The Prevalence of Chronic Kidney Disease in Hypertensive Patients in Primary Care in Hong Kong: a Cross-Sectional Study
Article Information
Shaowei Xu*, Yim Chu Li, Catherine Xiaorui Chen
Department of Family Medicine and General Outpatient Clinic, Queen Elizabeth Hospital, Hong Kong
*Corresponding author: Shaowei Xu, Department of Family Medicine and General Outpatient Clinic, Queen Elizabeth Hospital, Hong Kong.
Received: 05 May 2022; Accepted: 13 May 2022; Published: 30 May 2022
Citation: Shaowei Xu, Yim Chu Li, Catherine Xiaorui Chen. The Prevalence of Chronic Kidney Disease in Hypertensive Patients in Primary Care in Hong Kong: a Cross-Sectional Study. Cardiology and Cardiovascular Medicine 6 (2022): 268-283.
View / Download Pdf Share at FacebookAbstract
Background: To identify the prevalence of Chronic Kidney Disease (CKD) in Chinese hypertensive population managed in a local public primary care clinic and to explore its associated risk factors.
Methods: Medical records of Chinese adult hypertensive patients (> 18 years of age) who had been followed up in a public General Outpatient Clinic (GOPC) from 1 Jan 2018 to 30 Jun 2018 were retrieved and reviewed, and a sample group was randomly selected. Demographic, clinical parameters including age, sex, smoking status, body weight, height, systolic and diastolic blood pressure, biochemical data, and comorbidities were collected from the Clinical Management System (CMS). Estimated glomerular filtration rate (eGFR) was calculated by using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. CKD was defined as eGFR < 60 ml/min/1.73m2 and staged according to Kidney Disease Improving Global Outcomes (KDIGO) 2012 criteria. Student's t-test was used to analyze continuous variables and the Chi-squared test was used for categorical data. Multivariate Logistic regression was used to examine the association between CKD and variable associated factors. All statistical tests were two-sided, and a P-value of <0.05 was considered significant.
Results: Among the 993 Chinese hypertensive patients included in the final analysis, 152 were found to have CKD, with overall prevalence being 15.3%. In addition, the prevalence of CKD increased with the ageing of the population. In multivariate analysis, associated factors for CKD included age (OR 3.6 for every 10 years increase), history of congestive heart failure (OR 6.3), diabetes mellitus (OR 1.7), gout (OR 3.0), low high-density lipoprotein cholesterol level (OR 0.29), and presence of proteinuria or albuminuria (OR 2.7).
Keywords
Associated factor; Chronic kidney disease; Hong Kong; Hypertension; Primary care
Article Details
Abbreviations:
ACEI: Angiotensin-converting enzyme inhibitor; AF: Atrial fibrillation; AFCKDI: Asian Forum for Chronic Kidney Disease Initiatives; ARB: Angiotensin receptor blocker; BMI: Body mass index; CG: Cockcroft-Gault formula; CHF: Congestive heart failure; CKD: Chronic Kidney Disease; CMS: Computer Management System; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration equation; COPD: Chronic obstructive pulmonary disease; DBP: Diastolic blood pressure; DM: Diabetes mellitus; eGFR: Estimated glomerular filtration rate; ESRD: End stage renal disease; GOPC: General outpatient clinic; HDL: High density lipoprotein; HT: Hypertension; ICD -9: International Classification of Diseases -9; ICPC: International Classification of Primary Care; IHD: Ischaemic heart disease; KDIGO: Kidney Disease Improving Global Outcomes; LDL: low density lipoprotein; MDRD: Modification of Diet in Renal Disease; NHANES: National Health and Nutrition Examination Survey; NKDEP: National Kidney Disease Education Program; NSAIDs: Non-steroid anti-inflammatory drugs; OR: odds ratio; PVD: Peripheral vascular disease; RFT: Renal function test; SBP: Systolic blood pressure; SPSS: Statistical Product and Service Solutions; TIA: Transient ischaemic attack; USRDS: United States Renal Data System
1. Introduction
Chronic Kidney Disease (CKD) is a worldwide public health problem [1]. In Kidney Disease Improving Global Outcome (KDIGO) 2012 clinical guideline [2], CKD is defined as abnormalities of kidney structure or function, present for more than 3 months, with implications for health. It is confirmed to be associated with an increased risk of cardiovascular comorbidities and mortality [3,4] as well as progression to dialysis dependent End Stage Renal Disease (ESRD) [5,6] . In our daily practice, CKD refers to CKD stage 3 to 5 in the KDIGO CKD staging system, which is defined as Estimated Glomerular Filtration Rate (eGFR) being less than 60 ml/min/1.73m2. Extensive studies have shown that this group of patients carries a particularly high risk for complications and adverse outcomes [7,8] . The prevalence of CKD in the general population varies in regions, e.g. 8.7% in selected countries in Africa, 13.1% in Indian subcontinent, 14.7% in Australia, 15.5% in North America, 18.4% in Europe, 13.7% in Japan and South Korea, 13.2% in Greater China region, with considerable international variation [9]. In Hong Kong, a screening study showed the prevalence of positive (≥1+) urine dipstick for protein, glucose, blood, protein or blood, any urine abnormality was 3.2%, 1.7%, 13.8%, 16%, 17.4%, respectively in apparently “healthy” (asymptomatic and without history of DM, HT, or CKD) individuals [10]. Hypertension (HT) is a well-recognized risk factor for CKD. According to the United States Renal Data System (USRDS) 2019 Annual Data Report [11], hypertension is the second leading cause of ESRD. As suggested by the Asian Forum for Chronic Kidney Disease Initiatives (AFCKDI) [12], hypertensive patients are the target population for CKD screening. Various studies reported CKD prevalence in HT patients among 1.7-26.0% in different ethnic population in Europe [13], US [14], and Taiwan [15]. A recent study in Hong Kong [16] reported 22.0 per 1000 person-years for the incidence rate of CKD in local hypertensive patients without pre-existing diabetes mellitus, cardiovascular disease, or chronic kidney disease. However, the study of CKD prevalence in HT patients in Hong Kong is still lacking despite the fact that many hypertensive patients followed-up in primary care have co-existing renal impairment already.
2. Method
2.1. Aim
The objective of this study is to explore the prevalence of CKD in hypertensive patients in a public primary care clinic and to identify the possible associated factors.
2.2. Study design and setting
It is a cross-sectional study in a public primary care clinic. Yau Ma Tei General Outpatient Clinic is one of three public primary care clinics mainly serving but not restricted to an urban district with population around 330000 (Census and Statistics Department Hong Kong Special Administrative Region 2019).
2.3. Study population
2.3.1. Inclusion criteria
Chinese HT patients with International Classification of Primary Care (ICPC) code K86 (uncomplicated HT) or K87 (complicated HT) in the Clinical Management System (CMS), who had at least one follow up in a public primary care clinic from 01/01/2018 to 30/06/2018 and had at least two sets of serum renal function tests (RFT) done 3 months apart in the previous 3 years were included.
2.4. Definition of CKD and staging
2.4.1. Calculation of eGFR
eGFR was calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [17]:
eGFR =141 x min(SCr/κ, 1)α x max(SCr /κ, 1)-1.209 x 0.993Age x1.018 [if female] x1.159 [if Black]; κ = 0.7 (females) or 0.9 (males); α = -0.329 (females) or -0.411 (males); min = indicates the minimum of SCr/κ or 1; max = indicates the maximum of SCr/κ or 1; age = years; Scr in mg/dl.
2.4.2. Persistence of kidney abnormality
The latest two serum creatinine levels were retrieved from the CMS, which were at least 3 months apart. The mean eGFR was used for diagnosis and staging of CKD.
2.4.3. CKD definition and staging
CKD was defined as eGFR < 60 mL/min/1.73 m². According to KDIGO 2012 criteria [2], patients were further staged into:
CKD 3a: eGFR 45-59 ml/min/1.73m2;
CKD 3b: eGFR 30-44 ml/min/1.73m2;
CKD4: eGFR 15-29 ml/min/1.73m2;
CKD5: eGFR <15 ml/min/1.73m2.
All laboratory assays were performed in accredited laboratories by the College of American Pathologists, the Hong Kong Accreditation.
2.5. Determination of variables
Each recruited patients' age, biological sex, smoking status, body mass index (BMI), systolic and diastolic blood pressure (SBP and DBP), fasting sugar level and lipid profile were retrieved from the CMS. The patient was considered a smoker if he/she currently smoked or was within the first 6 months of quitting. The BMI was calculated as body weight (kg)/ body height2 (m2). BMI>=25 kg/m2 was defined as obesity (Centre for Health Protection Hong Kong 2010 criteria). SBP and DBP were averaged for all the outpatient encounters from 01/01/2018 to 30/06/2018. The most recent blood tests for glucose and lipid profile were used for data analysis if more than one test had been performed during the study period.
2.6. Proteinuria / albuminuria
All hypertensive patients regularly followed-up in the clinic were invited to a dipstick urine test (Albustix®) for proteinuria every 1-2 years, the results were manually read and reported as -ve, trace, or +ve. If trace or +ve, a spot urine Protein / Creatinine ratio (PCR) would be ordered; if negative, then the next urine dipstick test would be repeated 1 to 2 years later. Either dipstick protein +ve or PCR≥ 45 mg/mmol Creatinine was considered as proteinuria positive. For patients with HT and DM at the same time, the urine albumin / Creatinine ratio (ACR) was ordered directly instead of dipstick test. ACR >2.5mg/mmol(male) or >3.5mg/mmol(female) was defined as albuminuria positive, and the test would be repeated 3 months later. If there were 2 or more times of positive results in either dipstick, PCR or ACR tests, and they were taken more than 3 months apart, the patient was labeled “proteinuria or albuminuria” in this study.
2.7. Definition of comorbidities
The comorbidities were identified from both ICPC and International Classification of Diseases (ICD) -9 codes, as following:
Stroke / transient ischaemic attack (TIA): ICPC: K89, K90, K91; ICD-9: 430, 431, 432, 433, 434, 435, 436, 437, 438;
Ischaemic heart disease (IHD): ICPC: K74, K75, K76; ICD-9: 410, 411, 412, 413, 414 ;
Congestive heart failure (CHF): ICPC: K77; ICD-9: 428;
Atrial fibrillation (AF): ICPC: K78; ICD-9: 427.3;
Peripheral vascular disease (PVD); ICPC: K92; ICD-9: 443;
Diabetes mellitus (DM): ICPC: T89, T90; ICD-9: 250;
Gout: ICPC: T92; ICD-9: 274;
Chronic obstructive pulmonary disease (COPD): ICPC: R95; ICD-9: 491, 492, 494, 496.
2.8. Medications
Dispensary records were reviewed. The following medications were recorded: antihypertensive drugs, lipid-lowering agents, anti-platelet; urate-lowering agents; Non-Steroid Anti-Inflammatory Drugs (NSAIDs).
2.9. Sample size estimation
One proportion cross-sectional formula was used to calculate the sample size (website http://www2.ccrb.cuhk.edu.hk/stat/epistud.htm). Assume Probability of type 1 error is 0.01, prevalence proportion p is 0.15, estimated effect size is 1, desired level of absolute precision is 0.03, the required sample size is 940. To allow the room for sample exclusion (~20%), a total of 1200 patients was randomly selected by online (https://www.randomizer.org/) generated random numbers for data analysis. Briefly, all the included patients were listed in order of their outpatient case numbers, then a list of random numbers was generated from the research randomizer from which the 1200 patients to be included were selected.
2.10. Statistical analysis
Statistical calculations were completed using SPSS 19 (IBM SPSS Statistics version 19). Continuous variables were described as mean and standard deviation, while qualitative variables were expressed as numbers and percentage. T-test was used to compare quantitative variables and the Chi-squared test for categorical variables. Mantel-Haenszel test was used for trend between age groups and CKD prevalence. Multivariate logistic regression analysis was used to identify the risk factors for the presence of CKD. All statistical tests were two-sided, and a P value of less than 0.05 was considered significant.
3. Results
3.1. Study population and sampling process
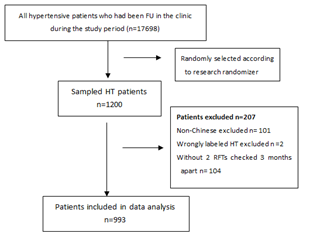
From 01/01/2018 to 30/06/2018, totally 17,698 HT patients had at least one follow-up visit in the clinic. Among them, 1200 patients were randomly selected, from which 207 cases were excluded, including 101 Non-Chinese, 2 wrongly labeled HT patients and 104 cases who had no repeated RFT tests 3 months apart. Therefore, the remaining 993 cases were included in the final analysis. The selection and sampling process was summarized in Figure 1.
The demographics and comorbidities of HT patients were demonstrated in Table 1. Among the 993 patients included in data analysis, 489 were female and 504 were male, with an average age of 68.9±10.9 years. The smoking status, BMI and comorbidities were retrieved.
|
Total (n=993) |
|
|
Age (year) |
68.9±10.9 |
|
Gender |
|
|
Female n(%) |
489 (49.2%) |
|
Male n(%) |
504(50.8%) |
|
BMI (kg/m2) |
25.7±4.1 |
|
Obesity n (%) |
514(51.8%) |
|
Smoking status n(%) |
|
|
Smoker |
73(7.4%) |
|
Ex-smoker |
165(16.6%) |
|
Non-smoker |
755(76.0%) |
|
Comorbidities n (%) |
|
|
cardiovascular disease |
|
|
Stroke / TIA |
100(10.1%) |
|
IHD |
54(5.4%) |
|
CHF |
12(1.2%) |
|
AF |
25(2.5%) |
|
PVD |
3(0.3%) |
|
Metabolic disorder |
|
|
Diabetes |
438(44.1%) |
|
Gout |
69(6.9%) |
|
COPD |
21(2.1%) |
|
Data are shown as mean ± standard deviation or No. (%) of cases |
|
Table 1: Demographics and comorbidities of HT patients included in data analysis .
3.2. Prevalence of CKD and distribution in age groups
As shown in Table 2, as defined by eGFR < 60 ml/min/1.73m2, the prevalence of CKD was 17.5% in male, 13.1% in female, and 15.3% overall. Male patients seemed to have a higher prevalence of CKD than female, but the difference was not significant (p = 0.065). The prevalence of CKD stage 3a, 3b, 4 and 5 in hypertensive patients were 10.1%, 4.4%, 0.6% and 0.2% respectively.
|
Male (n=504) |
Female (n=489) |
Total (n=993) |
||||
|
Creatinine (μmol/L) |
92.4±25.2 |
68.8±19.9 |
80.9±25.6 |
|||
|
eGFR (mL/min/1.73m2) |
76.0±18.2 |
80.4±17.5 |
78.2±18.0 |
|||
|
N |
% |
N |
% |
N |
% |
|
|
Non-CKD(eGFR >=60) |
416 |
82.5 |
425 |
86.9 |
841 |
84.7 |
|
CKD (eGFR<60) |
88 |
17.5 |
64 |
13.1 |
152 |
15.3 |
|
CKD3a (45-59) |
55 |
10.9 |
45 |
9.2 |
100 |
10.1 |
|
CKD3b (30-44) |
29 |
5.8 |
15 |
3.1 |
44 |
4.4 |
|
CKD4 (15-29) |
3 |
0.6 |
3 |
0.6 |
6 |
0.6 |
|
CKD5 (<15) |
1 |
0.2 |
1 |
0.2 |
2 |
0.2 |
|
Data are shown as mean ± standard deviation |
||||||
Table 2: CKD prevalence among HT patients in the primary care setting
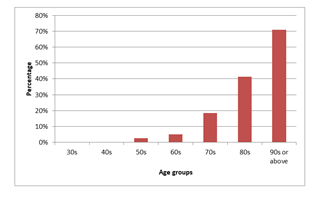
Figure 2 showed the prevalence of CKD in various age groups with apparent increasing trend of prevalence of CKD as age increased, the elder the age group, the higher the prevalence of CKD (trend p<0.001).
3.3. Factors associated with CKD
Table 3 summarized the univariate analysis of risk factors associated with CKD among HT patients. It showed that HT patients with CKD were older, longer hypertensive duration, had higher SBP but lower DBP than non-CKD group. They were more likely to have stroke / TIA, CHF, AF, DM and gout. Presence of albuminuria or proteinuria were apparently more in CKD patients. Patients with CKD were found to have lower concentration of total cholesterol, LDL, HDL level. They were on more anti-hypertensive medications, statin, anti-platelet and urate-lowering drug use. Multi-variate Logistic regression (Table 4) showed associated factors for CKD after controlling confounding factors were older age, history of CHF, DM, gout, lower level of HDL and presence of proteinuria or albuminuria.
|
Non CKD |
CKD |
P value |
|
|
(eGFR>=60) |
(eGFR < 60) |
||
|
(n=841) |
(n=152) |
||
|
Age (years) |
67.0±10.1 |
79.7±8.7 |
<0.001 |
|
Gender (Female %) |
425(50.5%) |
64(42.1%) |
0.064 |
|
BMI (kg/m2) * |
25.7±4.0 |
25.8±4.2 |
0.901 |
|
Obesity(BMI >=25)% |
257/506(50.8%) |
56/98(57.1%) |
0.27 |
|
Smoking status n (%) |
0.263 |
||
|
Smoker |
65(7.7%) |
8(5.3%) |
|
|
Ex-smoker |
134(15.8%) |
31(20.4%) |
|
|
Non-smoker |
642(76.3%) |
113(74.3) |
|
|
BP |
|||
|
SBP(mmHg) |
127.4±8.8 |
129.6±10.9 |
0.008 |
|
DBP(mmHg) |
73.2±8.5 |
67.7±8.9 |
<0.001 |
|
HT duration (year) |
10.0±4.9 |
12.9±5.0 |
<0.001 |
|
Cardiovascular disease n (%) |
|||
|
Stroke / TIA |
73(8.7%) |
27(17.8%) |
0.002 |
|
IHD |
45(5.4%) |
9(5.9%) |
0.701 |
|
CHF |
3(0.4%) |
9(5.9%) |
<0.001 |
|
AF |
17(2.0%) |
8(5.3%) |
0.042 |
|
PVD |
1(0.1%) |
2(1.3%) |
0.063 |
|
Metabolic disorder n (%) |
|||
|
Diabetes |
342(40.7%) |
96(63.2%) |
<0.001 |
|
Gout |
45(5.4%) |
24(15.8%) |
<0.001 |
|
COPD n (%) |
17(2.0%) |
4(2.6%) |
0.548 |
|
Fasting Glucose (mmol/L) |
6.1±1.4 |
6.3±1.3 |
0.085 |
|
Albuminuia / proteinuria** |
99(11.8%) |
54(35.5%) |
<0.001 |
|
Lipid |
|||
|
TG (mmol/L) |
1.5±0.9 |
1.4±0.8 |
0.881 |
|
TC (mmol/L) |
4.5±0.8 |
4.1±0.7 |
<0.001 |
|
LDL (mmol/L) |
2.4±0.7 |
2.1±0.6 |
<0.001 |
|
HDL (mmol/L) |
1.4±0.4 |
1.3±0.4 |
0.002 |
|
Medication use |
|||
|
Anti-Hypertensive |
|||
|
Number of medications |
1.7±0.8 |
2.2±1.0 |
<0.001 |
|
ACEI/ARB |
412(49.0%) |
93(61.2%) |
0.006 |
|
CCB |
652(77.5%) |
121(79.6%) |
0.598 |
|
Diuretics |
42(5.0%) |
12(7.9%) |
0.171 |
|
Beta-blocker |
232(27.6%) |
53(34.9%) |
0.053 |
|
Alpha-blocker |
104(12.4%) |
50(32.9%) |
<0.001 |
|
Statin |
494(58.7%) |
107(70.4%) |
0.007 |
|
Anti-platelet |
128(15.2%) |
47(30.9%) |
<0.001 |
|
Urate-lowering drugs |
21(2.5%) |
14(9.2%) |
<0.001 |
|
BMI, body mass index; SBP, systolic BP; DBP, diastolic BP; TIA, transient ischaemic attack; IHD, ischaemic heart disease; CHF, congestive heart failure; AF, atrial fibrillation; PVD, peripheral vascular disease; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; TG, triglyceride; TC, total cholesterol; LDL, low density lipoprotein; HDL, high density lipoprotein; ACEI/ ARB, ACEI angiotensin converting enzyme inhibitor/angiotensin receptor blocker. |
|||
Table 3: Univariate analysis of associated factors for CKD among HT cases
|
Covariates |
95% C.I. |
P-value |
||
|
OR |
Lower limit |
Upper limit |
||
|
Age (every 10 yrs increase) |
3.56 |
2.63 |
4.82 |
<0.001 |
|
CHF |
6.26 |
1.18 |
33.4 |
0.032 |
|
DM |
1.73 |
1.05 |
2.83 |
0.031 |
|
Gout |
3.02 |
1.16 |
7.88 |
0.021 |
|
HDL level |
0.29 |
0.14 |
0.62 |
0.001 |
|
Proteinuria or albuminuria |
2.72 |
1.6 |
4.62 |
<0.001 |
|
CHF, congestive heart failure; AF, atrial fibrillation; DM, diabetes mellitus; HDL, high density lipoprotein; OR, odds ratio. Only significant factors were listed (p-value <0.05) |
||||
Table 4: Multivariate Logistic regression analysis of associated factors for CKD among HT patients
4. Discussion
To the best of our knowledge, this is the first study to describe the CKD prevalence among unselected HT cases managed in the primary care setting in Hong Kong. We found that defined by eGFR < 60 ml/min/1.73m2, the prevalence of CKD was 17.5% in male, 13.1% in female, and 15.3% overall. Older age, history of CHF, DM, gout, lower level of HDL and presence of proteinuria or albuminuria are the predisposing factor of existence of CKD. Renal function test is essential to the diagnosis and staging of CKD. However, serum creatinine alone is not reliable to assess the renal function, as the serum creatinine concentration is influenced by GFR and other “non-GFR determinants” including the muscle bulk, dietary intake, renal tubular secretion and extra-renal creatinine elimination by the gastrointestinal tract [18]. Creatinine based eGFR estimation has evolved from Cockcroft-Gault (CG) formula [19], Modification of Diet in Renal Disease (MDRD) [20], to CKD-EPI equation [17]. KDIGO 2012 guideline recommended the use of the CKD-EPI equation for evaluation of eGFR in adults [2]. Hospital Authority in Hong Kong has completely turned to the CKD-EPI equation from the MDRD equation to report eGFR values since 2017. To identify comorbidities, we used both ICPC and ICD codes to retrieve comorbidities information, to cover all diagnoses in primary care setting, specialist outpatient clinic setting and hospital setting, which included more diagnosis information than ICPC code alone, as comparing percentage of comorbidities expressed ICPC/ICD combination versus ICPC coding alone: DM (44.1% vs 44.0%), stroke / TIA (10.1% vs 8.8%), IHD (5.4% vs 5.2%), AF (2.5% vs 2.2%), CHF (1.2% vs 0.6%), gout (6.9% vs 6.3%), COPD (2.1% vs 1.8%), and PVD (0.3% vs 0.2%). (data not shown in the results section). Defined by eGFR < 60ml/min/1.73m2, the prevalence of CKD varies throughout the world. In Europe, it varies between 1.7 to 11.5% in the general population, and 2.2-14.3% in hypertensive patients [13]. In the United States, USRDS reported the CKD prevalence of 6.9% in the general population and 16.1% in the hypertensive population among participants of the National Health and Nutrition Examination Survey (NHANES) 2013-2016 [14]. In Taiwan, a local cohort based study found 9.1% CKD prevalence in the general population and 26.0% in hypertensive individuals [15]. There were heterogenicity in patient source and sampling (electoral rolls, general practitioners lists, cohort, etc.), age ranges (all ages or elderly), eGFR calculation methods (CG, MDRD, CKD-EPI equations), test frequencies (single test or repeated tests), and definition of CKD (by eGFR calculation or by diagnostic codes). In our study, we found that CKD was present among 15.3% adult Chinese hypertensive patients, that was similar with those reported in the US and some European countries, but higher than other European countries and lower than Taiwan. The discrepancy could be due to the true difference or method diversity.
Although univariate analysis showed more possible associated factors could be related to CKD, the multivariate analysis after adjustment showed that significant factors associated with CKD were older age (OR 3.56 for every 10 years increase, p<0.001), history of CHF (OR 6.26, p 0.032), DM (OR 1.73, p 0.031), gout (OR 3.02, p 0.021), lower HDL (OR 0.29, p 0.001), and presence of proteinuria or albuminuria (OR 2.72, p <0.001). Firstly, our study showed a strong positive correlation between older age and increased risk of CKD, which is consistent with previous studies both in the general population [21] and in hypertensive patients [22-24]. There is a debate whether decreased GFR in older people represents an actual disease or a “normal ageing” phenomenon, as GFR declines steadily with ageing, beginning at age 30–40 years, with an apparent acceleration in the rate of decline after age 65–70 years [25]. The subdivision of CKD stage 3 (eGFR 30-59 ml/min/1.73 m2) into 3a (eGFR 45-59 ml/min/1.73 m2) and 3b (30-44 ml/min/1.73 m2), partially reflected the concept of the latter being more “pathologic” with more complications. Furthermore, glomerular sclerosis, tubular atrophy and vascular sclerosis are associated with ageing [26]. Given the fact that there appears to be increased risk of complications associated with decreased eGFR in older people irrespective of cause, KDIGO considers all individuals with persistently decreased GFR less than 60 ml/min/1.73m2 to have CKD, which is still the current standard of practice and research. History of CHF was found to be a strong associated factor for CKD, a similar finding as supported by other studies [27, 28]. Actually sometimes CHF and CKD are considered concurrent chronic disease epidemics [29]. CHF as the primary syndrome can experience secondary CKD, and vice versa, or both can coexist on the basis of shared risk factors. In this cross-sectional study, it is hard to tell which disease is primary and which is secondary. It was not unexpected that DM was an associated factor with CKD, as DM itself is the leading cause of CKD and ESRD in developed countries. As a well-recognized microvascular complication, kidney impairment develops in approximately 30% of Type 1 DM patients and 40% Type 2 DM patients. Among the risk factors for Diabetic kidney disease initiation and progression, hyperglycemia and hypertension are the two most prominent factors [30]. An association between gout and CKD has been recognized for many years [31-33]. The association could be bidirectional, with CKD as an independent risk factor for gout [34] and gout patients potentially predisposing to CKD possibly by hyperuricemia, chronic inflammation or NSAIDs drug therapy. Some interventional study suggested urate lowering treatment may have a beneficial role in renal function protection [35,36]. Dyslipidemia is common but not universal in CKD patients. The presence of dyslipidaemia was affected by eGFR, presence of DM, the severity of proteinuria and nutrition [37]. While KDIGO Work Group no longer recommended LDL-Cholesterol as the single indication or target for pharmacological therapy, some studies supported the role of low HDL-cholesterol in the development and progression of CKD [38,39]. However, the protective role of HDL in CKD is being challenged and needs further evidence [40]. It was not surprising to find proteinuria or albuminuia was a strong associated factor of CKD. Actually albuminuria has been recognized essential in cardiovascular risk stratification in CKD patients. Proteinuria or albuminuria is not only a marker of kidney injury, but also a potential toxic contributing to renal function decline [41]. KDIGO [2] suggests initial testing of proteinuria in the following descending order: 1) urine Albumin-to-Creatinine Ratio (ACR); 2) urine Protein-to-Creatinine Ratio (PCR); 3) reagent strip urinalysis for total protein with automated reading; 4) reagent strip urinalysis for total protein with manual reading. A spot urine protein sample for protein with standard urine Dipstick test was recommended by AFCKDI [12] as a convenient and cost-effective tool for proteinuria detection in the primary care setting. Although not all patients were tested for albuminuria in our retrospective study, the data on presence of either proteinuria or albuminuria apparently implied the positive relationship between them and CKD.
3.1. Limitations of the study
There were limitations to this study. Firstly, not all HT patients were checked for urine albumin. Dipstick for urine protein was used instead, which could be less accurate, and limited further risk stratification according to ACR levels. Secondly, this was a single centre data from a public primary care clinic. Although the sex ratio and median age are similar among different urban districts in Hong Kong, and the ethnic distribution imbalance was offset by excluding non-Chinese population, selection bias could still exist. Larger scale multicenter study was expected to overcome this limitation. Thirdly, patients in more advanced CKD stages would be referred to secondary care, thus the percentage of CKD 4/5 patients could be underestimated. The results may not be applicable to the private sector or secondary care setting. Lastly, given the cross-sectional design of the study, it could not establish a causal relationship between associated factors and CKD development. Prospective cohort study or interventional study would help provide more information on this regard.
4. Conclusion
We conclude that in Chinese hypertensive patients followed-up in the public primary care clinic in Hong Kong, the prevalence of chronic kidney disease with eGFR being less than 60ml/min/1.73m2 was 15.3% by CKD-EPI equation. The prevalence showed an apparently increasing trend in elderly age groups. The associated factors for CKD were older age, history of CHF, DM, gout, low HDL level, and presence of proteinuria or albuminuria. Since CKD is a well-established risk factor for several clinical outcomes, family physicians should enhance their awareness of the high prevalence of CKD among HT patients and pay particular attention to the presence of the above associated factors. A concerted effort should be made in early recognition of risky CKD group in HT patients.
Declarations
Ethical approval
The study was approved by the Cluster Research Ethics Committee. Ref: KC/KE-18-0196/ER-1. This is an observational study collecting existing data via Clinical Management System Retrieving Software without sensitive or identifiable personal information (name or ID), without affecting patient’s management, and reported in aggregate level. So exemption of consent was applied and approved by the Institutional Review Board (IRB) or Research Ethics Committee (REC) of Hospital Authority in Hong Kong.
Consent for publication
Not applicable
Availability of data
The data was stored and retrievable in the computer system of Hospital Authority Hong Kong, with restricted access according to “need to care” policy, therefore not accessible to the public. Researchers are allowed to retrieve and analyze data under approval from Ethical committee.
Competing interests
The authors declare that they have no competing interest.
Funding
No funding.
Author’s contributions
Xu collected, analyzed and interpreted the patient data. Li supervised the overall research, clinical work and patient care. Chen gave comments and major revisions on the design of the research and writing the manuscript. All authors read and approved the final manuscript.
Acknowledgements
I am indebted to Dr. Andrew Leung, and Prof. Shelly Tse from CUHK for their continuous support and advice on the study design, data review and manuscript preparation. All the colleagues in the GOPC contributed to the patient care and record maintenance. I would also like to thank the Risk Assessment Management Program (RAMP) hypertension team from the Department of Family Medicine and GOPC of KCC for their data entry and support throughout the study.
References
- Inker LA, Coresh J, Levey AS, et al . Estimated GFR, albuminuria, and complications of chronic kidney disease. J Am Soc Nephrol 22 (2011): 2322-2331.
- Stevens PE, Levin A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158 (2013): 825-830.
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351 (2004): 1296-1305.
- Chronic Kidney Disease Prognosis Consortium, Matsushita K, van der Velde M, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 375 (2010): 2073-2081.
- Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 80 (2011): 93-104.
- Mahmoodi BK, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet 380 (2012): 1649-1661.
- Hsu CY, Ordoñez JD, Chertow GM, et al. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int 74 (2008): 101-107.
- Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 79 (2011): 1331-1340.
- Hill NR, Fatoba ST, Oke JL, et al. Global Prevalence of Chronic Kidney Disease - A Systematic Review and Meta-Analysis. PLoS One 11 (2016): 0158765.
- Li PK, Kwan BC, Leung CB, et al. Prevalence of silent kidney disease in Hong Kong: the screening for Hong Kong Asymptomatic Renal Population and Evaluation (SHARE) program. Kidney Int Suppl (2005): S36-40.
- System USRD. US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States.
- Li PK, Chow KM, Matsuo S, et al. Asian chronic kidney disease best practice recommendations: positional statements for early detection of chronic kidney disease from Asian Forum for Chronic Kidney Disease Initiatives (AFCKDI). Nephrology (Carlton) 16 (2011): 633-641.
- Brück K, Stel VS, Gambaro G, et al. CKD Prevalence Varies across the European General Population. J Am Soc Nephrol 27 (2016): 2135-2147.
- System USRD. US Renal Data System 2018 Annual Data Report. Chapter 1: CKD in the General Population.
- Tsai MH, Hsu CY, Lin MY, et al. Incidence, Prevalence, and Duration of Chronic Kidney Disease in Taiwan: Results from a Community-Based Screening Program of 106,094 Individuals. Nephron 140 (2018): 175-184.
- Wan E, Yu E, Chin WY, et al. Association of Blood Pressure and Risk of Cardiovascular and Chronic Kidney Disease in Hong Kong Hypertensive Patients. Hypertension 74 (2019): 331-340.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 150 (2009): 604-612.
- Stevens LA, Levey AS. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol 20 (2009): 2305-2313.
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 16 (1976): 31-41.
- Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130 (1999): 461-470.
- Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health 8 (2008): 117.
- Rahman M, Brown CD, Coresh J, et al. The prevalence of reduced glomerular filtration rate in older hypertensive patients and its association with cardiovascular disease: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Arch Intern Med 164 (2004): 969-976.
- Ravera M, Noberasco G, Weiss U, et al. CKD awareness and blood pressure control in the primary care hypertensive population. Am J Kidney Dis 57 (2011): 71-77.
- Leoncini G, Viazzi F, Rosei EA, et al. Chronic kidney disease in hypertension under specialist care: the I-DEMAND study. J Hypertens. 2010. 28(1): 156-62.
- Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc 120 (2009): 419-28.
- Sobamowo H, Prabhakar SS. The Kidney in Aging: Physiological Changes and Pathological Implications. Prog Mol Biol Transl Sci 146 (2017): 303-340.
- Kottgen A, Russell SD, Loehr LR, et al. Reduced kidney function as a risk factor for incident heart failure: the Atherosclerosis Risk In Communities (ARIC) study. J Am Soc Nephrol 18 (2007): 1307-1315.
- Bagshaw SM, Cruz DN, Aspromonte N, et al. Epidemiology of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant 25 (2010): 1406-1416.
- McCullough PA, Philbin EF, Spertus JA, et al. Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study. J Am Coll Cardiol 39 (2002): 60-69.
- Alicic RZ, Rooney MT, Tuttle KR. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol 12 (2017): 2032-2045.
- Fessel WJ. Renal outcomes of gout and hyperuricemia. Am J Med 67 (1979): 74-82.
- Stack AG, Johnson ME, Blak B, et al. Gout and the risk of advanced chronic kidney disease in the UK health system: a national cohort study. BMJ Open 9 (2019): 031550.
- Roughley M, Sultan AA, Clarson L, et al. Risk of chronic kidney disease in patients with gout and the impact of urate lowering therapy: a population-based cohort study. Arthritis Res Ther 20 (2018): 243.
- Wang W, Bhole VM, Krishnan E. Chronic kidney disease as a risk factor for incident gout among men and women: retrospective cohort study using data from the Framingham Heart Study. BMJ Open 5 (2015): 006843.
- Soletsky B, Feig DI. Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension 60 (2012): 1148-1156.
- Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol 5 (2010): 1388-1393.
- Kasiske BL. Hyperlipidemia in patients with chronic renal disease. Am J Kidney Dis 32 (1998): S142-S156.
- Zoppini G, Targher G, Chonchol M, et al. Higher HDL cholesterol levels are associated with a lower incidence of chronic kidney disease in patients with type 2 diabetes. Nutr Metab Cardiovasc Dis 19 (2009): 580-586.
- Kawachi K, Kataoka H, Manabe S, Mochizuki T, Nitta K. Low HDL cholesterol as a predictor of chronic kidney disease progression: a cross-classification approach and matched cohort analysis. Heart Vessels 34 (2019): 1440-1455.
- Kronenberg F. HDL in CKD-The Devil Is in the Detail. J Am Soc Nephrol 29 (2018): 1356-1371.
- Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest 116 (2006): 288-296.