The Role of miR-181c in the Development of the tri-metabolic Disorders: Diabetes, Obesity and Aging
Article Information
Nura A Mohamed*, 1, Tanveer A. Tabish2, Hana A Mohamed1, Sarah I Mazi3, Fahad A Alrashed3
1Biomedical Research Center (BRC), Qatar University, Doha-Qatar.
2Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, Oxford-United Kingdom.
3Department of Cardiac Sciences, College of Medicine, King Saud University, P.O. Box 7805, Riyadh 11472, Saudi Arabia
*Corresponding author: Nura A Mohamed, Biomedical Research Center (BRC), Qatar University, Doha-Qatar.
Received: 27 September 2023; Accepted: 03 October 2023; Published: 05 December 2023
Citation: Nura A Mohamed, Tanveer A. Tabish, Hana A Mohamed, Sarah I Mazi, Fahad A Alrashed. The Role of miR-181c in the Development of the tri-metabolic Disorders: Diabetes, Obesity and Aging. Archives of Clinical and Biomedical Research. 7 (2023): 604-613.
View / Download Pdf Share at FacebookAbstract
In the past decades, miRNAs were considered pivotal cellular rheostats that control many critical and fundamental signaling pathways. These small noncoding RNAs play a fundamental role in regulating gene expression, with accumulating evidence of the miRNAs ability to modulate diverse signaling pathways that once altered can lead to the development of many metabolic disorders. In addition, miRNAs were shown to have a crucial role in maintaining mitochondrial homeostasis and inflammatory responses. Of the many studied miRNAs, the highly conserved miR-181 family, particularly miR-181c represents an interesting research area. In the current review, we will provide an overview of the tri-pathways miR-181c is involved in, its function, and its role in developing diabetes, obesity, impaired angiogenesis, and aging. Additionally, this review will provide key insights into the key markers miR-181c triggers or targets in these disease models. Finally, this review aims to provide a better understanding of the miR-181c family with the hope that it may open new therapeutic avenues for different metabolic conditions.
Keywords
MicroRNA; Diabete; Ageing; Obesity; Metabolic Disorders
Article Details
1. Introduction
MicroRNAs (miRNAs) are endogenous small non-coding RNAs (20–22 nucleotides in length); they are pivotal for cellular behavior as they regulate various fundamental biological pathways that maintain the developmental processes and homeostasis of mature tissues/organs; by regulating the gene expression. miRNAs usually down-regulate the expression of their target genes, through their imperfect binding to the seed regions of the target sequences often located in 3′-untranslated regions [1]. Depending on the recognition site, the binding of the miRNA-induced silencing complex to the cognate target can have different outcomes [2]. In the majority of cases, the binding is partially complementary to the target sites and leads to the repression of translation, whereas when it is fully complementary, it leads to the degradation of the target transcript [1, 3, 4]. In the vast majority of cases, miRNAs are grouped in families consisting of several members that only differ in few nucleotides outside the seed region. Subsequently, each family member is clustered in specific chromosomal regions that can be present in two or more copies [5]. Of the different miRNA families, lately the miR-181 [6] family has been drawing a lot of interest in the biomedical research. Intriguinely, it was revealed that miR-181 family modulates various important cellular events including cellular proliferation, mitochondrial function, autophagy, programmed cell death, and immune responses [7-12]. MiR-181 family consists of four different 5p mature forms: (i) miR-181a-5p; (ii) miR-181b-5p; (iii) miR-181c-5p, and (iv) miR-181d-5p [13]. It is noteworthy that this miRNA family is an evolutionarily family that is well conserved across all vertebrate species; with miR-181a, and miR-181b being the original family members. Whereas the “c” and “d” paralogs appeared more recently through evolution [14].
MiR-181c’s important roles start early in life from the embryo implantation stage and continue until the end of the individual’s life [14-19]. In vascular biology, miR-181c is involved in regulating the following vascular genes and inflammatory markers BMPR2 Li et al. [20], mitochondrial cytochrome c oxidase subunit 1 (mt-COX1) [21], and IL-2 [22]. Remarkably, studies have shown that miR-181c targets the mt-COX1 expression through the interaction with its mRNA, thereby inhibiting the mt-COX1 3′-UTR activity. Subsequently the overexpression of miR-181c causes an increase in mitochondrial respiration, and intern increasing the reactive oxygen species (ROS). Revealing the potentially important role of miR-181c in the regulation of mitochondrial gene expression and function. MiR-181c also has well-known roles in the inflammatory response; Xue et.al [22], showed that miR-181c acts as a suppressor of CD4+ T cell activation by binding to the 3′-UTR of IL-2 [22].
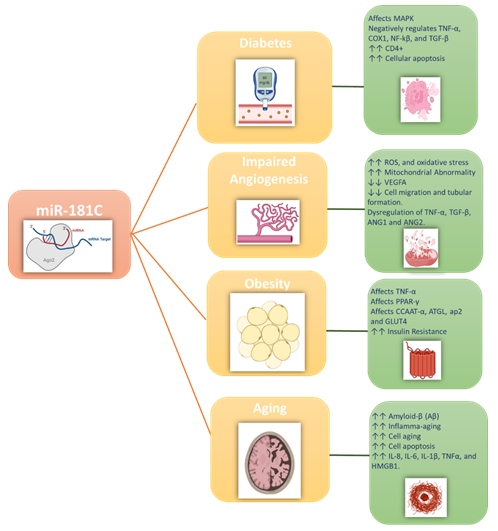
Additionally, miR-181c plays a critical role in immunosenescence in the context of aging, with older individuals showing reduced expression levels of the miR-181 family members. Such lower expression also causes a reduction in the lymphocyte count, particularly both T and B cells in the peripheral blood [23]. In this review, we will summarize the emerging roles of miR-181c and the crosstalk between metabolic and inflammatory pathways involved in the development of four closely related metabolic disorders including diabetes, angiogenesis, obesity, and metabolic aging. Figure 1 illustrates the major effects of miR-181c in the different metabolic disorders discussed in this review.
2. MiR-181c role in diabetes
The effect insulin has on almost every cell, and tissue type is well recognized. Such effects are mediated via the insulin receptors present on the membranes of different cell types and the many signaling cascades it exerts. The outcomes of these effects regulate the systemic fuel metabolism, energy consumption, and cellular survival and replication. Although insulin's effect varies depending on the cell type; it generally maintains nutrients distribution, in addition to the distribution of cytokines, hormones, and other signaling molecules, as well as removing the generated by-products [24-28]. Insulin resistance, a crucial feature and risk factor for Type 2 diabetes (T2D) [29], causes a disturbance in the nutrients metabolism and energy homeostasis. Chronic inflammation is a major contributor to the development of insulin resistance [30, 31]. Furthermore, the biochemical consequences of insulin resistance affect both the classical peripheral target organs (e.g. adipose tissue, liver, and skeletal muscle) and the non-classical target organs (e.g., brain) [32].
The main inflammatory pathways that play key roles in the progression of insulin resistance are: (i) mitogen-activated protein kinase (MAPKs), (ii) nuclear factor-kappa B (NF-κB), and (iii) TGF-β. MAPKs are a family of three mammalian protein kinases: p38, extracellular signal-related kinase (ERK), and c-Jun NH2-terminal kinase (JNK). The activation of the MAPKs leads to the serine phosphorylation of the insulin receptor-1 (IRS-1), which results in the downstream impairment of the PI3K-AKT pathway, decreasing the transduction of the insulin-signaling pathway [33]. Moreover, pro-inflammatory cytokines such as TNF-α was shown to mediate the activation of the IκB kinase (IKK)-β inhibition, which then directly targets the serine residue of the IRS-1 or rapidly removes tyrosine phosphate groups from IRS-1 in an NF-κB-dependent manner. Such actions were later found to link metabolic inflammation and the development of insulin resistance [34-37]. Finally, the TGF-β pathway has widely been implicated in the regulation of many cellular functions including angiogenesis, cell adhesion, immunosuppression, apoptosis induction, and cell growth and differentiation [38, 39]. Active TGF-β ligands promote receptor phosphorylation through binding to the TGF-β receptors type I (TGFβR1) and II (TGFβR2). Such binding activates the SMAD pathway; then, the SMAD proteins act as transcription factors that mediate gene expressions through the SMAD cascade [39]. This cascade is critically important for regulating cell development and growth. Therefore, TGF-β dysregulation affects the SMAD cascade in T2D, which underpins the development of many related complications [40].
Recent studies demonstrated that alterations in miRNAs were proven to be relevant to the development of insulin resistance in T2D. miRNAs, including miR-181, tune the activity of innate immune cells, thereby indirectly modulating the inflammation-derived insulin resistance in target tissues. Being abundantly expressed in many cells and tissues, miR-181c was also implicated in various diseases, including T2D [41]. miR-128c has been demonstrated to negatively control TNFα, a cytokine contributing to inflammation in T2D [32, 42]. MiR-181c has a dual action as under normal conditions, it was found to enhance the insulin-signaling pathway, but under diseased conditions, it was shown to worsen the insulin resistance and cause several diabetes-related complications such as impaired angiogenesis and impaired adipogenesis. This is due to the fact that miR-181c can affect the metabolism of both glucose and fatty acid metabolism via glycolytic and mitochondrial fluxes, which indirectly alters many cellular functions [24, 43]. Accumulating evidences showed that the expression of miR-181c tightly controls energy expenditure, inflammation, and insulin sensitivity [44]. Additionally, miR-181c was shown to play a role in CD4+ T-cell activation [22]. Furthermore, miR-181c was shown to regulate the cellular response to oxidative stress in the context of myocardial function [45], it was also shown to correlate to those of COX1 Intriguingly negatively, miR-181c levels were shown to be significantly upregulated under hypoxia condition, which was positively correlated with the severity of apoptosis [46]. Finally, emerging evidences suggests that miR-181c is implicated in the regulation of both the NF-κB and TGF- β pathways in a negative feedback fashion [47].
3. MiR-181c role in angiogenesis
Angiogenesis is the physiological process of new blood vessels formation from preexisting ones48. Traditionally, other processes such as vasculogenesis, intussusception, and vascular mimicry, differentiation into arteries (arteriogenesis) are also involved in the process of vessel formation. Since vessels nourish, deliver oxygen, and uptake by-products in nearly every cell in the body, any changes in the angiogenesis process (i.e. increase or decrease) can impact the body's function. Despite being beneficial for tissue growth and regeneration, angiogenesis can also enhance the inflammatory response such as seen in cancer as well as contributing to tumorigenesis leading to mortality49. Insulin has pleiotropic effects on endothelial cells [50]. For instance, it induces vasorelaxation, and enhances the uptake of amino acids (e.g. L-arginine) [51, 52], the expression of the pro-angiogenic factors such as the vascular endothelial growth factor (VEGF) [48]. It also increases the endothelial cells' survival and migration rate [51, 52], and increases the pericytes’ survival rate and reduces their expression of the anti-angiogenic proteins [48]. Taken together, abnormalities in insulin signaling (e.g., insulin resistance and T2D) can indeed cause impaired angiogenesis. Such impairment involves endothelial cell dysfunction with poor angiogenic responses, abnormal expression of the angiopoietin-1 and 2 (ANG-1, ANG-2), VEGF, ephrin-B2, fibroblast growth factors (FGFs), Notch signaling, platelet-derived growth factor B (PDGF-B), TGF-β, endothelial growth factors (EGF) inflammatory markers (e.g. TNFα), and chemokines [53, 54]. Impaired angiogenesis is integral to physiological and pathological processes of wound healing, atherosclerosis, and aging [55]. The impaired vascular function stems from the chronic hyperglycemia in T2D and underpinning the high morbidity and mortality of the diseases. T2D is well known for its poor recovery from ischaemia-driven angiogenesis [56]. Poor wound healing significantly contributes to the high rates of lower/upper limb amputations in T2D patients.
miR181-c is important in regulating insulin signaling. However, the insulin resistance associated with T2D was shown to cause overexpression of the miR-181c to compensate for the dysregulation in the insulin signaling pathway. Unfortunately, such increase unleashes the other effects of the miR-181c as it orchestrates the development of the impaired angiogenesis associated with T2D. In vitro studies showed that miR-181c expression levels were overexpressed in human umbilical vein endothelial cells (HUVECs) in response to a high glycemic environment [57, 58]. Moreover, the overexpression of miR-181c significantly increased ROS production, enhanced oxidative stress causing mitochondrial dysfunction, and increased cell death [58]. Generally, in T2D the increases in ROS levels often precede endothelial and vascular dysfunction, suggesting that dysregulation of miR-181c can be used as a marker for the onset of the cellular dysfunction. Furthermore, these results showed that miR-181c has anti-angiogenic properties [59], particularly with miR-181c inhibiting the endothelial cells from forming vascular networks, expressing the pro-angiogenic mediator VEGFA, cell migration and tubule formation [59]. Moreover, miR-181c was shown to dysregulate the TNF-α, TGFβ, ANG1, and ANG2, as well as being divergently regulated by hypoxia [59, 60]. Therefore, inhibiting miR-181c can potentially promote angiogenesis and improve wound healing.
4. MiR-181c role in obesity
Metabolic disorders such as obesity, T2D, and ageing often are caused by imbalances in the metabolic homeostasis or system that links them. Such system consists of the sophisticated crosstalk between several organs and signaling pathways [61]. Obesity is one of the metabolic disorders that reached epidemic dimensions. It affects all age groups, it has been recognized as one of the major health challenges owing to its association with high morbidity and mortality rates and the high associated medical costs [62]. Advanced research improved our understanding of the adipose tissue, previously, adipose tissue was considered merely a fat storage depot. Today, adipose tissue and the process of adipocyte development are considered an essential energy metabolism process. Dysfunction of adipose tissue underpins the development of a series of metabolic syndromes, including fatty liver disease, insulin resistance, T2D, and cardiovascular disease [63]. In normal, healthy individuals, miR-181c was shown to enhance the insulin signaling pathway. However, as the body mass index (BMI) increases it causes impaired adipogenesis. Also, miR-181c was reported to play critical roles in adipose tissues, including lipid metabolism, adipogenesis, inflammation, paracrine and endocrine communication. Additionally, miR-181c was shown to induce obesity and be upregulated in the presence of adipogenesis dysfunction [64, 65]. Moreover, miR-181 was shown to be involved in the obesity-related inflammation and to target and influence the function of the TNF-α [64]. TNF-α is a multifunctional cytokine that has a regulatory effects on energy metabolism, as it is involved in almost every aspect of adipose biology. TNF- α is expressed and secreted by adipose tissue, and it affects adipocyte differentiation. It also inhibits adipogenesis by preventing the early induction of the adipogenic transcription factors PPARγ and CCAAT/enhancer-binding protein-α (C/ EBPα).
Furthermore, it downregulates the genes responsible for the mature adipocyte phenotype, including the adipose triglyceride lipase (ATGL), the aP2, and glucose transporter type 4 (Glut4) [64]. TNF-α was shown to be highly elevated in adipose tissue isolated from obese people. Subsequently, circulating levels of TNF-α seem to be well correlated to individuals' BMI [63, 65]. Finally, recent studies have shown that lowering miR-181c expression would protect against obesity and induced cardiovascular complications [66].
5. MiR-181c role in ageing
Aging is an extremely complex and inexorable process characterized by the gradual accumulation of biological damages. The overtime accumulation of the biological damages gradually causes impairment in the cellular homeostasis, and it decreases the organ mass, ultimately leading to the loss of the body systems [67]. Medically speaking, aging is not considered a disease, but it can increase the risk factors for developing different metabolic disorders68. Experimental investigations suggest that the downregulation of the aging process can improve survival rate and delay disease onset [69-71]. Thus, suggesting that chronic diseases can be prevented through intervening and delaying the aging process [72, 73]. Of the prominent organs affected by aging is the brain, with many diseases such as Alzheimer's developing late in life. Recent evidence suggests that neurons often release miRs into the bloodstream [74, 75], with the elevation of certain miRs indicating the developing of different neurological disorders. A study conducted by Schonrock et.al. revealed that miR-181c regulates amyloid-β (Aβ) deposition [76], with the expression of miR-181c, especially miR-181c-5p, having a direct correlation with the increased Aβ levels [77]. It also acts as a mediator of the complex crosstalk between the nucleus and the mitochondria.
Moreover, the elevation in the miR-181c levels in the plasma precedes the development of neurological disorders. Suggesting that miR-181c-5p is associated with the development of cognitive and brain changes in asymptomatic elderly subjects. Therefore, lowering miR-181c-5p levels should parallel changes in plasma Aβ concentrations, which might delay cognitive and brain changes. In addition, miR-181c modulates mitochondrial activity, making them closely involved in inflamm-aging, and cell aging. Both in vivo and in vitro aging studies suggested the different modulatory effects of different miRs, associated with inflamm-aging through the modulation of key pro-inflammatory molecules. Of note, the number of these miRs is increasing, and miR-181c was shown also to affect the mitochondrial respiration, metabolism, and genome stability. A mounting body of evidence demonstrates that miR-181c is encoded in and released by the nucleus, then processed to the mature form in the cytosol, and finally translocated to mitochondria, where it regulates mitochondrial gene expression [21].
Additionally, miR-181c co-immunoprecipitates with the mRNAs of both the Ago-2 [78] and its mitochondrial target COX-1 (COX subunit I). Alternatively, the overexpression of miR-181c results in translationally repressing the production of the mitochondrial COX-1 [21]. MiR-181c is likely to play a substantial role in modulating mitochondrial processes, thus influencing the aging process by altering the inflamm-aging pathway as it undergoes further modulation in peripheral blood cells of aged individuals which enhances the expression of different proinflammatory cytokines such as IL-8, IL-6, IL-1β, and TNF-α; as well as the expression of the High mobility group box 1 (HMGB1) [11]. Disruption of this crosstalk could lead to ROS overproduction and increased mitochondrial permeability, initiating the hallmarks of the cellular senescence. This was evidenced by the disturbance in the mitochondrial homeostasis caused by the extensive changes in miR-181c expression and distribution that was demonstrated in senescent cells. Such disturbance was seen in the form of ROS overproduction, mtDNA mutation, protein misfolded, cellular depolarization, accumulation of phosphatase and tensin (PTEN) induced kinase 1 (PINK1) on the mitochondrial outer membrane, which phosphorylates Parkin (PARK2). After that autophagy machinery recognize the proteins that are ubiquitination by the Parkin [79].
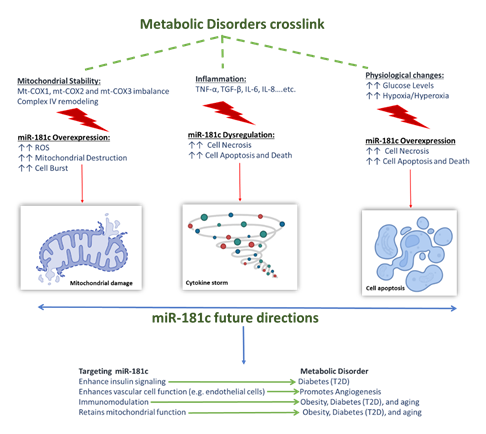
6. miR-181c: the trigger and center for the development of different metabolic disorders
miRNAs, in general, were shown to play a role in almost all aspects of cellular functions, such as cellular homeostasis, cell proliferation, differentiation, apoptosis, as well as their involvement in embryonic development [20, 80]. Accordingly, miR-181 family, including miR-181c was suggested to have important roles in embryonic and organ system development [20], immunity, mitochondrial function, cell apoptosis, and inflammation [81]. miR-181c is expressed in different cells and organs; it has a protective role under normal and healthy conditions. Nonetheless, studies showed that under disease conditions and specific physiological changes, miR-181c has a reversed role as it worsens disease progression. Such dual effect was seen in diabetes, obesity, aging-related disorders and impaired angiogenesis. Emerging evidence showed that despite their diversity, these diseases share three common co-factors that underpin their development and progression: i) involvement of inflammation, ii) mitochondrial dysfunction, and iii) physiological changes.
The first co-factor is inflammation; one of the major pro-inflammatory cytokine that is affected by miR-181c dysregulation and was shown to be linked to the development of the above-mentioned metabolic disorders is the TNF-α. Within the cells, the biological functions and diverse range of TNF-α signaling events as well as its mechanism of action are somewhat complex. On one hand TNF-α has several therapeutic roles within the body, as it is involved in embryonic development [20], it provides resistance to certain types of infections and tumors, immunostimulation, and helps regulate sleep patterns. On the one hand, it is known to have contradictory roles in some pathological complications, which is seen in its association with a number of autoimmune diseases such as graft rejection, rheumatoid arthritis, and its activation of the cellular necrosis/apoptosis pathways. These contradictory effects are attributed to the fact that TNF-α is involved in the activation of many related signaling pathways. Furthermore, other inflammatory markers affected by miR-181c are TGF-β, IL-8, IL-6, and IL-1β [11], dysregulation of these pro-inflammatory cytokines was proven to underpin the onset of obesity, T2D and inflamma-aging associated disorders. Secondly is the mitochondrial dysfunction, miR-181c has a role in mitochondrial metabolism; overexpression of miR-181c perturbates a loss of mt-COX1 protein, and an increase in both the mt-COX2 and mt-COX3. The binding of the miR-181c to mt-COX1 disturbs the balance among the mitochondrially encoded subunits in complex IV, causing complex IV remodeling which also promotes ROS generation. Such events alter the mitochondrial stability, with the worst-case scenario being mitochondrial destruction. Moreover, if the situation propagated from mitochondrion to mitochondrion, this could cause cell burst [21] which marks the onset of different metabolic disorders. Interestingly, physiological changes can affect the expression levels and behavior of the miR-181c in diabetes, obesity, aging related diseases and angiogenesis. Such effects maybe so determinantal that they cause cell apoptosis and death.
For instance, in recent T2D studies, miR-181c was found to significantly enhance the high glucose-induced oxidative stress injury, causing apoptosis in HUVECs; such action is mediated by targeting the LIF pathway. Another physiological change that was shown to alter miR-181c’s function was the CO2/O2 levels, both hypoxia [82] and hyperoxia [81] were shown to significantly up regulate miR-181c expression in vascular endothelial cells, promoting endothelial damage and apoptosis. Furthermore, the hypoxia [82]/hyperoxia [81] related overexpression of miR-181c was linked to the development of impaired angiogenesis seen in diabetes and increased cell apoptosis seen inaging-related disorders such as Alzheimer [77]. The association between these three factors provides valuable clues for a deeper understanding of the miR-181c mechanism of action in normal vs. disease conditions. Such deep understanding will help us use the miR-181c level to determine the onset of different metabolic disorders. It will also help us identify the potential benefits silencing miR-181c can have in such models, making it a multi-action target that can provide solutions for various conditions. Figure 2 summarizes the effect of the three co-factors and the potential therapeutic benefits silencing miR-181c might have.
7. Conclusion
miR-181c is shown to be a critical player in the homeostasis of the tri-linked pathways underlining the development of diabetes, obesity, and aging and the associated complications such as impaired angiogenesis. Despite the diversity of the metabolic disorders, they all share the implication of the miR-181 in their onset, with accumulating evidence suggesting its involvement in the development of insulin resistance, and inflammatory processes that underpin the development of impaired adipogenesis, impaired adipogenesis angiogenesis, and aging. Such involvement is then further centered to the miR-181c’s effect in dysregulating the mitochondrial activity and enhancing the inflammatory processes involved in each disorder. In the contrary, some studies showed that miR-181c might have dual effects under normal and diseases models; however, what causes the sudden switch in its activity remains largely unknown—indicating the urgent need for further investigations to determine te cause of the miR-181c’s dual effect in different metabolic disorders. As well as understanding the conditions at which inhibiting or enhancing the miR-181c expression could be beneficial.
Funding
NAM is a recipient of the Early Career Research Award (ECRA; ECRA03-006-3-004) from the Qatar National Research Fund (QNRF), and the L’Oréal-UNESCO for Women in Science Middle East Regional Young Talents ward 2021. Also, the authors extend their appreciation to the Deputyship for Research & Innovation, “Ministry of Education“in Saudi Arabia for funding this research work through the project no.( IFKSURG-2-861)".
Conflicts of Interest
The authors declare no conflict of interest
Author Contributions
Conceptualization, N.A.M; data curation, N.A.M, and T.A.T; writing—original draft prep-aration, N.A.M; writing—article and editing, all authors; funding acquisition, N.A.M. All authors have read and agreed to the published version of the manuscript.
References
- DP B. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004; 116(2): 281-97.
- Pillai RS BS, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol 17 (2007): 118-26.
- Filipowicz W, Jaskiewicz L, Kolb FA, Pillai RS. Post-transcriptional gene silencing by siRNAs and miRNAs. Curr Opin Struct Biol 15 (2005): 331-41.
- Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11 (2010): 597-610.
- Olena AF, Patton JG. Genomic organization of microRNAs. J Cell Physiol 222 (2010): 540-5.
- Ji J, Yamashita T, Budhu A, et al. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology 50 (2009): 472-80.
- Ouyang YB, Lu Y, Yue S, Giffard RG. miR-181 targets multiple Bcl-2 family members and influences apoptosis and mitochondrial function in astrocytes. Mitochondrion 12 (2012): 213-9.
- Rodriguez-Ortiz CJ, Baglietto-Vargas D, Martinez-Coria H, LaFerla FM, Kitazawa M. Upregulation of miR-181 decreases c-Fos and SIRT-1 in the hippocampus of 3xTg-AD mice. J Alzheimers Dis 42 (2014): 1229-38.
- Henao-Mejia J, Williams A, Goff LA, et al. The microRNA miR-181 is a critical cellular metabolic rheostat essential for NKT cell ontogenesis and lymphocyte development and homeostasis. Immunity 38 (2013): 984-97.
- He L, Yao H, Fan LH, et al. MicroRNA-181b expression in prostate cancer tissues and its influence on the biological behavior of the prostate cancer cell line PC-3. Genet Mol Res 12 (2013): 1012-21.
- Hutchison ER, Kawamoto EM, Taub DD, et al. Evidence for miR-181 involvement in neuroinflammatory responses of astrocytes. Glia 61 (2013): 1018-28.
- Tekirdag KA, Korkmaz G, Ozturk DG, Agami R, Gozuacik D. MIR181A regulates starvation- and rapamycin-induced autophagy through targeting of ATG5. Autophagy 9 (2013): 374-85.
- Karali M, Persico M, Mutarelli M, et al. High-resolution analysis of the human retina miRNome reveals isomiR variations and novel microRNAs. Nucleic Acids Res 44 (2016): 1525-40.
- Carrella S, D'Agostino Y, Barbato S, et al. miR-181a/b control the assembly of visual circuitry by regulating retinal axon specification and growth. Dev Neurobiol 75 (2015): 1252-67.
- Chu B, Zhong L, Dou S, et al. miRNA-181 regulates embryo implantation in mice through targeting leukemia inhibitory factor. J Mol Cell Biol 7 (2015): 12-22.
- Garcia-Riart B, Lorda-Diez CI, Marin-Llera JC, Garcia-Porrero JA, Hurle JM, Montero JA. Interdigital tissue remodelling in the embryonic limb involves dynamic regulation of the miRNA profiles. J Anat 231 (2017): 275-86.
- Bhushan R, Grünhagen J, Becker J, Robinson PN, Ott CE, Knaus P. miR-181a promotes osteoblastic differentiation through repression of TGF-β signaling molecules. Int J Biochem Cell Biol 45 (2013): 696-705.
- Gabler J, Ruetze M, Kynast KL, Grossner T, Diederichs S, Richter W. Stage-Specific miRs in Chondrocyte Maturation: Differentiation-Dependent and Hypertrophy-Related miR Clusters and the miR-181 Family. Tissue Eng Part A 21 (2015): 2840-51.
- McAlinden A, Varghese N, Wirthlin L, Chang LW. Differentially expressed microRNAs in chondrocytes from distinct regions of developing human cartilage. PLoS One 2013; 8: e75012.
- Li J, Cao Y, Ma XJ, et al. Roles of miR-1-1 and miR-181c in ventricular septal defects. Int J Cardiol 168 (2013): 1441-6.
- Das S, Ferlito M, Kent OA, et al. Nuclear miRNA regulates the mitochondrial genome in the heart. Circ Res 110 (2012): 1596-603.
- Xue Q, Guo ZY, Li W, et al. Human activated CD4 (+) T lymphocytes increase IL-2 expression by downregulating microRNA-181c. Mol Immunol 48 (2011): 592-9.
- Sun X SA, Feinberg MW. Role of miR-181 family in regulating vascular inflammation and immunity. Trends Cardiovasc Med 24 (2014): 105-12.
- King GL, Park K, Li Q. Selective Insulin Resistance and the Development of Cardiovascular Diseases in Diabetes: The 2015 Edwin Bierman Award Lecture. Diabetes 65 (2016): 1462-71.
- King GL. The role of hyperglycaemia and hyperinsulinaemia in causing vascular dysfunction in diabetes. Ann Med 28 (1996): 427-32.
- Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev 28 (2007): 463-91.
- Muniyappa R QM. Insulin action and insulin resistance in vascular endothelium. Curr Opin Clin Nutr Metab Care 10 (2007): 523-30.
- Mather KJ, Steinberg HO, Baron AD. Insulin resistance in the vasculature. J Clin Invest 123 (2013): 1003-4.
- BJ G. Insulin resistance as the core defect in type 2 diabetes mellitus. Am J Cardiol 90 (2002): 3G-10G.
- Nandipati KC, Subramanian S, Agrawal DK. Protein kinases: mechanisms and downstream targets in inflammation-mediated obesity and insulin resistance. Mol Cell Biochem 426 (2017): 27-45.
- Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112 (2003): 1821-30.
- Nigi L, Grieco GE, Ventriglia G, et al. MicroRNAs as Regulators of Insulin Signaling: Research Updates and Potential Therapeutic Perspectives in Type 2 Diabetes. Int J Mol Sci 19 (2018).
- Fujishiro M, Gotoh Y, Katagiri H, et al. Three mitogen-activated protein kinases inhibit insulin signaling by different mechanisms in 3T3-L1 adipocytes. Mol Endocrinol 17 (2003): 487-97.
- Arkan MC, Hevener AL, Greten FR, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 11 (2005): 191-8.
- Fernández-Veledo S, Nieto-Vazquez I, Rondinone CM, Lorenzo M. Liver X receptor agonists ameliorate TNFalpha-induced insulin resistance in murine brown adipocytes by downregulating protein tyrosine phosphatase-1B gene expression. Diabetologia 49 (2006): 3038-48.
- Gao Z, Hwang D, Bataille F, et al. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem 277 (2002): 48115-21.
- Zabolotny JM, Kim YB, Welsh LA, Kershaw EE, Neel BG, Kahn BB. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J Biol Chem 283 (2008): 14230-41.
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425 (2003): 577-84.
- Vander Ark A, Cao J, Li X. TGF-β receptors: In and beyond TGF-β signaling. Cell Signal 52 (2018): 112-20.
- Fekrazad R, Sarrafzadeh A, Kalhori KAM, Khan I, Arany PR, Giubellino A. Improved Wound Remodeling Correlates with Modulated TGF-beta Expression in Skin Diabetic Wounds Following Combined Red and Infrared Photobiomodulation Treatments. Photochem Photobiol 94 (2018); 94: 775-9.
- Oh YS, Bae GD, Park EY, Jun HS. MicroRNA-181c Inhibits Interleukin-6-mediated Beta Cell Apoptosis by Targeting TNF-α Expression. Molecules 24 (2019).
- Tonyan ZN, Nasykhova YA, Danilova MM, Glotov AS. Genetics of macrovascular complications in type 2 diabetes. World J Diabetes 12 (2021): 1200-19.
- Sousa GR PD, Galderisi A, Lv H, Yu L, Pereira AC, Doria A, et al. Glycemic Control, Cardiac Autoimmunity, and Long-Term Risk of Cardiovascular Disease in Type 1 Diabetes Mellitus. Circulation 139 (2019): 730-43.
- Virtue AT, McCright SJ, Wright JM, et al. The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci Transl Med 11 (2019).
- Borja-Gonzalez M, Casas-Martinez JC, McDonagh B, Goljanek-Whysall K. Aging Science Talks: The role of miR-181a in age-related loss of muscle mass and function. Transl Med Aging 4 (2020): 81-5.
- Ge L, Cai Y, Ying F, et al. miR-181c-5p Exacerbates Hypoxia/Reoxygenation-Induced Cardiomyocyte Apoptosis via Targeting PTPN4. Oxid Med Cell Longev (2019): 1957920.
- Redshaw N, Camps C, Sharma V, et al. TGF-β/Smad2/3 signaling directly regulates several miRNAs in mouse ES cells and early embryos. PLoS One 8 (2013): e55186.
- Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem 153 (2013): 13-9.
- Cumsille P, Coronel A, Conca C, Quiñinao C, Escudero C. Proposal of a hybrid approach for tumor progression and tumor-induced angiogenesis. Theor Biol Med Model 12 (2015): 13.
- Westermeier F, Salomón C, González M, et al. Insulin restores gestational diabetes mellitus-reduced adenosine transport involving differential expression of insulin receptor isoforms in human umbilical vein endothelium. Diabetes 60 (2011): 1677-87.
- Dubó S, Gallegos D, Cabrera L, Sobrevia L, Zúñiga L, González M. Cardiovascular Action of Insulin in Health and Disease: Endothelial L-Arginine Transport and Cardiac Voltage-Dependent Potassium Channels. Front Physiol 7 (2016): 74.
- Sobrevia L, Salsoso R, Fuenzalida B, et al. Insulin Is a Key Modulator of Fetoplacental Endothelium Metabolic Disturbances in Gestational Diabetes Mellitus. Front Physiol 7 (2016): 119.
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res 97 (2005): 512-23.
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 473 (2011): 298-307.
- Budi EH, Mamai O, Hoffman S, Akhurst RJ, Derynck R. Enhanced TGF-β Signaling Contributes to the Insulin-Induced Angiogenic Responses of Endothelial Cells. iScience 11 (2019): 474-91.
- Abaci A, Oguzhan A, Kahraman S, et al. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation 99 (1999): 2239-42.
- Zitman-Gal T, Green J, Pasmanik-Chor M, Golan E, Bernheim J, Benchetrit S. Vitamin D manipulates miR-181c, miR-20b and miR-15a in human umbilical vein endothelial cells exposed to a diabetic-like environment. Cardiovasc Diabetol 13 (2014): 8.
- Shen XLY, Sun G, et al. miR-181c-3p and -5p promotes high-glucose-induced dysfunction in HUVECs by regulating leukemia inhibitory factor. Int J Biol Macromol 115 (2018): 509-17.
- Hourigan STSE, Nankivell VA, Ridiandries A, Weimann BM, Henriquez R, Tepper ER, et al. The regulation of miRNAs by reconstituted high-density lipoproteins in diabetes-impaired angiogenesis. Scientific Reports 8 (2018): 13596.
- Deng Y, Li S, Yu C, Huang D, Chen H, Yin X. CircPDE4B inhibits retinal pathological angiogenesis via promoting degradation of HIF-1α though targeting miR-181c. IUBMB Life 72 (2020): 1920-9.
- Stapleton PA, James ME, Goodwill AG, Frisbee JC. Obesity and vascular dysfunction. Pathophysiology 15 (2008): 79-89.
- González-Muniesa P, Mártinez-González MA, Hu FB, et al. Obesity. Nat Rev Dis Primers 3 (2017): 17034.
- Cawthorn WP SE, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res 53 (2012): 227-46.
- Wu D, Xi QY, Cheng X, et al. miR-146a-5p inhibits TNF-α-induced adipogenesis via targeting insulin receptor in primary porcine adipocytes. J Lipid Res 57 (2016): 1360-72.
- Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett 582 (2008): 117-31.
- Roman B, Kaur P, Ashok D, et al. Nuclear-mitochondrial communication involving miR-181c plays an important role in cardiac dysfunction during obesity. J Mol Cell Cardiol 144 (2020): 87-96.
- Kirkwood TB, Austad SN. Why do we age? Nature 408 (2000): 233-8.
- Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol 22 (2012): R741-52.
- Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444 (2006): 337-42.
- Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325 (2009): 201-4.
- Wilkinson JE, Burmeister L, Brooks SV, et al. Rapamycin slows aging in mice. Aging Cell 11 (2012): 675-82.
- Blagosklonny MV. Disease or not, aging is easily treatable. Aging (Albany NY) 10 (2018): 3067-78.
- Partridge L. Intervening in ageing to prevent the diseases of ageing. Trends Endocrinol Metab 25 (2014): 555-7.
- Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 105 (2008): 10513-8.
- Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res 39 (2011): 7223-33.
- Schonrock N, Humphreys DT, Preiss T, Götz J. Target gene repression mediated by miRNAs miR-181c and miR-9 both of which are down-regulated by amyloid-β. J Mol Neurosci 46 (2012): 324-35.
- Schonrock N, Ke YD, Humphreys D, et al. Neuronal microRNA deregulation in response to Alzheimer's disease amyloid-beta. PLoS One 5 (2010): e11070.
- Chendrimada TP, Gregory RI, Kumaraswamy E, et al. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 436 (2005): 740-4.
- Giordani C, Silvestrini A, Giuliani A, Olivieri F, Rippo MR. MicroRNAs as Factors in Bidirectional Crosstalk Between Mitochondria and the Nucleus During Cellular Senescence. Front Physiol 12 (2021): 734976.
- Yang Z, Wan X, Gu Z, et al. Evolution of the mir-181 microRNA family. Comput Biol Med 52 (2014): 82-7.
- Wu J, Zhang G, Xiong H, Zhang Y, Ding G, Ge J. miR-181c-5p mediates apoptosis of vascular endothelial cells induced by hyperoxemia via ceRNA crosstalk. Sci Rep 11 (2021): 16582.
- Hourigan ST, Solly EL, Nankivell VA, et al. The regulation of miRNAs by reconstituted high-density lipoproteins in diabetes-impaired angiogenesis. Sci Rep 8 (2018): 13596.