The Role of Exhaled Nitric Oxide Fraction in Sarcoidosis Patients
Article Information
Suphi Aydin1,*, Aydin Balci2
1Afyonkarahisar Health Sciences University, Departmen of Chest Surgery, Afyonkarahisar, Turkey
2Afyonkarahisar Health Sciences University, Department of Pulmonology, Afyonkarahisar, Turkey
*Corresponding Author: Suphi Aydin, Afyonkarahisar Health Sciences University, Departmen of Chest Surgery, Afyonkarahisar, Turkey
Received: 19 November 2020; Accepted: 27 Novemberber 2020; Published: 04 December 2020
Citation: Suphi Aydın, Aydın Balcı. The Role of Exhaled Nitric Oxide Fraction in Sarcoidosis Patients. Journal of Surgery and Research 3 (2020): 445-443.
View / Download Pdf Share at FacebookAbstract
Nitric oxide is a molecule that has important roles in various biological processes such as apoptosis, angiogenesis and endothelial permeability and therefore its disorder plays a role in the pathogenesis of various disorders. We aimed to investigate fractional exhaled nitric oxide values in sarcoidosis, a multi-systemic non-caseified granulomatous disease that commonly affects all organs, especially the pulmonary system. A total of 107 individuals, including 54 sarcoidosis patients and 53 healthy controls were included in this study. Data were analyzed retrospectively. Gender, age, body mass index, exhaled nitric oxide fraction values, respiratory function parameters, diagnostic methods and additional disease states were recorded.
Exhaled nitric oxide fraction values in sarcoidosis patients and the control group were measured using Bedfont NObreath (UK) device, respiratory function parameters were measured using ZAN GPI.3.00 (Germany) device and the results were recorded. Sarcoidosis patients were graded as stage 0, 1, 2, 3, 4 according to chest x-ray findings. Exhaled nitric oxide fraction values detected in sarcoidosis patients and fractional exhaled nitric oxide values detected in the control group were compared. The sensitivity and specificity of exhaled nitric oxide fraction values in sarcoidosis were investigated. The relationship between respiratory function parameters and exhaled nitric oxide fraction values was examined.
There was a significant difference between the exhaled nitric oxide fraction values detected in sarcoidosis patients and the exhaled nitric oxide fraction values detected in the control group and the specificity and sensitivity of the exhaled nitric oxide fraction value was found to be high in patients with and sarcoidosis. There was no relationship between respiratory function parameters and exhaled nitric oxide fraction values. Exhaled nitric oxide fraction values, which have been studied in
Keywords
Exhaled nitric oxide fraction; Sarcoidosis; Exhalation; Lung
Exhaled nitric oxide fraction articles; Sarcoidosis articles; Exhalation articles; Lung articles
Exhaled nitric oxide fraction articles Exhaled nitric oxide fraction Research articles Exhaled nitric oxide fraction review articles Exhaled nitric oxide fraction PubMed articles Exhaled nitric oxide fraction PubMed Central articles Exhaled nitric oxide fraction 2023 articles Exhaled nitric oxide fraction 2024 articles Exhaled nitric oxide fraction Scopus articles Exhaled nitric oxide fraction impact factor journals Exhaled nitric oxide fraction Scopus journals Exhaled nitric oxide fraction PubMed journals Exhaled nitric oxide fraction medical journals Exhaled nitric oxide fraction free journals Exhaled nitric oxide fraction best journals Exhaled nitric oxide fraction top journals Exhaled nitric oxide fraction free medical journals Exhaled nitric oxide fraction famous journals Exhaled nitric oxide fraction Google Scholar indexed journals Sarcoidosis articles Sarcoidosis Research articles Sarcoidosis review articles Sarcoidosis PubMed articles Sarcoidosis PubMed Central articles Sarcoidosis 2023 articles Sarcoidosis 2024 articles Sarcoidosis Scopus articles Sarcoidosis impact factor journals Sarcoidosis Scopus journals Sarcoidosis PubMed journals Sarcoidosis medical journals Sarcoidosis free journals Sarcoidosis best journals Sarcoidosis top journals Sarcoidosis free medical journals Sarcoidosis famous journals Sarcoidosis Google Scholar indexed journals Exhalation articles Exhalation Research articles Exhalation review articles Exhalation PubMed articles Exhalation PubMed Central articles Exhalation 2023 articles Exhalation 2024 articles Exhalation Scopus articles Exhalation impact factor journals Exhalation Scopus journals Exhalation PubMed journals Exhalation medical journals Exhalation free journals Exhalation best journals Exhalation top journals Exhalation free medical journals Exhalation famous journals Exhalation Google Scholar indexed journals Lung articles Lung Research articles Lung review articles Lung PubMed articles Lung PubMed Central articles Lung 2023 articles Lung 2024 articles Lung Scopus articles Lung impact factor journals Lung Scopus journals Lung PubMed journals Lung medical journals Lung free journals Lung best journals Lung top journals Lung free medical journals Lung famous journals Lung Google Scholar indexed journals angiogenesis articles angiogenesis Research articles angiogenesis review articles angiogenesis PubMed articles angiogenesis PubMed Central articles angiogenesis 2023 articles angiogenesis 2024 articles angiogenesis Scopus articles angiogenesis impact factor journals angiogenesis Scopus journals angiogenesis PubMed journals angiogenesis medical journals angiogenesis free journals angiogenesis best journals angiogenesis top journals angiogenesis free medical journals angiogenesis famous journals angiogenesis Google Scholar indexed journals apoptosis articles apoptosis Research articles apoptosis review articles apoptosis PubMed articles apoptosis PubMed Central articles apoptosis 2023 articles apoptosis 2024 articles apoptosis Scopus articles apoptosis impact factor journals apoptosis Scopus journals apoptosis PubMed journals apoptosis medical journals apoptosis free journals apoptosis best journals apoptosis top journals apoptosis free medical journals apoptosis famous journals apoptosis Google Scholar indexed journals vasoconstriction articles vasoconstriction Research articles vasoconstriction review articles vasoconstriction PubMed articles vasoconstriction PubMed Central articles vasoconstriction 2023 articles vasoconstriction 2024 articles vasoconstriction Scopus articles vasoconstriction impact factor journals vasoconstriction Scopus journals vasoconstriction PubMed journals vasoconstriction medical journals vasoconstriction free journals vasoconstriction best journals vasoconstriction top journals vasoconstriction free medical journals vasoconstriction famous journals vasoconstriction Google Scholar indexed journals impaired tissue perfusion articles impaired tissue perfusion Research articles impaired tissue perfusion review articles impaired tissue perfusion PubMed articles impaired tissue perfusion PubMed Central articles impaired tissue perfusion 2023 articles impaired tissue perfusion 2024 articles impaired tissue perfusion Scopus articles impaired tissue perfusion impact factor journals impaired tissue perfusion Scopus journals impaired tissue perfusion PubMed journals impaired tissue perfusion medical journals impaired tissue perfusion free journals impaired tissue perfusion best journals impaired tissue perfusion top journals impaired tissue perfusion free medical journals impaired tissue perfusion famous journals impaired tissue perfusion Google Scholar indexed journals non-smokers articles non-smokers Research articles non-smokers review articles non-smokers PubMed articles non-smokers PubMed Central articles non-smokers 2023 articles non-smokers 2024 articles non-smokers Scopus articles non-smokers impact factor journals non-smokers Scopus journals non-smokers PubMed journals non-smokers medical journals non-smokers free journals non-smokers best journals non-smokers top journals non-smokers free medical journals non-smokers famous journals non-smokers Google Scholar indexed journals idiopathic pulmonary fibrosis articles idiopathic pulmonary fibrosis Research articles idiopathic pulmonary fibrosis review articles idiopathic pulmonary fibrosis PubMed articles idiopathic pulmonary fibrosis PubMed Central articles idiopathic pulmonary fibrosis 2023 articles idiopathic pulmonary fibrosis 2024 articles idiopathic pulmonary fibrosis Scopus articles idiopathic pulmonary fibrosis impact factor journals idiopathic pulmonary fibrosis Scopus journals idiopathic pulmonary fibrosis PubMed journals idiopathic pulmonary fibrosis medical journals idiopathic pulmonary fibrosis free journals idiopathic pulmonary fibrosis best journals idiopathic pulmonary fibrosis top journals idiopathic pulmonary fibrosis free medical journals idiopathic pulmonary fibrosis famous journals idiopathic pulmonary fibrosis Google Scholar indexed journals
Article Details
1. Introduction
The incidence of sarcoidosis varies according to geographic region, race and gender. It has the highest prevalence in the world with 50-60 / 100,000 in Scandinavian countries. In the United States, the prevalence of the disease is 10-40 / 100,000, 20-64 / 100,000 in northern European countries and 1.4 / 100,000 in Spain and Japan. It is more common in women than men. Sarcoidosis with two peaks between the ages of 25-35 and 45-65 [1]. Sarcoidosis is a systemic disease of unknown etiology characterized by non-caseating granulomas that can involve all organs, especially lung and mediastinal lymph nodes [2].
Although the immunopathogenesis of sarcoidosis is not fully understood, it has been shown that it is similar to other granulomatous diseases such as chronic beryllium disease. That is, some antigens enter the host and are phagocytosed by antigen presenting cells (APC), macrophages or dendritic cells. APCs process the antigen and then present it to T cell receptors on T lymphocytes from the CD41 class via human leukocyte antigen (HLA) class II molecules [3]. Giant multinucleated cells formed by epithelioid histiocytes and CD41 T lymphocytes are responsible for granuloma formation in sarcoidosis. Contributes to the formation of granulomas in fibroblasts, CD81 T lymphocytes, regulatory T cells and B lymphocytes [3].
The diagnosis is made in the presence of clinical, radiological findings and histological detection of non-caseating granulomas after excluding other granulomas and factors causing local reactions [4]. Symptoms may range from systemic symptoms such as shortness of breath, cough, chest pain, low-grade fever, fatigue, weight loss and night sweats to Lofgren's Syndrome accompanied by bilateral hilar lymphadenopathy and erythema nodosum [5]. Pulmonary sarcoidosis can be examined radiologically in five stages. Stage 0. Chest X-ray normal, Stage 1. Only hilar and mediastinal lymphadenopathy, Stage 2. Lymphadenopathy (LAP) and pulmonary infiltration, Stage 3. Pulmonary infiltration alone, Stage 4. Pulmonary fibrosis. Staging provides information about the prognosis of the disease[6] Pulmonary involvement is not observed in Stage 0 and Stage 1 and spontaneous remission is seen in most of them. Symptomatic treatment is given. Steroids are used in the main treatment of sarcoidosis. Immunosuppressive therapy and anti-Tumor Necrosis Factor (TNF) agents can also be used in cases with severe clinical picture [7].
Fractional exhaled nitric oxide values (FeNO) were first determined 20 years ago [8] and it was observed that FeNO concentration increased in allergic diseases of the respiratory tract [9]. FeNO mostly originates from the upper respiratory tract and the lower respiratory tract contributes less [10] and NO is synthesized in the vascular endothelium from the amino acid L-arginine via the enzyme nitric oxide synthase
(NOS) in the human body. It is an unstable molecule with an average life of as short as 3-5 seconds. Due to its lipophilic property, it can easily pass through the membranes. When administered by inhalation, it easily crosses the alveolar epithelial cell barrier, stimulates the guanilyl cyclase enzyme and causes relaxation and vascular dilatation in smooth muscle cells via cyclic guanosine monophosphate (cGMP) [11]. In addition, inhaled NO may show pro-inflammatory or anti-inflammatory properties on the pulmonary system [12]. In patients with lung damage, NO can cause vasodilation in areas of the lung that are not well ventilated, thus increasing ventilation-perfusion mismatch and causing systemic arterial hypoxemia. Depletion of NO can lead to vasoconstriction, impaired tissue perfusion and inflammation [13].
In granulomatous lung diseases, an increase was observed in the amount of inducible NO synthase detected in the lung tissue [14]. An increase in FeNO concentration is observed in pathologies in which sarcoidosis and exogenous allergic alveolitis and idiopathic pulmonary fibrosis develop [15]. Also, smoking affects NO metabolism. Smokers have lower exhaled NO values than non-smokers [16].
2. Methods
For our study, 74 patients diagnosed with sarcoidosis were identified. Twenty sarcoidosis patients were excluded due to smoking. A total of 107 individuals, including 54 sarcoidosis patients and 53 healthy individuals, were included in the study. Data of patients diagnosed with sarcoidosis were retrospectively analyzed. Gender, age, body mass index, pulmonary function parameters, FeNO, diagnostic methods and additional disease states were recorded. Of the sarcoidosis patients, 3 were diagnosed with mediastinoscopy-guided biopsy, 45 with flexible fiberoptic bronchoscopy-guided biopsy and 6 with clinical findings. Sarcoidosis patients were staged according to chest X-ray findings. Among the sarcoidosis patients, those with normal chest x-ray were considered as stage 0, those with hilar lymphadenopathy (LAP) as stage 1, those with hilar LAP and parenchymal involvement as stage 2, those with only parenchymal involvement as stage 3 and those with pulmonary fibrosis as stage 4.
2.1 Ethic approval
The study was conducted in accordance with good clinical practice and the Declaration of Helsinki. The study was approved by the ethics committee of Afyonkarahisar Health Sciences University Medical Faculty Hospital (No: 2011- KAEK-2 2020/10).
2.2 Performing the respiratory function tests
Respiratory function tests (PFT), in the presence of 3 experienced laboratory technicians for at least 8 hours, short-acting bronchodilator and cromoline, long-acting bronchodilator and theophylline and nedocromil for at least 48 hours, leukotriene receptor antagonist (LTRA) for at least 24 hours, at least 3-4 It was paid attention that he had not been using antihistamines for days. ZAN GPI.3.00 (Germany) device was used in the measurements. On the day of the test, they were asked to eat or not drink chocolate, tea, coffee or coke. Before the test, the patients were rested for at least 5 minutes. From the measurement results, forced expiratory volume in 1 second (FEV1 as percentage of predicted value), forced vital capacity (FVC as percentage of predicted value), FEV1 / FVC ratio (as percentage of predicted value) were used in our study.
2.3 Exhaled nitric oxide fraction measurement
For FeNO measurements, using Bedfont NObreath (England) device, different disposable mouthpieces were used for each patient and the patient was told to perform a deep inhalation first and then exhalation for 10-12 seconds and measurements were made. Measurements were made three times at intervals and 2 measurements that were identical with each other were accepted as correct and entered into the study data. In cases where different results were obtained in all 3 measurements, up to 5 measurements were made in total. The most same value was accepted as true.
In order for the measurements to be accurate, it was paid attention that the persons to be measured did not eat or drink anything at least 1 hour before the measurements and that they rinse their mouths before the measurement. Since FeNO levels were affected by smoking, attention was paid that the persons to be measured were not smoking.
2.4. Statistical analysis
Statistical evaluation was performed using the Statistical Package for Social Sciences for Windows V 20.0 (SPSS Inc., Chicago, IL, USA) program. Kolmogorov-Smirnov test was used to evaluate the distribution of continuous variables.
Continuous variables were expressed as median (minimum-maximum) if not normally distributed, and as mean ± standard deviation if normally distributed. Categorical variables were expressed as number and percentage (n (%)). Chi square test was used to compare the rates of the groups. Multiple groups (> 2) were compared with the Kruskall-Wallis test. When a statistically significant difference was detected with the Kruskall-Wallis test, Bonferroni-corrected Mann-Whitney U-test was used for post hoc analysis. Statistical significance level was accepted as p <0.05. "Reciever Operator Characteristics Curve" (ROC) analysis method was used to evaluate the specificity and sensitivity of the obtained results.
3. Results
In our study, 74 patients diagnosed with sarcoidosis were identified. 20 sarcoidosis patients were excluded from the study because of smoking. A total of 107 individuals including 54 sarcoidosis patients and 53 healthy individuals were included in the study. Of the sarcoidosis patients 39 (72.2%) were female and 15 (27.8%) were male.
In the control group 34 (64.2%) were female and 19 (35.8%) were male. The mean age was 46.5 (20-70) in sarcoidosis patients and 32 (20-48) in the control group. Body mass index (BMI) (kg / cm2) was 26.28 ± 4.88 in sarcoidosis patients and 24.69 ± 3.47 in the control group. Of the sarcoidosis patients 24 (44.4%) were stage 1, 25 (46.3%) were stage 2, 4 (7.4%) were stage 3, and 1 (1.9%) were stage 4 (Table 1).
|
|
Sarcoidosis (n=54) |
Control (n=53) |
Average |
|
Gender |
|
|
|
|
Female n (%) |
39(%72.2) |
34(%64.2) |
- |
|
Male n (%) |
15(%27.8) |
19(%35.8) |
- |
|
Age |
46.5(20-70) |
32(20-48) |
37(20-70) |
|
Height (cm) |
166.44±9.14 |
169.24±8.84 |
167.83±9.06 |
|
Weight (kg) |
72.57±12.89 |
71.13±13.36 |
71.85±13.09 |
|
BMI (kg/cm2) |
26.28±4.88 |
24.69±3.47 |
25.49±4.29 |
|
Comorbid disease n (%) |
16 (%29.6) |
0 |
- |
|
Sarcoidosis stage |
|
|
|
|
Stage 1 |
24(%44.4) |
- |
- |
|
Stage 2 |
25(%46.3) |
- |
- |
|
Stage 3 |
4(%7.4) |
- |
- |
|
Stage 4 |
1(%1.9) |
- |
- |
BMI: Body mass index
Table 1: Demographic characteristics of the study participants.
|
|
Stage 1 |
Stage 2 |
Stage 3 |
Stage 4 |
Control |
Total |
|
|
(n=24) |
(n=25) |
(n=4) |
(n=1) |
(n=53) |
(n=107) |
|
Gender |
|
|
|
|
|
|
|
Male n (%) |
8(%23.5) |
5(%14.7) |
2(%5.9) |
0 |
19(%55.9) |
34(%100) |
|
Female n (%) |
16(%21.9) |
20(%27.4) |
2(%2.7) |
1(%1.4) |
34(%46.6) |
73(%100) |
|
Previous steroid treatment history |
6(%22.2) |
16(%59.3) |
4(%14.8) |
1(%3.7) |
0 |
27(%100) |
|
n (%) |
|
|
|
|
|
|
|
Currently taking steroid therapy |
0 |
7(%58.3) |
4(%33.3) |
1(%8.3) |
0 |
12(%100) |
|
n (%) |
|
|
|
|
|
|
|
Organ involvement |
|
|
|
|
|
|
|
Heart involvement |
0 |
0 |
0 |
1 |
0 |
1 |
|
Eye involvement |
0 |
3 |
0 |
0 |
0 |
3 |
|
Lymph node involvement |
24 |
25 |
4 |
1 |
0 |
54 |
|
Other |
2 |
0 |
0 |
0 |
0 |
2 |
|
Co-morbid illness |
2 |
12 |
1 |
1 |
0 |
16 |
Table 2: Characteristics of sarcoidosis patients.
6 (22.2%) of stage 1 patients, 16 (59.3%) of stage 2 patients, 4 (14.8%) of stage 3 patients, 1 (3.7%) of stage 4 patients had previously received steroid treatment. 7 (58.3%) patients in stage 2, 4 (33.3%) patients in stage 3, 1 (8.3%) patient in stage 4 were still on steroid treatment. Eye involvement due to sarcoidosis was detected in 3 patients and all patients were in stage 2. Of the patients with lymph node involvement, 24 were observed in stage 1 sarcoidosis, 25 in stage 2 sarcoidosis, 4 in stage 3 sarcoidosis, and 1 in stage 4 sarcoidosis. It was not detected in stage 3 sarcoidosis. Other system involvements such as liver and neurological system involvement were observed in 2 of the stage 1 patients. Chronic diseases such as diabetes mellitus, hypertension and cardiovascular diseases were observed in 2 patients in stage 1, 12 patients in stage 2, stage 3 and 1 patient each in stage 4 (Table 2).
Other: liver and neurological system involvement, Comorbid disease: Chronic diseases such as diabetes mellitus, hypertension and cardiovascular diseases. When respiratory function parameters such as % FEV1,
FVC and % FEV1 / FVC in sarcoidosis patients were compared with the control group, the FEV1% in sarcoidosis patients was 3.03 ± 1.10 and 3.48 ± 0.75 in the control group and was statistically significant (p <0.001). When compared in FEV1 / FVC%, 80.5 (55-100) in sarcoidosis patients and 84 (60-107) in the control group were observed and statistically significant (p = 0.002). There was no significant difference in % FVC values.
FeNO values were compared between sarcoidosis patients and control group. FeNO was measured as 17.85 ± 10.72 ppb (parts per bilion) in sarcoidosis patients, while it was measured as 10.69 ± 6.05 ppb in the control group and was statistically significant (p <0.001). When the respiratory function parameters and FeNO values were compared, no correlation was observed between them.
|
|
Sarcoidosis (n=54) |
Control Group (n=53) |
Average |
p value |
|
FEV1 (LT) |
3.03±1.10 |
3.48±0.75 |
3.25±0.96 |
P<0.001 |
|
FVC (LT) |
3.30±0.95 |
4.18±0.94 |
3.73±1.04 |
0.882 |
|
FEV1/FVC |
80.5(55-100) |
84(60-107) |
82(55-107) |
0.002 |
|
Saturation % |
93.50(87-99) |
96(89-99) |
95(87-99) |
p<0.001 |
|
Pulse heart beat / minute |
72.50(57-119) |
75(58-120) |
74(57-120) |
0.760 |
|
FeNO (ppb) |
17.85±10.72 |
10.69±6.05 |
14.30±9.40 |
p<0.001 |
FEV1: Forced Expiratory Volume in 1. Second, FVC: Forced Vital Capacity, Ppb: parts per bilion
Table 3: Comparison of sarcoidosis patients and the control group.
When the results found in sarcoidosis patients were evaluated by ROC (Reciever Operator Characteristics Curve) analysis, it was found that the sensitivity of FeNO in sarcoidosis was 64.8%, its specificity was
69.8% and the cutt of value was 12.50 Ppb. These results were found to be statistically significant (p <0.001) (Graphic 1).
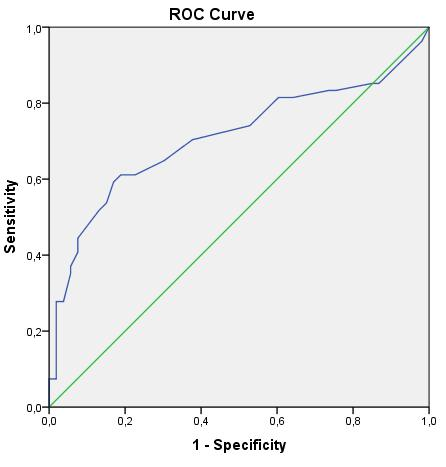
Graphic 1: The specificity and sensitivity of exhaled nitric oxide fraction in sarcoidosis.
4. Discussion
Some studies have shown that it may be useful to evaluate FeNO as a leading marker in proximal airway and / or distal airway inflammation [17]. It has also been found that the amount of NO in exhaled air increases with the increase of eosinophils in sputum in airway inflammation [16]. This suggests that NO has a role in airway inflammation. In a study by Rutgers et al., They found that NO levels in exhaled air and supernatant NO2 / NO3 levels in healthy smokers were lower than healthy non-smokers [16]. In our study, we did not include smoking patients in order not to affect FeNO values.
It has been found that the NO concentration in the lower respiratory tract in healthy people is 20 times lower than in the upper respiratory tract [18]. However, there is an increased concentration of NO in the lower respiratory tract of asthmatic patients [19]. In sarcoidosis and systemic sclerosis, together with pulmonary involvement, it was observed that NO
concentration increased in the breath of patients, but no correlation was shown with disease activity in sarcoidosis [20]. Tiev et al. Similar results were obtained by [21].
In our study, we found that the FeNO value increased significantly in sarcoidosis patients compared to the healthy control group. We found no significant difference in FeNO values between stages in sarcoidosis patients. A negative correlation was observed between the high concentration of alveolar NO and inspiratory vital capacity in the market by Schildge [22]. In our study, there was no correlation between breathing and FeNO.
5. Conclusion
Diseases in which lung parenchyma is affected, bronchoalveolar inflammation states and sarcoidosis cause changes in pulmonary NO metabolism. This is followed by an increase in FeNO levels. FeNO analysis can be used as a marker in detecting these processes.
Conflict of Interest
All authors declare that they have no conflict of interest.
Financial Support
No financial support was received for study.
Author Contributions
All authors contributed equally.
References
- Tekin NS. Sarcoidosis. Turk J Dermatol 6 (2012): 80-86.
- Gomez NS, Peters JI, Namb?ar Diagnosis and Management of Sarcoidosis. American Family Physician 93 (2016): 10.
- Baughman RP, Culver DA, Judson MA. A Concise Review of Pulmonary Sarcoidosis. Am J Respir Crit Care Med 183 (2011): 573-581.
- Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, et al. American Thoracic Society/ European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis 16 (1999): 149-173.
- Jain R, Yadav D, Puranik N, Guleria R, OJin J. Sarcoidosis: Causes, Diagnosis, Clinical Features, and Treatments. J. Clin. Med 9 (2020): 1081.
- Sulica R, Teirstein AS, Kakarla S, Nemani N, Behnegar A, et al. Distinctive clinical, radiographic, and functional characteristics of patients with sarcoidosis-related pulmonary hypertension. Chest 128 (2005): 1483-1489.
- Baughman RP, Lower EE, Drent M. Inhibitors of tumor necrosis factor (TNF) in sarcoidosis: who, what and how to use them. Sarcoidosis Vasc Diffuse Lung Dis 25 (2008): 76-89.
- Gustafsson LE, Leone AM, Person MG, et al. Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Commun 181 (1991): 852-857.
- Yates DH, Kharitonov SA, Robbins RA, et al. Effect of a nitric oxide synthase inhibitor and a glucocorticosteroid on exhaled nitric oxide. Am J Respir Crit Care Med 152 (1995): 892-896.
- Arnal JF, Flores P, Rami J, Murris-Espin M, Bremont F, et al. Nasal nitric oxide concentration in paranasal sinus inflammatory diseases. Eur Respir J 13 (1999): 307-312.
- Monsalve-Naharro jÁ, Doming -Chiva E, Castillo SG, Cuesta-Montero P, Jiménez-Vizuete JM. Inhaled nitric oxide in adult patients with acute respiratory distress syndrome. Farm Hosp 41 (2017): 292-312, 293.
- Creagh-Brown BC, Griffiths MJ, Evans TW. Bench-to-bedside review: Inhaled nitric oxide therapy in adults. Crit Care 13 (2009): 221.
- Yu B, Ichinose F, Bloch DB, Zapol WM. Inhaled nitric oxide. British Journal of Pharmacology 176 (2019): 246-255.
- Lakari E, Soini Y, Säily M, et al. Inducible nitric oxide synthase, but not xanthine oxidase, is highly expressed in interstitial pneumonias and granulomatous diseases of human lung. Am J Clin Pathol 117 (2002):132-142.
- Tiev KP, Coste J, Ziani M, et al. Diagnostic value of exhaled nitric oxide to detect interstitial lung disease in systemic sclerosis. Sarcoidosis Vasc Diffuse Lung Dis 26 (2009): 32-38.
- Rutgers SR, Mark TW, Coers W, Moshage H, Timens W, et al. Markers of nitric oxide metabolism in sputum and exhaled air are not increased in chronic obstructive pulmonary disease. Thorax 54 (1999): 576-580.
- García-Río F, Casitas R, Romero D. Utility of two-compartment models of exhaled nitric oxide in patients with asthma. J Asthma 48 (2011): 329-334.
- Kharitonov S, Alving K, Barnes P. Exhaled and nasal nitric oxide measurements: recommendations. Eur Respir J 10 (1997): 1683-1693.
- Sardón O, Corcuera P, Aldasoro A, Korta J, Mintegui J, et al. Alveolar nitric oxide and its role in pediatric asthma control assessment. Sardón et al. BMC Pulmonary Medicine 14 (2014): 126.
- Paredi P, Kharitonov SA, Loukides S, et al. Exhaled nitric oxide is increased in active fibrosing alveolitis. Chest 115 (1999): 1352-1356.
- Tiev KP, Cabane J, Aubourg F, et al. Severity of scleroderma lung disease is related to alveolar concentration of nitric oxide. Eur Respir J 30 (2007): 26-30.
- Schildge J. Nitric Oxide in Exhaled Breath of Patients with Interstitial Lung Diseases. Stickstoffmonoxyd in der Atemluft von Patienten mit interstitiellen Lungenkrankheiten Pneumologie 65 (2011): 143-148.
