivery and permeation of drugs, the development of new methods of local drug application aimed at reducing possible adverse effects, and the thorough investigation of drug pharmacokinetic properties to ensure the achievement of the target cell, could represent crucial starting points for the development of new therapeutic approaches. Moreover, the exploitation of the knowledge acquired so far on NTs and on their role in ear physiology and pathophysiology, together with the implementation of animal-based experimental research, might improve the knowledge we have already gained in the field and pave the way to new clinical studies that will provide solid support on the safety and efficacy of NT treatment in ear disorders. In conclusion, the development of optimized formulations in combination with the rational choice of the administration route could be the key factor for the selection of new NT-based approaches that can be moved to clinical application for the treatment of hearing loss disorder.
Author Contributions
PC, CG, RN and LB wrote the manuscript. MA and AA substantially revised it. All the authors contributed to the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Italian Ministry of Economic Development, Grant “Piattaforma tecnologica integrata per l′identificazione e lo sviluppo di nuovi farmaci per il trattamento di patologie rare o ad elevato bisogno di cura insoddisfatto (PON I&C 2014/2020 D.M. 1 giugno 2016 F/090033/01-03-04/X36)”.
Conflicts of Interest
The authors PC, CG, RN, AA, MA, LB declare the following competing interest: they are employees of Dompé Farmaceutici s.p.a.
References
- Olusanya BO, Davis AC, Hoffman HJ. Hearing loss grades and the International classification of functioning, disability and health. Bull. World Health Organ 97 (2019): 725-728.
- Müller U, Barr-Gillespie PG. New treatment options for hearing loss. Nat. Rev. Drug Discov. 14 (2015): 346-365.
- Qing Z, Mao-li D. Anatomy and physiology of peripheral auditory system and commen causes of hearing loss. J Otol 4 (2009): 7-14.
- Hunter AGW, Yotsuyanagi T. The external ear: More attention to detail may aid syndrome diagnosis and contribute answers to embryological questions. Am J Med Genet A 135 (2005): 237-250.
- Goode RL, Killion M, Nakamura K, et al. New knowledge about the function of the human middle ear: development of an improved analog model. Am. J Otol 15 (1994): 145-154.
- Kleinlogel S, Vogl C, Jeschke M, et al. Emerging Approaches for Restoration of Hearing and Vision. Physiol Rev 100 (2020): 1467-1525.
- Ma Y, Wise AK, Shepherd RK, et al. New molecular therapies for the treatment of hearing loss. Pharmacol Ther 200 (2019): 190-209.
- Wagner EL, Shin JB. Mechanisms of Hair Cell Damage and Repair. Trends Neurosci 42 (2019): 414-424.
- Tu NC, Friedman RA. Age-related hearing loss: Unraveling the pieces. Laryngoscope Investig. Otolaryngol 3 (2018): 68-72.
- Liu XZ, Yan D. Ageing and hearing loss. J Pathol 211 (2007): 188-197.
- Kamil RJ, Betz J, Powers BB, et al. Association of Hearing Impairment with Incident Frailty and Falls in Older Adults. J. Aging Health 28 (2016): 644-660.
- Rutherford BR, Brewster K, Golub JS, et al. Sensation and Psychiatry: Linking Age-Related Hearing Loss to Late-Life Depression and Cognitive Decline. Am J Psychiatry 175 (2018): 215-224.
- Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet Lond. Engl 390 (2017): 2673-2734.
- Yamasoba T, Lin FR, Someya S, et al. Current concepts in age-related hearing loss: epidemiology and mechanistic pathways. Hear Res 303 (2013): 30-38.
- Nadol JB. The Laryngoscope 106 (1996): 1327-1329.
- Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol 102 (1993): 1-16
- Ohlemiller KK. Age-related hearing loss: the status of Schuknecht’s typology. Curr. Opin. Otolaryngol. Head Neck Surg 12 (2004): 439-443.
- Scholtz AW, Kommen-Jolley K, Felder E, et al. Selective aspects of human pathology in high-tone hearing loss of the aging inner ear. Hear Res 157 (2001): 77-86.
- Fransen E, Bonneux S, Jason JC, et al. Genome-wide association analysis demonstrates the highly polygenic character of age-related hearing impairment. Eur J Hum Genet EJHG 23 (2015): 110-115.
- Wolber LE, Girooto G, Buniello A, et al. Salt-inducible kinase 3, SIK3, is a new gene associated with hearing. Hum Mol Genet 23 (2014): 6407-6418.
- Kutz JW, Simon LM, Chennupati SK, et al. Clinical predictors for hearing loss in children with bacterial meningitis. Arch. Otolaryngol. Head Neck Surg 132 (2006): 941-945.
- Cohen BE, Durstenfeld A, Roehm PC. Viral causes of hearing loss: a review for hearing health professionals. Trends Hear 18 (2014): 2331216514541361
- Cadoni, G, Agostino S, Scipione S, et al. Sudden sensorineural hearing loss: our experience in diagnosis, treatment, and outcome. J Otolaryngol 34 (2005): 395-401.
- Lee JY, Bowden DS. Rubella Virus Replication and Links to Teratogenicity. Clin Microbiol Rev 13 (2000): 571-587
- Prasad HKC, Bhojwani KM, Shenoy V, et al. HIV manifestations in otolaryngology. Am J Otolaryngol 27 (2006): 179-185.
- Van der Westhuizen Y, Swanepoel DW, Heinze B, et al. Auditory and otological manifestations in adults with HIV/AIDS. Int J Audiol 52 (2013): 37-43.
- Yaseen NK, Al-Ani RM, Ali Rashid R. COVID-19-related sudden sensorineural hearing loss. Qatar Med J 2021 (2021): 58.
- Natalie S, Alida N. An overview of pharmacotherapy-induced ototoxicity. South Afr Fam Pract 55 (2013): 357-365.
- Yorgason JG, Fayad JN, Kalinec F. Understanding drug ototoxicity: molecular insights for prevention and clinical management. Expert Opin Drug Saf 5 (2006): 383-399.
- Trendowski MR, et al. Clinical and genetic risk factors for radiation-associated ototoxicity: A report from the Childhood Cancer Survivor Study and the St. Jude Lifetime Cohort. Cancer 127 (2021): 4091-4102.
- Rybak LP, Ramkumar V, Mukherjea D. Ototoxicity of Non-aminoglycoside Antibiotics. Front Neurol 12 (2021) 652674.
- Laurell G, Ekborn A, Viberg A, et al. Effects of a single high dose of cisplatin on the melanocytes of the stria vascularis in the guinea pig. Audiol Neurootol 12 (2007): 170-178.
- Selimoglu E. Aminoglycoside-induced ototoxicity. Curr Pharm Des 13 (2007): 119-126.
- McGhan LJ, Merchant SN. Erythromycin ototoxicity. Otol Neurotol Off Publ Am Otol Soc Am Neurotol. Soc Eur Acad Otol Neurotol 24 (2003): 701-702.
- Forouzesh A, Moise PA, Sakoulas G. Vancomycin ototoxicity: a reevaluation in an era of increasing doses. Antimicrob. Agents Chemother 53 (2009): 483-486.
- Lo SH, Kotabe S, Mitsunaga L. Azithromycin-induced hearing loss. Am. J. Health-Syst. Pharm. AJHP Off. J Am Soc Health-Syst Pharm 56 (1999): 380-383.
- Kolkman W, Groeneveld JHM, Baur HJCM, et al. Ototoxicity induced by clarithromycin. Ned Tijdschr Geneeskd 146 (2002): 1743-1745.
- Brock PR, Knight KR, Freyer DR, et al. Platinum-Induced Ototoxicity in Children: A Consensus Review on Mechanisms, Predisposition, and Protection, Including a New International Society of Pediatric Oncology Boston Ototoxicity Scale. J Clin Oncol 30 (2012): 2408-2417.
- Knight KR, Chen LJ, Freyer D, et al. Group-Wide, Prospective Study of Ototoxicity Assessment in Children Receiving Cisplatin Chemotherapy (ACCL05C1): A Report From the Children’s Oncology Group. J Clin Oncol Off J Am Soc Clin Oncol 35 (2017): 440-445.
- Clemens, Vries AC, Tissing WJ, et al. Hearing loss after platinum treatment is irreversible in noncranial irradiated childhood cancer survivors. Pediatr Hematol Oncol 34 (2017): 120-129
- Waissbluth S, Chuang A, Del Valle Á, et al. Long term platinum-induced ototoxicity in pediatric patients. Int J Pediatr Otorhinolaryngol 107 (2018): 75-79.
- Paken J, Govender CD, Pillay M, et al. Cisplatin-Associated Ototoxicity: A Review for the Health Professional. J Toxicol 2016 (2016): 1809394.
- Callejo A, Durochat A, Bresseiux S, et al. Dose-dependent cochlear and vestibular toxicity of trans-tympanic cisplatin in the rat. Neurotoxicology 60 (2017): 1-9.
- Lin FR, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med 173 (2013): 293-299.
- Gurney JG, Tersak JM, Ness KK, et al. Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: a report from the Children’s Oncology Group. Pediatrics 120 (2007): e1229-1236.
- Schreiber JE, Gurney JG, Palmer SL, et al. Examination of risk factors for intellectual and academic outcomes following treatment for pediatric medulloblastoma. Neuro-Oncol 16 (2014): 1129-1136.
- Olivier TW, Bass JK, Ashford JM, et al. Cognitive Implications of Ototoxicity in Pediatric Patients with Embryonal Brain Tumors. J Clin Oncol Off J Am Soc Clin Oncol 37 (2019): 1566-1575.
- Clemens E, Marry MHE, Mulder RL, et al. Recommendations for ototoxicity surveillance for childhood, adolescent, and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCare Consortium. Lancet Oncol 20 (2019): e29-e41.
- Gordon JS, et al. Audiologic characteristics in a sample of recently-separated military Veterans: The Noise Outcomes in Servicemembers Epidemiology Study (NOISE Study). Hear Res 349 (2017): 21-30.
- Tagoe T, Barker M, Jones A, et al.. Auditory nerve perinodal dysmyelination in noise-induced hearing loss. J Neurosci Off J Soc Neurosci 34 (2014): 2684-2688.
- Coyat C, Cazieveillei C, Baudoux V, et al. Morphological consequences of acoustic trauma on cochlear hair cells and the auditory nerve. Int J Neurosci 129 (2019): 580-587.
- Rask-Andersen H, Ekvall L, Scholtz A, et al. Structural/audiometric correlations in a human inner ear with noise-induced hearing loss. Hear Res 141 (2000): 129-139.
- Aedo C, Aguilar E. Cochlear synaptopathy: new findings in animal and human research. Rev Neurosci 31 (2020): 605-615.
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after ‘temporary’ noise-induced hearing loss. J Neurosci Off J Soc Neurosci 29 (2009): 14077-14085.
- Wan G, Corfas G. Transient auditory nerve demyelination as a new mechanism for hidden hearing loss. Nat Commun 8 (2017): 14487.
- Kyle ME, Wang JC, Shin JJ. Impact of nonaspirin nonsteroidal anti-inflammatory agents and acetaminophen on sensorineural hearing loss: a systematic review. Otolaryngol-Head Neck Surg Off J Am Acad Otolaryngol.-Head Neck Surg 152 (2015): 393-409.
- Gonzalez-Gonzalez S. The role of mitochondrial oxidative stress in hearing loss. Neurol Disord Ther 1 (2017).
- Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science 240 (1988): 1774-1776.
- Tomassini A, Spinelli D, Jacono M, et al. Rhythmic oscillations of visual contrast sensitivity synchronized with action. J Neurosci Off J Soc Neurosci 35 (2015): 7019-7029.
- Moffat DA, Ramsden RT. Profound bilateral sensorineural hearing loss during gentamicin therapy. J Laryngol Otol 91 (1977): 511-516.
- Fee WE Jr. Gentamicin and Tobramycin: Comparison of Ototoxicity. Rev Infect Dis 5 (1983): S304-S313.
- Shu Y, Li W, Huang M, et al. Renewed proliferation in adult mouse cochlea and regeneration of hair cells. Nat Commun 10 (2019): 5530.
- Bramhall NF, Shi F, Arnold K, et al. Lgr5-positive supporting cells generate new hair cells in the postnatal cochlea. Stem Cell Rep 2 (2014): 311-322.
- Maass JC, Gu R, Basch ML, et al. Changes in the regulation of the Notch signaling pathway are temporally correlated with regenerative failure in the mouse cochlea. Front Cell Neurosci 9 (2015): 110.
- Menendez L, et al. Generation of inner ear hair cells by direct lineage conversion of primary somatic cells. eLife 9 (2020): e55249.
- Oshima K, Senn P, Heller S. Isolation of sphere-forming stem cells from the mouse inner ear. Methods Mol Biol Clifton NJ 493 (2009): 141-162.
- Cox BC, Chai R, Lenoir A, et al. Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development 141 (2014): 1599.
- Bucks SA, Cox BC, Vlocish BA, et al. Supporting cells remove and replace sensory receptor hair cells in a balance organ of adult mice. eLife 6 (2017): e18128.
- Okano T, Kelley MW. Stem cell therapy for the inner ear: recent advances and future directions. Trends Amplif 16 (2012): 4-18.
- Tateya I, Nakagawa T, Iguchi F, et al. Fate of neural stem cells grafted into injured inner ears of mice. Neuroreport 14 (2003): 1677-1681.
- Oshima K, Shin K, Mac D, et al. Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell 141 (2010): 704-716.
- Lee HS, Kim WJ, Gong JS, et al. Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients. J Audiol Otol 22 (2018): 105-109.
- Bas E, Water TR, Lumbreras V, et al. Adult human nasal mesenchymal-like stem cells restore cochlear spiral ganglion neurons after experimental lesion. Stem Cells Dev 23 (2014): 502-514.
- Choi BY, Song JJ, Chang SO, et al. Intravenous administration of human mesenchymal stem cells after noise- or drug-induced hearing loss in rats. Acta Otolaryngol. (Stockh.) 132 (2012: 94-102.
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24 (2001): 677-736.
- Levi-Montalcini R, Hamburger V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exp Zool 116 (1951): 321-361.
- Vilar M, Mira H. Regulation of Neurogenesis by Neurotrophins during Adulthood: Expected and Unexpected Roles. Front Neurosci 10 (2016): 26.
- Hennigan A, O’Callaghan RM, Kelly AM. Neurotrophins and their receptors: roles in plasticity, neurodegeneration and neuroprotection. Biochem Soc Trans 35 (2007): 424-427.
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci 6 (2005): 603-614.
- Fritzsch B, Tessarollo L, Coppola E, et al. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog. Brain Res 146 (2004): 265-278.
- Fritzsch B, Fariñas I, Reichardt LF. Lack of Neurotrophin 3 Causes Losses of Both Classes of Spiral Ganglion Neurons in the Cochlea in a Region-Specific Fashion. J Neurosci 17 (1997): 6213-6225.
- Fritzsch B, Pirvola U, Ylikoski J. Making and breaking the innervation of the ear: neurotrophic support during ear development and its clinical implications. Cell Tissue Res 295 (1999): 369-382.
- Bianchi LM, Conover JC, Fritzch B, et al. Degeneration of vestibular neurons in late embryogenesis of both heterozygous and homozygous BDNF null mutant mice. Dev Camb Engl 122 (1996): 1965-1973.
- Ernfors P, Van De Water T, Loring J, et al. Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron 14 (1995): 1153-1164.
- Li Q, Chen M, Zhang C, et al. Opposite Roles of NT-3 and BDNF in Synaptic Remodeling of the Inner Ear Induced by Electrical Stimulation. Cell Mol Neurobiol 41 (2021): 1665-1682.
- Zheng JL, Stewart RR, Gao WQ. Neurotrophin-4/5 enhances survival of cultured spiral ganglion neurons and protects them from cisplatin neurotoxicity. J Neurosci off J Soc Neurosci 15 (1995): 5079-5087.
- Gillespie LN, Shepherd RK. Clinical application of neurotrophic factors: the potential for primary auditory neuron protection. Eur J Neurosci 22 (2005): 2123-2133.
- Kashyap MP, Roberts C, Waseem M, et al. Drug Targets in Neurotrophin Signaling in the Central and Peripheral Nervous System. Mol Neurobiol 55 (2018): 6939-6955.
- Richardson RT, Noushi F, O’Leary S. Inner ear therapy for neural preservation. Audiol Neurootol 11 (2006): 343-356.
- Ramekers D, Versnel H, Strahl SB, et al. Temporary Neurotrophin Treatment Prevents Deafness-Induced Auditory Nerve Degeneration and Preserves Function. J Neurosci 35 (2015): 12331-12345.
- Miller JM, Miller AL, Yamagata T, et al. Protection and Regrowth of the Auditory Nerve after Deafness: Neurotrophins, Antioxidants and Depolarization Are Effective in vivo. Audiol. Neurotol 7 (2002): 175-179.
- Sly DJ, Cambel L, Uschakov A, et al. Applying Neurotrophins to the Round Window Rescues Auditory Function and Reduces Inner Hair Cell Synaptopathy After Noise-induced Hearing Loss. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol 37 (2016): 1223-1230.
- Suzuki J, Corfas G, Liberman MC. Round-window delivery of neurotrophin 3 regenerates cochlear synapses after acoustic overexposure. Sci Rep 6 (2016): 24907.
- Sly DJ, Hampson AJ, Minter Rl, et al. Brain-derived neurotrophic factor modulates auditory function in the hearing cochlea. J Assoc Res Otolaryngol JARO 13 (2012): 1-16.
- Agterberg MJH, Versnel H,Groot JCM, et al. Morphological changes in spiral ganglion cells after intracochlear application of brain-derived neurotrophic factor in deafened guinea pigs. Hear Res 244 (2008): 25-34.
- Blakley BW, Seaman M, Alenezi A. Brain-derived nerve growth factor in the cochlea – a reproducibility study. J Otolaryngol-Head Neck Surg 49 (2020): 37.
- Leake PA, Hradek GT, Hetherington AM, et al. Brain-derived neurotrophic factor promotes cochlear spiral ganglion cell survival and function in deafened, developing cats. J Comp Neurol 519 (2011): 1526-1545.
- Leake PA, Rebscher SJ, Dore’ C, et al. AAV-Mediated Neurotrophin Gene Therapy Promotes Improved Survival of Cochlear Spiral Ganglion Neurons in Neonatally Deafened Cats: Comparison of AAV2-hBDNF and AAV5-hGDNF. J Assoc Res Otolaryngol JARO 20 (2019): 341-361.
- McGuinness SL, Shepherd RK. Exogenous BDNF rescues rat spiral ganglion neurons in vivo. Otol. Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol 26 (2005): 1064-1072.
- Scheper V, Seidel-Effenberg I, Lenarz T, et al. Consecutive Treatment with Brain-Derived Neurotrophic Factor and Electrical Stimulation Has a Protective Effect on Primary Auditory Neurons. Brain Sci 10 (2020): 559.
- Xie J, Pak K, Evans A, et al. Neurotrophins differentially stimulate the growth of cochlear neurites on collagen surfaces and in gels. Neural Regen Res 8 (2013): 1541-1550.
- Khan AM, Whiten DM, Nadol JB, et al. Histopathology of human cochlear implants: correlation of psychophysical and anatomical measures. Hear Res 205 (2005): 83-93.
- Hu Z, Ulfendahl M, Olivius NP. Survival of neuronal tissue following xenograft implantation into the adult rat inner ear. Exp Neurol 185 (2004): 7-14.
- Hu Z, Ulfendahl M, Olivius NP. NGF stimulates extensive neurite outgrowth from implanted dorsal root ganglion neurons following transplantation into the adult rat inner ear. Neurobiol Dis 18 (2005): 184-192.
- Gao L, Ge R, Xie G, et al. Hearing Improvement in A/J Mice via the Mouse Nerve Growth Factor. Clin Exp Otorhinolaryngol 10 (2017): 303-308.
- Shah SB, Gladstone HB, Williams H, et al. An extended study: protective effects of nerve growth factor in neomycin-induced auditory neural degeneration. Am J Otol 16 (1995): 310-314.
- Pinyon JL, Georg VJ, Edward NC, et al. Neurotrophin gene augmentation by electrotransfer to improve cochlear implant hearing outcomes. Hear Res 380 (2019): 137-149.
- Schilder AGM, et al. Early phase trials of novel hearing therapeutics: Avenues and opportunities. Hear Res 380 (2019): 175-186.
- Salvinelli F, Frari V, Rocco ML, et al. Enhanced presence of NGF and mast cells number in nasal cavity after autologous stimulation: relation with sensorineural hearing deficit. Eur Rev Med Pharmacol Sci 19 (2015): 381-391.
- Jung SY, Yoo J, Yang KJ, et al. Intratympanic administration of alpha-lipoic acid-loaded pluronic F-127 nanoparticles ameliorates acute hearing loss. Nanomedicine Nanotechnol. Biol Med 32 (2021): 102329.
- Darge HF, Andrgie AT, Tsai HC, et al. Polysaccharide and polypeptide based injectable thermo-sensitive hydrogels for local biomedical applications. Int J Biol Macromol 133 (2019): 545-563.
- Aksit A, et al. Drug delivery device for the inner ear: ultra-sharp fully metallic microneedles. Drug Deliv Transl Res 11 (2021): 214-226.
- Rathnam C, Chueng STD, Ying YLM, et al. Developments in Bio-Inspired Nanomaterials for Therapeutic Delivery to Treat Hearing Loss. Front. Cell Neurosci 13 (2019): 493.
- Ahmed EM. Hydrogel: Preparation, characterization, and applications: A review. J Adv Res 6 (2015): 105-121.
- Schmidt BVKJ. Hydrophilic Polymers. Polymers 11 (2019): E693.
- Xu X, Zheng J, He Y, et al. Nanocarriers for Inner Ear Disease Therapy. Front Cell Neurosci 15 (2021): 791573.
- Havenith S, Versnal H, Martijn JHA, et al. Spiral ganglion cell survival after round window membrane application of brain-derived neurotrophic factor using gelfoam as carrier. Hear Res 272 (2011): 168-177.
- Noushi F, Richardson RT, Hardman J, et al. Delivery of neurotrophin-3 to the cochlea using alginate beads. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol 26 (2005): 528-533.
- Xu X, Wang S, Shu Y, et al. Nanoparticle-based inner ear delivery systems for the treatment of hearing loss. Smart Mater Med 2 (2021): 350-353.
- Poe DS, Pyykkö I. Nanotechnology and the treatment of inner ear diseases. Wiley Interdiscip. Rev Nanomed Nanobiotechnol 3 (2011): 212-221.
- Roy S, Alex HJ, Tracey AN, et al. Cell-specific targeting in the mouse inner ear using nanoparticles conjugated with a neurotrophin-derived peptide ligand: potential tool for drug delivery. Int J Pharm 390 (2010): 214-224.
- Kempfle JS, et al. Bisphosphonate-Linked TrkB Agonist: Cochlea-Targeted Delivery of a Neurotrophic Agent as a Strategy for the Treatment of Hearing Loss. Bioconjug Chem 29 (2018): 1240-1250.
- Kempfle JS, Duro MV, Zhang A, et al. A Novel Small Molecule Neurotrophin-3 Analogue Promotes Inner Ear Neurite Outgrowth and Synaptogenesis In vitro. Front Cell Neurosci 15 (2021): 666706.
- Fernandez KA, et al. Trk agonist drugs rescue noise-induced hidden hearing loss. JCI Insight 6 (2021).
- Vink HA, van Dorp WC, Thomeer HGXM, et al. BDNF Outperforms TrkB Agonist 7,8,3′-THF in Preserving the Auditory Nerve in Deafened Guinea Pigs. Brain Sci 10 (2020): 787.
- Szobota S, Msthur PD, Siegel S, et al. BDNF, NT-3 and Trk receptor agonist monoclonal antibodies promote neuron survival, neurite extension, and synapse restoration in rat cochlea ex vivo models relevant for hidden hearing loss. PloS One 14 (2019): e0224022.
- Schindler RA, Gladstone HB, Scott N, et al. Enhanced preservation of the auditory nerve following cochlear perfusion with nerve growth factors. Am J Otol 16 (1995): 304-309.
- Staecker H, Kopke R, Malgrange B, et al. T. R. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport 7 (1996): 889-894.
- Ernfors P, Li Duan M, Elshamy WM, et al. Protection of auditory neurons from aminoglycoside toxicity by neurotrophin-3. Nat Med 2 (1996): 463-467.
- Gillespie LN, Clark GM, Bartlett PF, et al. BDNF-induced survival of auditory neurons in vivo: Cessation of treatment leads to accelerated loss of survival effects. J Neurosci Res 71 (2003): 785-790.
- Gillespie LN, Clark GM, Marzella PL. Delayed neurotrophin treatment supports auditory neuron survival in deaf guinea pigs: NeuroReport 15 (2004): 1121-1125.
- Richardson RT, O’Leary S, Wise A, et al. A single dose of neurotrophin-3 to the cochlea surrounds spiral ganglion neurons and provides trophic support. Hear Res 204 (2005): 37-47.
- Shepherd RK, Coco A, Epp SB, et al. Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol 486 (2005): 145-158.
- Wise AK, Richardson R, Hardman J, et al. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J Comp Neurol 487 (2005): 147-165.
