The Left Atrial Function as a Marker for the Severity of Heart Failure with Preserved Ejection Fraction
Article Information
Mingxue Sun1,2, Zhiyuan Shui3, Yunzhi Wang1, Yiqun Gao1, Shunji Liang1, Yiran Wang1, Qin Yu1, Li Liu1*
1Department of Cardiology, Affiliated Zhongshan Hospital of Dalian University, Dalian, Liaoning Province, China
2Department of Cardiology, The People’s Hospital of Sanya City, Hainan Province, China
3Department of Cardiology, Sanmenxia Central Hospital, Henan Province, China
*Corresponding author: Li Liu, Department of Cardiology, Affiliated Zhongshan Hospital of Dalian University, Dalian, Liaoning Province, 620061, China
Received: 28 April 2021; Accepted: 06 May 2021; Published: 12 May 2021
Citation: Mingxue Sun, Zhiyuan Shui, Yunzhi Wang, Yiqun Gao, Shunji Liang, Yiran Wang, Qin Yu, Li Liu. The Left Atrial Function as a Marker for the Severity of Heart Failure with Preserved Ejection Fraction. Cardiology and Cardiovascular Medicine 5 (2021): 300-314.
View / Download Pdf Share at FacebookAbstract
Background: Heart failure with preserved ejection fraction (HFpEF) is a complex syndrome characterized by heart failure symptoms and signs, but normal or near-normal left ventricular ejection fraction (LV-EF). There are objective evidences of left ventricular systolic/diastolic dysfunctions and alteration of left atrial (LA) structure and functions. However, limited data are available on the association of LA functions with the severity of HFpEF.
Methods: We assessed and analyzed LA/LV structure and functions in 61 patients with HFpEF with 2D echocardiography and two-dimensional speckle tracking echocardiographic technology (2D-STE). LA triphasic functions in subgroups with different classification of cardiac functions (NYHA II – IV) were compared. The correlation analysis of LA triphasic functions with LV systolic/diastolic functions was made with Pearson test.
Results: Patients with HFpEF had impaired LV systolic/diastolic functions, and impaired LA triphasic functions compared with control subjects. LA global longitudinal strain (LA-GLS) and the left atrial systolic strain rate (LA-mSRs, reflecting LA reservoir function) were positively correlated with global LV longitudinal strain (LV-GLS) and E/e’; the longitudinal strain rate of the left atrium at the early diastole (LA-mSRe, reflecting LA conduit function) and the longitudinal strain rate of the left atrium at the late diastole (LA-mSRa, reflecting LA pump function) were inversely correlated with the LV-GLS and E/e’. The LA function is closely related with the NYHA classification in patients with HFpEF.
Conclusion: LA phasic functions were significantly impaired in patients with HFpEF. It can be used as a marker for scaling the severity of HFpEF.
Keywords
Heart failure with preserved ejection fraction; Echocardiography; Two-dimensional speckle tracking echocardiography; Left atrial function; Strain; Strain rate
Article Details
Abbreviations:
HFpEF: Heart failure with preserved ejection fraction; LV-EF: Left ventricular ejection fraction; LA: Left atrial; 2D-STE: Two-dimensional speckle tracking echocardiographic technology; LA-GLS: LA global longitudinal strain; LA-mSRs: The left atrial systolic strain rate; LA-mSRe: The longitudinal strain rate of the left atrium at the early diastole; LA-mSRa: The longitudinal strain rate of the left atrium at the late diastole; HFrEF: Heart failure and reduced ejection fraction; LAD: Left atrial inner diameter; LA-EF: Left atrial ejection fraction; eGFR: estimated glomerular filtration rate; BUN: Blood urea nitrogen; NT-proBNP: N-terminal pro b-type natriuretic peptide
1. Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) is a complex syndrome characterized by heart failure symptoms and signs, but normal or near-normal left ventricular ejection fraction (LV-EF). Epidemiological studies indicated that the prevalence of HFpEF within the population varies from 1.14% to 5.5%, and appears to be rising [1-3]. At least one-half of patients with HF indeed have preserved ejection fraction, and it is more likely seen in women, elderly, people with a history of hypertension, obesity and other cardiovascular risk factors [1, 3, 4]. Patients with HFpEF experience similar patterns of morbidity and functional decline as do those with heart failure and reduced ejection fraction (HFrEF) [3, 4], but different from HFrEF which has classes of drugs to improve patients’ symptoms and outcome, the effective treatments for HFpEF are lacking [4, 5]. One of the reasons is thought to be due to incomplete understanding of its pathophysiology therefore poor matching of therapeutic mechanisms and primary pathophysiological processes.
The diagnosis and classification of its severity of HFpEF remain challenging due to the complicated pathophysiological processes, phenotypic manifestations and frequent multiple concomitant illnesses [3, 6]. LV-EF, along with the Doppler parameters, is still commonly used as a cut-off for inclusion/exclusion criteria because of convenience and ease of noninvasive assessment for the diagnosis of HFpEF in clinical settings, although it has several limitations, such as pre- and afterload-dependent, using two-dimensional non-tomographic technique [6, 7]. Recently, the change of LA structure and functions in patients with HFpEF attracted intensive attention [8, 9]. However, limited data are available on the association of LA function with the severity of HFpEF. In this study we used two-dimensional speckle tracking echocardiographic technology (2D-STE), a novel, feasible and sensitive method for evaluating the LA deformation in patients with HFpEF. Our purpose is to investigate the potential association of LA functions with the severity of HFpEF.
2. Materials and Methods
2.1 Patients
Patients with heart failure hospitalized in the Department of Cardiology, Affiliated Zhongshan Hospital of Dalian University from January 2017 to November 2018 were enrolled in this study. The following patients were excluded if they have (1) Congenital heart diseases; (2) Heart valve diseases; (3) Cardiomyopathy; (4) Severe liver and kidney dysfunction; (5) Pericardial diseases; (6) Thyroid disease; (7) Severe infection; (8) Malignant tumor; (9) Anemia; (10) Atrial fibrillation; (11) Left bundle branch block or paced rhythm; (12) Poor echocardiographic images. Patients’ general clinical information, including age, gender, height, weight, blood pressure, heart rate, medication history, as well as laboratory test results were collected. All patients underwent routine echocardiography within 48 hours after hospitalization. The diagnostic criteria of HFpEF is as followings (Materials and methods: Echocardiographic studies). We further divided HFpEF patients into three subgroups according to the New York Heart Association's (NYHA) cardiac function classification as 1) HFpEF subgroup A: patients with NYHA grade II and 2) HFpEF subgroup B: patients with NYHA grade III and 3) HFpEF subgroup C: patients with NYHA grade IV. A group of individuals matched with age and gender were recruited as control. Written informed consent was obtained from all patients before enrollment. The study protocol was approved by the Dalian University Ethics Committee and was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki.
2.2 Echocardiographic studies
Echocardiography was performed in all patients using Phillips Color Doppler Ultrasound Scanner (Phillips Medical System EPIC 7C, S5-1 probe) in a supine and left lateral decubitus position. Two-dimensional grayscale images were acquired in the standard parasternal and apical (apical 4, apical 2) views, and 3 cardiac cycles were recorded. In the apical 4-chamber view, mitral inflow was recorded at end expiration. Mitral annulus tissue Doppler velocities were measured at the septal and lateral sides of the mitral annulus using pulsed wave Doppler. All images acquirement and cardiac structure measurement methods are according to the recommendations of 2016 Chinese Adult Echocardiography Examination Measurement Guidelines [10]. The inner diameter of the left atrium was measured at the end of the ventricular systole, including the antero-posterior diameter (LA-ap), the long diameter (LA-l) and the transverse diameter of the left atrium (LA-t). Calculate the average value of the left atrial inner diameter as mean LAD (mLAD), mLAD = (LA-ap + LA-l + LA-t)/3. The two-plane Simpson method was used to calculate left ventricular and left atrial volume and ejection fraction. All LV and LA size and volume measurement were the average of three different cardiac cycles. Images and cine loops were stored digitally for subsequent offline analysis for strain. The diagnostic criteria for HFpEF is based on The 2016 ESC Guidelines for Diagnosis and Treatment of Acute and Chronic Heart Failure [11]: (1) symptoms and/or signs of heart failure; (2) LVEF ≥50%; (3) elevated brain natriuretic peptide levels ; (4) At least one of the following: 1) Associated structural heart disease (left ventricular hypertrophy and/or left atrial hypertrophy); 2) Diastolic dysfunction. The diagnostic criteria of left ventricular diastolic dysfunction follow the recommendations of American Society of Echocardiography/European Association of Cardiovascular Imaging (ASE/EACVI) for the evaluation of Left Ventricular
Diastolic Function by Echocardiography [12].
2.3 Two-dimensional speckle tracking echo-cardiography (2D-STE)
For 2D-STE, echocardiographic images were stored at 60–100 frames per second in three cardiac cycles. The analysis of strain and strain rate were performed offline using Qlab10.5 workstation at apical 4- and 2-chamber views respectively, as described previously [13]. Briefly, the endocardial border of the LA was manually traced in aCMQ mode at end ventricular systole, the epicardial borders of the LA wall were automatically defined by software, then manually adjust the width so that the tracking area covers the full-thickness myocardium. After the adjustment and confirmation of these traced borders, the software will automatically calculate and display the results. In patients with adequate image quality, 6 segments were analyzed. The left atrial global longitudinal strain (LA-GLS) was read on the strain curves of AP4 and AP2 respectively. The longitudinal LA strain rate curve had a positive systolic peak (LA-SRs), an early negative peak at early diastole (LA-SRe), and a late negative peak at late diastole (LA-SRa). LA-GLS and LA-SRs represented the LA reservoir function, LA-SRe reflected the LA conduit, and LA-SRa was the indices of the LA booster function. The value is the average of the AP-4 and AP-2 measurement, presenting as LA longitudinal average strain (LA-mGLS), and average strain rate: the longitudinal strain rate of the left atrium at systole (LA-mSRs), the longitudinal strain rate of the left atrium at the early diastole (LA-mSRe) and the longitudinal strain rate of the left atrium at the late diastole (LA-mSRa). All the image and strain anal-yses were done by a single expert echocardiologists.
2.4 Statistics
Continuous data are presented as mean ± SD, and count data is expressed as a percentage. The two continuous quantitative indicators that meet the normal distribution use independent sample t test for inter-group test; qualitative indicators use χ2 test. Pearson correlation coefficients test was used for correlation. All statistical analysis was performed by statistical software SPSS 20.0. A probability value ≤ 0.05 was used to define a significant result.
3. Results
3.1 Patients’ clinical characteristics
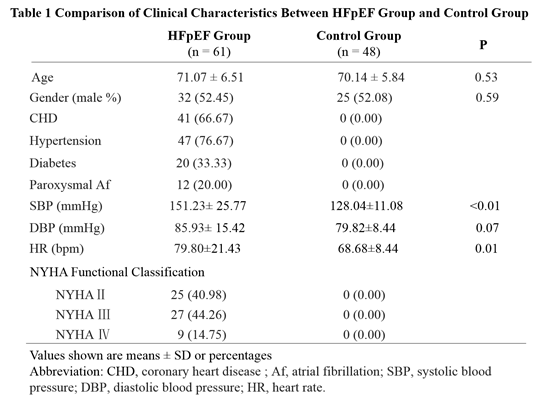
Out of 154 screened patients with HF, 61 patients met with the criteria for HFpEF. Among them, 25 patients with NYHA class II were sub-grouped as HFpEF group A, 27 patients with NYHA class III were sub-grouped as HFpEF group B and 9 patients with NYHA class IV were sub-grouped as HFpEF group C. Forty-eight normal individuals with age- and gender-matched were recruited as control group. There was no significant difference in age, gender, diastolic blood pressure between the HFpEF group and the control group (p> 0.05), but a significant diff-erence was observed in systolic BP and heart rate between the two groups. There were more patients with hypertension, diabetes, paroxysmal atrial fibrillation in HFpEF group that that in control group (Table 1).
3.2 Laboratory tests
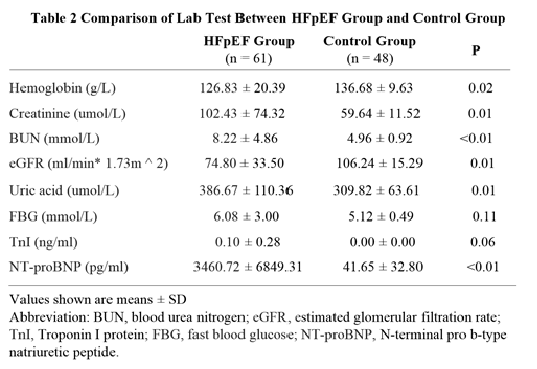
There was lower hemoglobin, estimated glomerular filtration rate (eGFR), but higher creatinine, blood urea nitrogen (BUN), uric acid and N-terminal pro b-type natriuretic peptide (NT-proBNP) in HFpEF group than that in control group (p<0.05). No significant difference was observed in hemoglobin and troponin I protein between the two groups (p>0.05) (Table 2).
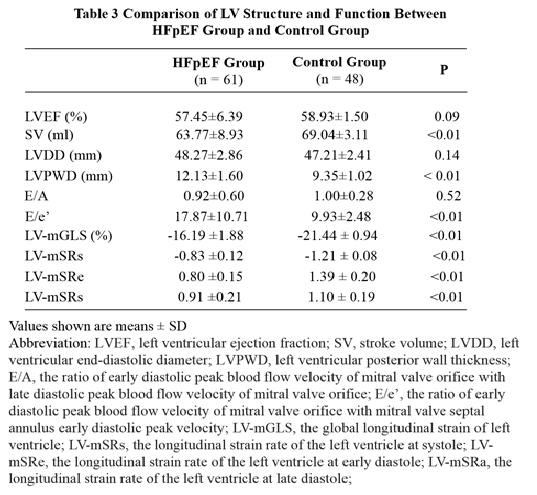
3.3 Left ventricular/Left atrial structure and function
It is not surprised to observe impairment of left
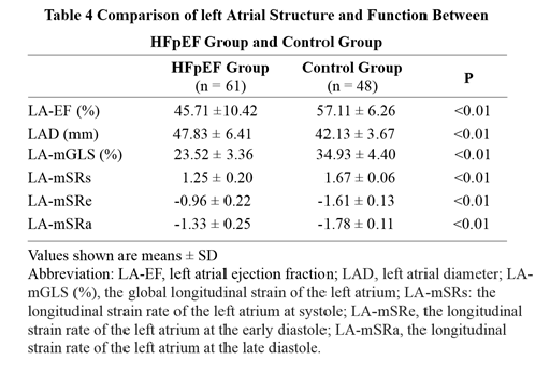
ventricular systolic and diastolic functions assessed by 2D-STE in HFpEF group although there is no significant difference in LV-EF between the two groups (Table 3). A significant differences in LA function was observed regarding the 2D-STE-derived indices between the two groups, including left atrial ejection fraction (LA-EF), LA-mGLS, LA-mSRs, LA-mSRe and LA-mSRa, indicating impairment of whole left atrial functions (Table 4).
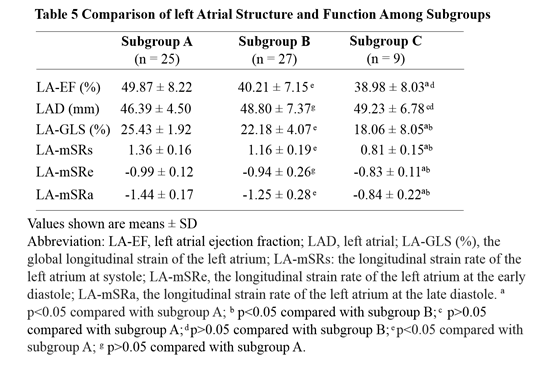
3.4 The Comparison of left atrial structure and function in patients with different cardiac function classification
We sub-grouped the patients with HFpEF according to the NYHA functional classification as subgroup A (NYHA II, n = 25), subgroup B (NYHA III, n = 27) and subgroup C (NYHA IV, n = 9). We compared LA function among the three subgroups to observe the relationship of LA function with the severity of heart failure. Our results showed that there is significant difference of LA-EF, LA-mGLS, LA-mSRs, LA-mSRe and LA-mSRa among the subgroups (p<0.05) (Table 5).
3.5 Correlation analysis of left atrial strain with Left ventricular strain
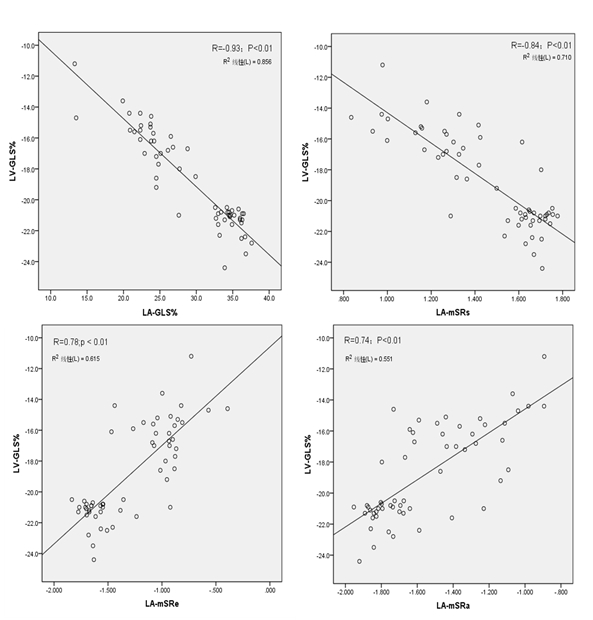
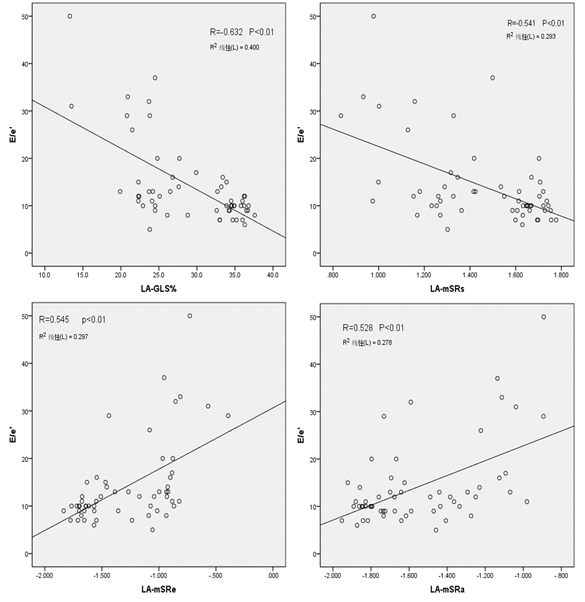
To further explore the relationship of LA functions with LV systolic/diastolic functions, Pearson test was used to analyze the correlation of these LA continuous variables with LV-GLS and E/e’. Our results showed that LA-mGLS and LA-mSRs were positively correlated with LV-GLS and negatively correlated with E/e’, LA-mSRe and LA-mSRa were negatively correlated with LV-GLS and positively correlated with E/e’. (Figure 1, Figure 2).
Values shown are means ± SD
Abbreviation: LV-GLS, the global longitudinal strain of left ventricle; LA-GLS, the global longitudinal strain of the left atrium; LA-mSRs: the longitudinal strain rate of the left atrium at systole; LA-mSRa, the longitudinal strain rate of the left atrium at the late diastole.
Values shown are means ± SD
Abbreviation: LV-GLS, the global longitudinal strain of left ventricle; LA-GLS, the global longitudinal strain of the left atrium; LA-mSRs: the longitudinal strain rate of the left atrium at systole; LA-mSRa, the longitudinal strain rate of the left atrium at the late diastole.
4. Discussion
HFpEF was not caused simply by LV diastolic dysfunction, its pathophysiology is much more complex. The essence of the pathophysiology of HFpEF is an increase of LV filling pressure (LVFP) [12, 14]. Elevation in LVFP alter the Starling forces across the pulmonary capillaries through left atrium, favoring filtration of water out of the vascular space and into the interstitium of lungs [15, 16], induces alterations in gas exchange and pulmonary ventilation, and therefore reductions in aerobic capacity [17, 18]. These prompt patients’ symptoms of dyspnea, especially during the stress of exercise. There are several echocardiographic indices that have been applied for the estimation of LVFP, with the ratio of early diastolic transmitral inflow velocity to mitral annular tissue velocity (E/e’) mostly used [19-21]. However, the accuracy of the E/e’ ratio in HFpEF was recently reported only a modest correlation between E/e’ and invasively-obtained resting filling pressures (pooled r = 0.56) [22]. Therefore a simple and more accurate parameter is required in clinic setting for evaluating LVFP.
Left atrium plays an important role in ensuring proper performance of left ventricular function and the systemic circulation [23]. It is not just simply a conduit for left ventricular (LV) filling. From hemodynamic perspective left atrium is divided into three phases, LA reservoir, conduit, and pump function, all of which contribute to LV filling [23-25]. Conversely, LV function influences LA function. LA reservoir function is affected by LV contraction and LA compliance. LA pump function is influenced by LV end-diastolic pressure, LV compliance, and LA contractile properties, while LA conduit function is dependent on LV diastolic properties [25, 26]. In patients with HFpEF, as LV diastolic filling pressures are elevated intermittently over time, there is secondary remodeling and dysfunction that develops in the left atrium. LA size and functions provide incremental clinical and prognostic information in patients with HFpEF [23-28]. Accurate evaluation of LA function has important significance. In clinical practice, LA function is usually assessed by 2D-echocardiography through analysis of pulmonary venous and transmitral flows by Doppler echocardiography, and LA myocardial velocities by tissue-Doppler echocardiography. However, these quantitative methods are affected by myocardial tethering, hemodynamic loading and acquisition angle [24, 28, 29]. 2D-STE is a relatively new echocardiographic technique which tracks the spatial dislocation of speckles (natural acoustic reflections) for regional and global myocardial function analysis [30, 31]. 2D-STE analysis gives an excellent assessment of the atrial deformation profile during an entire cardiac cycle, closely following the LA physiology [32]. In contrast to Doppler derived parameters, speckle tracking has the advantages of being angle-independent, less load-dependent, less affected by reverberations, side lobes and drop out artifacts. 2D-STE was found to be a feasible, reproducible and sensitive method to assess LA function [32-34]. Several studies have shown that strain imaging can detect LA dysfunction before the manifestation of LA structural changes [35-37]. Reduction in LA strain was found to be an important predictor in separating patients with clinical HFpEF and asymptomatic diastolic dysfunction [38].
In this study, we observed significant impairment of LA functions, including reservoir function, conduct function and pump function (reflecting with LA-mSRs, LA-mSRe, LA-mSRa) in the HFpEF group compared with the control group (p <0.01), which is consistent with most previous studies [8, 35-38]. With the cardiac functions further worsening (NYHA class II to class IV), we also noticed that the LA triphasic functions become even worse although the size of LA (LAD) has no significant changes. Correlation analysis indicated that LA triphasic function has strong correlation with LV systolic function (LV-GLS%), and modest correlation with the LV diastolic function (E/e’), suggesting that LA function can be used as a sensitive marker for scaling the severity of HFpEF. Further large-scale studies are needed to confirm this finding.
4.1 Limitations of this study
This study carried several limitations. The first was the patient sample. The sample size is relatively small, especially in subgroup C, large scale study is warranted for confirming our findings. Secondly, the follow-up data is lack to show the prognostic value of LA dysfunction in patients with HFpEF. Thirdly, the measurement of LA deformation was done by a single expert echocardiologist, although he is blinded to the study’s assignment.
5. Conclusion
In conclusion, there is significant impairment of LA function and structure changes (enlargement) in patients with HFpEF, and LA triphasic functions (reservoir, conduit and pump functions) are sensitive marker for scaling the severity of HFpEF. The improvement of left atrial dysfunction may be a potential therapeutic target for patients with HFpEF.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
References
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355 (2006): 251-259.
- Michele Senni, Walter J Paulus, Antonello Gavazzi, Alan G Fraser, Javier Díez, Scott D Solomon, et al. New strategies for heart failure with preserved ejection fraction: the importance of targeted therapies for heart failure phenotypes. Eur Heart J 35 (2014): 2797-2815.
- Mads J Andersen, Barry A Borlaug. Heart Failure with Preserved Ejection Fraction: Current Understandings and Challenges. Curr Cardiol Rep 16 (2014): 501-522.
- Peter Moritz Becher, Nina Fluschnik, Stefan Blankenberg, Dirk Westermann. Challenging aspects of treatment strategies in heart failure with preserved ejection fraction: “Why did recent clinical trials fail?”. World J Cardiol 7 (2015): 544-554.
- Marc A Pfeffer, Amil M Shah, Barry A Borlaug. Heart Failure with Preserved Ejection Fraction: In Perspective. Circ Res 124 (2019): 1598-1617.
- Olga Dzhioeva, Evgeny Belyavskiy. Diagnosis and Management of Patients with Heart Failure with Preserved Ejection Fraction (HFpEF): Current Perspectives and Recommendations. Ther Clin Risk Manag 16 (2020): 769-785.
- Sharifov OF, Schiros CG, Aban I, Denney TS, Gupta H. Diagnostic accuracy of tissue Doppler index E/e’ for evaluating left ventricular filling pressure and diastolic dysfunction/heart failure with preserved ejection fraction: a systematic review and meta-analysis. J Am Heart Assoc 5 (2016): e002530.
- Santos A, Kraigher-Krainer E, Gupta DK, Claggett B, Zile MR, Pieske B, et al. For the PARAMOUNT Investigators. Impaired left atrial function in heart failure with preserved ejection fraction. Eur J Heart Fail 16 (2014): 1096-1103.
- Frydas A, Morris DA, Belyavskiy E, Radhakrishnan A, Kropf M, Tadic M, et al. Left atrial strain as sensitive marker of left ventricular diastolic dysfunction in heart failure. ESC Heart Fail 7 (2020): 1956-1965
- Zhang Y, Yin LX, Ren WD, et al. The guidelines for echocardiographic measurement of adult Chinese. Chinese Journal of Ultrasonography 25 (2016): 645-665.
- Piotr Ponikowski, Adriaan A Voors, Stefan D Anker, Héctor Bueno, John G F Cleland, Andrew J S Coats, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 18 (2016): 891-975.
- Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 17 (2016): 1321-1360.
- Shui Z, Wang Y, Sun M, Gao Y, Liang S, Wang Y, et al. The effect of coronary slow flow on left atrial structure and function. Scientific Reports 11 (2021): 7511.
- Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 11 (2014): 507-515.
- Melenovsky V, Andersen MJ, Andress K, Reddy YN, Borlaug BA. Lung congestion in chronic heart failure: haemodynamic, clinical, and prognostic implications. Eur J Heart Fail 17 (2015): 1161-1171.
- Thompson RB, Pagano JJ, Chow K, Sekowski V, Ezekowitz J, Anderson TJ, et al. Subclinical Pulmonary Edema is Associated with Reduced Exercise Capacity in HFpEF and HFrEF. J Am Coll Card 70 (2017): 1827-1828.
- Obokata M, Olson TP, Reddy YN, Melenovsky V, Kane GC, Borlaug BA. Hemodynamics, Dyspnea, and Pulmonary Reserve in Heart Failure with Preserved Ejection Fraction. Eur Heart J 39 (2018): 2810-2821.
- Reddy YNV, Olson TP, Obokata M, Melenovsky V, Borlaug BA. Hemodynamic correlates and diagnostic role of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. JACC Heart Fail 6 (2018): 665-675.
- Nagueh SF, Mikati I, Kopelen HA, Middleton KJ, Quinones MA, Zoghbi WA. Doppler estimation of left ventricular filling pressure in sinus tachycardia. A new application of tissue doppler imaging. Circulation 98 (1998): 1644-1650.
- Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, et al. Clinical utility of doppler echocardiography and tissue doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous doppler-catheterization study. Circulation 102 (2000): 1788-1794.
- Andersen OS, Smiseth OA, Dokainish H, Abudiab MM, Schutt RC, Kumar A, et al. Estimating left ventricular filling pressure by echocardiography. J Am Coll Cardiol 69 (2017): 1937-1948.
- Nauta JF, Hummel YM, van der Meer P, Lam CSP, Voors AA, van Melle JP. Correlation with invasive left ventricular filling pressures and prognostic relevance of the echocardiographic diastolic parameters used in the 2016 esc heart failure guidelines and in the 2016 ASE/EACVI recommendations: A systematic review in patients with heart failure with preserved ejection fraction. Eur J Heart Fail 20 (2018): 1303-1311.
- Hoit BD. Assessing atrial mechanical remodeling and its consequences. Circulation 112 (2005): 304-306.
- Hoit BD. Left atrial size and function: Role in prognosis. J Am Coll Cardiol 63 (2014): 493-505.
- Thomas L, Marwick TH, Popescu BA, Donal E, Badano LP. Left atrial structure and function and left ventricular diastolic dysfunction: JACC state-of-the-art review. J Am Coll Cardiol 73 (2019): 1961-1977.
- Barbier P, Solomon SB, Schiller NB, Glantz S A. Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation 100 (1999): 427-436.
- Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol 90 (2002): 1284-1289.
- Dominic Y Leung, Anita Boyd, Arnold A Ng, Cecilia Chi, Liza Thomas. Echocardiographic evaluation of left atrial size and function: current understanding, pathophysiologic correlates, and prognostic implications. Am Heart J 156 (2008): 1056-1064.
- Arco J Teske, Bart WL De Boeck, Paul G Melman, Gertjan T Sieswerda, Pieter A Doevendans, Maarten JM Cramer. Echocardiographic quantification of myocardial function using tissue deformation imaging, a guide to image acquisition and analysis using tissue Doppler and speckle tracking. Cardiovasc Ultrasound 5 (2007): 27.
- Mondillo S, Galderisi M, Mele D, Cameli M, Lomoriello VS, Zacà V, et al. Echocardiography study group of the Italian society of cardiology: speckle tracking echocardiography: A new technique for assessing myocardial function. J Ultrasound Med 30 (2011): 71-83.
- Gary C H. Gan, Aaisha Ferkh, Anita Boyd, Liza Thomas. Left atrial function: evaluation by strain analysis. Cardiovasc Diagn Ther 8 (2018): 29-46.
- Cameli M, Lisi M, Righini FM, Mondillo S. Novel echocardiographic techniques to assess left atrial size, anatomy and function. Cardiovasc Ultrasound 10 (2012): 4.
- Matteo Cameli, Maria Caputo, Sergio Mondillo, Piercarlo Ballo, Elisabetta Palmerini, Matteo Lisi, et al. Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc Ultrasound 7 (2009): 6.
- Rita Vianna-Pinton, Carlos A Moreno, Christy M Baxter, Kwan S Lee, Teresa S M Tsang, Christopher P Appleton. Two-dimensional speckle-tracking echocardiography of the left atrium: Feasibility and regional contraction and relaxation differences in normal subjects. J Am Soc Echocardiogr 22 (2009): 299-305.
- Nobuaki Kokubu, Satoshi Yuda, Kazufumi Tsuchihashi, Akiyoshi Hashimoto, Tomoaki Nakata, Tetsuji Miura, et al. Noninvasive assessment of left atrial function by strain rate imaging in patients with hypertension: a possible beneficial effect of renin-angiotensin system inhibition on left atrial function Hypertens Res 30 (2007): 13-21.
- Ping Yan, Bin Sun, Haiming Shi, Wen Zhu, Qing Zhou, Yuwen Jiang, et al. Left atrial and right atrial deformation in patients with coronary artery disease: A velocity vector imaging-based study. PLoS One 7 (2012): e51204.
- Yonghuai Wang, Yan Zhang, Chunyan Ma, Zhengyu Guan, Shuang Liu, Weixin Zhang, et al. Evaluation of left and right atrial function in patients with coronary slow-flow phenomenon using two-dimensional speckle tracking echocardiography. Echocardiography 33 (2016): 871-880.
- Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail 8 (2015): 295-303.