The Impact of Universal Transport Media and Viral Transport Media Liquid Samples on a SARS-CoV-2 Rapid Antigen Test
Article Information
Jeff A. Mayfield, Jose Ortiz, David J. Ledden*
Siemens Healthcare Diagnostics Inc., Mishawaka, USA
*Corresponding Author: David J. Ledden, Siemens Healthcare Diagnostics Inc., 430 S. Beiger St. Mishawaka, IN 46544, USA
Received: 08 September 2022; Accepted: 20 September 2022; Published: 26 October 2022
Citation:
Jeff A. Mayfield, Jose Ortiz, David J. Ledden. The Impact of Universal Transport Media and Viral Transport Media Liquid Samples on a SARS-CoV-2 Rapid Antigen Test. Archives of Internal Medicine Research 5 (2022): 481- 487.
View / Download Pdf Share at FacebookAbstract
Background: SARS-CoV-2 rapid antigen test evaluations require the use of samples that have been collected and stored in a variety of media and tested using non-ideal methods. The issues with this are sample dilution (e.g., decreased analyte concentration), dilution of extraction buffer components (surfactant, pH, and ionic strength) and UTM/VTM formula components that may interfere with test results, often producing false positive outcomes.
Methods: To evaluate the impact from these issues, a variety of materials were tested on the CLINITEST® Rapid COVID-19 Antigen Test (Manufactured by Healgen Scientific LLC, distributed by Siemens Healthcare Diagnostics Inc.) for their ability to generate a positive result in the absence of sample. This includes multiple UTM/VTM solutions, dilution of the extraction buffer components, and individual UTM/VTM components.
Results: The most important factor in UTM/VTM liquid sample dilution of the extraction buffer was ionic strength as measured by conductivity. Dilutions with conductivity below ~ 17 mS/cm induce a false positive result.
Conclusions: Evaluation of rapid antigen tests using libraries of older samples and samples collected in UTM/VTM is the most likely reason for discrepant results. The over-dilution of extraction buffer with UTM/VTM liquid samples may drive non-specific electrostatic interactions between the antibodies in the assay.
Keywords
SARS-CoV-2, Rapid Antigen Test, Viral Transport Media, False positive, Universal Transport Media
Research Article
Article Details
Introduction
The introduction of COVID-19 rapid antigen tests has had a big impact on controlling the spread of SARS-CoV-2 infections during the 2019 pandemic [1]. Although these tests may be analytically less sensitive than the gold standard RT-PCR assay, they are fast (i.e., 10-20 minutes versus hours to days), much less expensive and do not require highly trained professionals to perform the testing. Rapid antigen testing requires the acquisition of sample via a nasal or nasopharyngeal swab followed by extraction of the viral particles to release the nucleocapsid protein (N-protein) antigen. The extracted sample is then placed in the sample well of the test device and the results can be detected visually or are instrument read.
Current upper respiratory sample types include nasopharyngeal swabs, nasal swabs, and oropharyngeal swabs. Alternative sample types like saliva, oral fluid, gargle and wash, and breath are being investigated [2]. It is preferable that the sample swabs are tested immediately (or within an hour) but in some cases these swabs which are also used for RT-PCR testing are stored in either universal transport medium (UTM) or viral transport medium (VTM), used interchangeably throughout. In these cases, the sample swab is placed in 1 to 3 mL of UTM/VTM. In retrospective evaluations of rapid antigen tests is where UTM/VTM liquid samples are being used. These sample types are problematic in that some manufacturers do not support the use of UTM/VTM (e.g., Becton Dickinson, Healgen, and Abbott) whereas other manufacturers recommend specific UTM/VTM (e.g., AccessBio, SD Biosensor, and Quidel), and some manufacturers specify that certain UTM/VTM products should not be used (e.g., Quidel) [2,3,4,5].
It is important for rapid antigen manufacturers to understand how UTM/VTM samples may impact assay performance [6]. The major issues with these samples are swab sample dilution (e.g., decreased analyte concentration), dilution of extraction buffer components (surfactant, pH, and ionic strength) and UTM/VTM formulations (e.g., fetal calf serum, bovine serum albumin, gelatin, amino acids, metabolites, and dyes). To understand how UTM/VTM samples impact the performance of the Healgen rapid antigen test four different studies were performed: 1) a study to determine the impact of UTM/VTM liquid sample dilution on the viral extraction solution surfactant concentration, pH and ionic strength, 2) a dilution study using several different UTM/VTMs, 3) a study to investigate concentrations of common UTM/VTM components, and 4) a study using SARS-CoV-2 negative nasopharyngeal swab remnants at varying dilutions.
Materials and Methods
The initial study focused on the impact of liquid UTM/VTM sample dilution on the pH, surfactant concentration, and ionic strength. Extraction buffer mimics were prepared using Tris buffer (Sigma Trizma PreSet Crystals) in ultrapure water (18.2 MΩ-cm at 25 °C) at pH values of 7 and 8. In addition, these extraction buffer mimics were prepared with and without 1.0% surfactants (Sigma, Triton X-100 and Tween-20) or with and without 0.2 M NaCl (Sigma). Conductivity and pH measurements were performed on a Metler Toledo Seven Compact Conductivity meter and a Fisher Scientific Accumet AE/50 pH meter, respectively. The conductivity meter was calibrated before use with VWR Symphony one-point calibrator at 1412 µS/cm. The pH meter was calibrated before use using multiple levels Fisher Chemical buffer solutions (pH 4, 7, and 10). The kit extraction buffer, extraction buffer dilutions (saline and water) and extraction buffer mimics were tested neat, 1:1, 1:2, 1:4, and1:6 dilution. In all cases, 100 µL of sample was applied to the sample well of the device via pipette (Rainin single channel manual pipette, 20 – 200 µL) and the results were read visually at 15 minutes. Duplicate tests were measured unless indicated.
In the second study, seven different UTM/VTMs from multiple vendors (BD, Hardy Diagnostics, TEKNOVA, Copan, Puritan, and Remel) were acquired and tested. All of these materials were mixed with assay extraction buffer in ratios of 1:6, 1:2, 1:1, 2:1, and 6:1 EB:VTM. Additionally, each experiment contained tests with 100% EB and 100% VTM for a total of seven levels. Each mixture was tested for conductivity on a Metler Toledo Seven Compact Conductivity meter that was calibrated before use with VWR Symphony one-point calibrator at 1412 µS/cm.
The third study examined the impact of the different components in the UTM/VTMs (e.g., fetal calf serum, bovine serum albumin, gelatin, amino acids, metabolites, and dyes). Preliminary studies included adding concentrations of BSA (Sigma 100 mg/mL), fetal bovine serum (Sigma, 50%), gelatin (Sigma, 1%), yeast extract (Sigma, 10%), and lactalbumin (Sigma, 100 mg/mL) to the existing extraction buffer. A viral transport media mimic developed according to Centers for Disease Control and Prevention procedure (3) using 1X Hank’s balanced salt solution (HBSS, Sigma) and 2% heat inactivated fetal bovine serum (FBS, Sigma) in water was prepared and tested similarly to commercially available VTM’s above. In all cases, 100 µL of sample was applied to the sample well of the device via pipette and the results were read visually at 15 minutes and images were recorded. Duplicate tests were measured unless indicated.
The final study examined SARS-CoV-2 negative (RT-PCR) swab remnant samples. Multiples swab remnant samples were acquired from Cantor Bioconnect and tested at multiple ratios with assay extraction buffer. The mixture conductivity was measured as described above and duplicate assay results were recorded.
The Coronavirus Ag Rapid Test Cassettes (Healgen, Lot 2009087, Exp. 2022-08-31, CLINITEST Rapid COVID-19 Antigen Test (Manufactured by Healgen Scientific LLC, distributed by Siemens Healthcare Diagnostics Inc.), Lot 2010185, Exp. 2022-09-30 and Lot2010187, Exp. 2022-09-30) were used in the first and third studies. CLINITEST Rapid COVID-19 Antigen Test Lot 2011054 (Exp. 2022-10-31) was used in the second and final study.
Results
As mentioned above there were four different studies performed to determine the impact of UTM/VTM liquid samples on the Healgen Coronavirus Ag Rapid Test. The results of these studies are summarized below.
Studies were performed to determine if these were due to changes in the extraction buffer properties via dilution (e.g., pH, surfactant concentration or ionic strength) or one or more of the components commonly found UTM/VTM formulations. The importance of surfactant, pH, and ionic strength in the extraction buffer was tested with respect to false positives. Results are shown below in Table 1.
|
Sample |
pH |
Surfactant |
Salt |
Result |
|
Tris Buffer |
8 |
Yes |
Yes |
Negative |
|
Tris Buffer |
8 |
Yes |
No |
Positive |
|
Tris Buffer |
8 |
No |
Yes |
Negative |
|
Tris Buffer |
8 |
No |
No |
Positive |
|
Tris Buffer |
7 |
Yes |
Yes |
Negative |
|
Tris Buffer |
7 |
Yes |
No |
Positive |
|
Tris Buffer |
7 |
No |
Yes |
Negative |
|
Tris Buffer |
7 |
No |
No |
Positive |
Table 1: Extraction Buffer Components. The presence of buffer components of surfactants, pH, ionic strength were tested for false positive results.
Buffer at pH 7 and 8 was prepared with and without the additives. Solutions with both additives yielded negative results at pH 7 and 8. The addition of surfactant without salt resulted in false positives at both pH 7 and 8. The absence of surfactant with salt produced negative test results at both pH 7 and 8. Absence of both additives also produces a positive test line at both pH levels. Solutions without salt added generate false positive results suggesting a critical role for the ionic strength.
False positive results due to the absence of sodium chloride suggests that extraction buffer ionic strength measured as conductivity (mS/cm) may play a role in reducing non-specific interactions. To test this hypothesis, several samples were prepared with ranges from high to low conductivity by mixing assay extraction buffer with ultrapure water (measured conductivity = 0.15 mS/cm) and 0.9% saline (measured conductivity = 17 mS/cm). Volume ratios tested were determined based on potential use cases for samples stored in VTM or buffer. Ultrapure water represents the most extreme case where the initial sample conductivity is very low. Saline samples represent cases where samples are stored in a higher conductivity solution. Results of three replicates and two rapid antigen test reagent lots are shown in Tables 2 and 3 below.
|
Extraction |
Water (µL) |
Lot 2010185/20201307 |
Lot 2010187/ 20201308 |
||
|
Buffer (µL) |
Conductivity (mS/cm) |
False Positive /Replicates |
Conductivity (mS/cm) |
False Positive/Replicates |
|
|
300 |
0 |
23.9 |
0/3 |
23.9 |
0/3 |
|
300 |
50 |
23.3 |
0/3 |
23 |
0/3 |
|
300 |
100 |
20.5 |
0/3 |
20 |
0/3 |
|
300 |
150 |
19.57 |
0/3 |
19.29 |
0/3 |
|
300 |
200 |
17.41 |
2/3 |
17.81 |
0/3 |
|
300 |
250 |
15.9 |
3/3 |
17.59 |
0/3 |
|
300 |
300 |
15.28 |
3/3 |
14.89 |
3/3 |
|
300 |
400 |
12.99 |
3/3 |
14.19 |
3/3 |
|
300 |
500 |
11.6 |
3/3 |
11.62 |
3/3 |
|
300 |
600 |
10.43 |
3/3 |
11.01 |
3/3 |
|
0 |
300 |
0.1479 |
3/3 |
0.1128 |
3/3 |
Table 2: False Positives with Water as Sample. Results from mixing extraction buffer with varying amounts of water and testing for false positives and conductivity. False positives occur when the water is mixed with extraction buffer making the conductivity <17.5 mS/cm.
|
Extraction Buffer (µL) |
Saline (µL) |
Lot 2010185/20201307 |
Lot 2010187/ 20201308 |
||
|
Conductivity (mS/cm) |
False Positive /Replicates |
Conductivity (mS/cm) |
False Positive /Replicates |
||
|
300 |
0 |
26.2 |
0/3 |
28.1 |
0/3 |
|
300 |
50 |
23.8 |
0/3 |
24.6 |
0/3 |
|
300 |
100 |
21.9 |
0/3 |
24.2 |
0/3 |
|
300 |
150 |
20.2 |
0/3 |
22.7 |
0/3 |
|
300 |
200 |
20 |
0/3 |
22.3 |
0/3 |
|
300 |
250 |
20 |
0/3 |
21.6 |
0/3 |
|
300 |
300 |
21.6 |
0/3 |
21.9 |
0/3 |
|
300 |
400 |
20 |
0/3 |
22.7 |
0/3 |
|
300 |
500 |
20 |
0/3 |
22.4 |
0/3 |
|
300 |
600 |
19.71 |
0/3 |
21.3 |
0/3 |
|
0 |
300 |
16.18 |
3/3 |
17.25 |
3/3 |
Table 3: False Positives with Saline as Sample. Results from mixing extraction buffer with varying amounts of 0.9% saline and testing for false positives and conductivity. False positives only occur when the saline is run without extraction buffer and conductivity is <17.5 mS/cm.
Samples with conductivity below ~17.5 mS/cm generated false positive test results. As expected, mixing extraction buffer (EB) with water, the low conductivity solution, generates false positives at a dilution of 2:3 (sample:EB). There are small differences between this cutoff for the two lots tested suggesting some variability. Mixing saline, a higher conductivity sample, with extraction buffer resulted in no false positives when mixed up to 2:1 (sample:EB). In each ratio the conductivity of the final solution is greater than 17.5 mS/cm. A false positive only occurs when saline is run without EB with conductivity at ~ 17 mS/cm.
To further support the role of sample conductivity in false positive results, seven VTM solutions were tested for conductivity at multiple dilution levels with extraction buffer. Five of the seven tested have conductivity of ~10-15 mS/cm, shown in Table 4, while two of the seven have a conductivity of ~ 3 mS/cm.
|
% UTM/VTM in EB Mixture |
Conductivity (mS/cm) |
|||||||
|
BD ESwab |
Hardy Diagnostics VTM R99 |
Teknova VTM (4V2020) |
Copan UTMRT |
BD UTM |
Puritan Unitranz |
Remel Microtest M4 RT |
% FP |
|
|
0 |
18.51 |
21.7 |
21.6 |
20.9 |
20.9 |
22.0 |
20.0 |
0 |
|
14.3 |
19.94 |
20.0 |
20.7 |
20.0 |
20.0 |
20.5 |
19.33 |
0 |
|
33.3 |
17.23 |
16.24 |
19.98 |
18.82 |
18.52 |
19.30 |
15.72 |
0 |
|
50 |
17.20 |
12.96 |
18.50 |
17.19 |
15.88 |
17.80 |
12.54 |
0 |
|
66.7 |
13.99 |
9.51 |
16.86 |
15.30 |
14.22 |
15.83 |
8.83 |
28.5 |
|
85.7 |
12.32 |
5.30 |
14.69 |
13.03 |
12.85 |
13.77 |
5.02 |
78.5 |
|
100 |
10.61 |
2.80 |
13.15 |
11.41 |
11.66 |
12.45 |
2.10 |
100 |
Table 4: Summary of conductivity results from Mixtures of different UTM/VTM vendors and extraction buffer. Data shows how some UTM/VTM materials are initially at lower conductivity and create false positives will less dilution by extraction buffer. The last column reflects the total number of false positives recorded for that mixture ratio across all UTM/VTM suppliers.
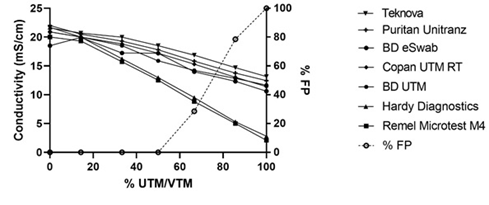
This variability suggests that VTMs can differ in conductivity and may give unique results depending on the sample dilution used. Figure 1 illustrates the conductivity shifts observed with varying percentage of VTM in extraction buffer and how the number of false positives increases with decreasing conductivity.
The conductivity results for each mixture of UTM/VTM with extraction buffer are plotted on the left y-axis and decrease with increasing percent of UTM/VTM in the mixture. Two of the seven UTM/VTM show lower conductivity. The open circles represent the number of false positives observed across all UTM/VTM types at that mixture, each mixture was tested in duplicated for a total of fourteen using seven UTM/VTM types. The percentage of false positives (%FP) is depicted on the right y-axis and shows that the false positive rate increases with decreasing conductivity and that UTM/VMT tested gave a false positive at 100% UTM/VTM.
All UTM/VTM’s tested gave false positives when run in the absence of extraction buffer. No false positives were observed with mixtures that contained more extraction buffer than UTM/VTM all the way up to a ratio of 1:1. Some UTM/VTM solutions began to give false positives when a ratio of 2:1 VTM:EB was used. Those were only the UTM/VTMs that had an initial conductivity of ~ 3 mS/cm and included Hardy Diagnostics VTM R99 and Remel Microtest M4 RT.
Viral transport media often contains additives that support viral preservation but could potentially interfere with immunoassays. To investigate this possibility, five of the common VTM components (e.g., bovine serum albumin, fetal bovine serum, gelatin, yeast extract, and lactalbumin) were tested. Solutions of these additives were prepared in assay extraction buffer and tested at levels at or above those observed in VTM. Table 5 shows all additives generated negative results.
|
Component |
Concentration |
Result |
|
Bovine Serum Albumin |
100 mg/mL |
Negative |
|
Fetal Bovine Serum |
50% (v/v) |
Negative |
|
Gelatin |
1% (v/v) |
Negative |
|
Yeast Extract |
10% (v/v) |
Negative |
|
Lactalbumin |
100 mg/mL |
Negative |
Table 5: UTM/ VTM Components. All solutions were prepared in extraction buffer and ran as a normal sample. Negative results were observed for all materials.
Laboratory recipes for viral transport media (VTM) are available in the literature and are known to contain 2% fetal bovine serum (FBS) and Hanks balanced salt solution (HBSS) with antimicrobial compounds [7]. One formulation of a laboratory VTM without antimicrobial compounds was mixed with extraction buffer at several dilutions and tested. Results are shown below in Table 6. All laboratory VTM solutions mixed with extraction buffer up to 1:1 by volume showed negative results. The laboratory VTM run without extraction buffer resulted in a false positive result. Conductivity of extraction buffer and VTM are also shown in Table 6.
|
Extraction Buffer (µL) |
VTM (µL) |
Conductivity (mS/cm) |
Result |
|
300 |
0 |
25.1 |
Negative |
|
300 |
50 |
ND* |
Negative |
|
300 |
100 |
ND |
Negative |
|
300 |
150 |
ND |
Negative |
|
300 |
300 |
ND |
Negative |
|
0 |
300 |
14.2 |
Positive |
|
*ND = not determined |
|||
Table 6: Laboratory VTM. VTM with 2% FBS was mixed with extraction buffer at varying volumes and tested for false positive results. Conductivity is shown when relevant.
Lastly the results of testing SARS-CoV-2 negative swabs with varying amounts of assay extraction buffer are summarized in Table 7.
|
Volume EB (µL) |
Volume Sample (µL) |
Ratio (Sample:EB) |
Sample 1 |
Sample 2 |
Sample 3 |
|||
|
Conductivity (mS/cm) |
FP/ Reps |
Conductivity (mS/cm) |
FP/ Reps |
Conductivity (mS/cm) |
FP/ Reps |
|||
|
300 |
0 |
- |
23.3 |
0/2 |
23.5 |
0/2 |
23.1 |
0/2 |
|
300 |
50 |
1:6 |
21.9 |
0/2 |
21.9 |
0/2 |
21.8 |
0/2 |
|
300 |
150 |
1:2 |
20.6 |
0/2 |
20.8 |
0/2 |
21.2 |
0/2 |
|
300 |
300 |
1:1 |
20.0 |
0/2 |
21.8 |
0/2 |
20.0 |
0/2 |
|
150 |
300 |
2:1 |
18.5 |
0/2 |
18.8 |
0/2 |
18.7 |
0/2 |
|
50 |
300 |
6:1 |
18.4 |
0/2 |
18.1 |
0/2 |
18.8 |
1/2 |
|
0 |
300 |
- |
14.5 |
2/2 |
16.2 |
2/2 |
14.7 |
2/2 |
Table 7: SARS-CoV-2 Negative Swabs. Remnant samples negative for SARS-CoV-2 (RT-PCR) stored in 0.9% saline were mixed with varying amounts of assay extraction buffer and tested in duplicate on the CLINITEST Rapid COVID-19 Antigen Test. Results for the conductivity of the mixture and the false positives are shown. False positives occur when the samples are tested without extraction buffer and in one case at a ration of sample to buffer of 6:1.
These data show that swab samples that tested negative for SARS-CoV-2 (RT-PCR) stored in 0.9% saline will yield a false positive result when not mixed with extraction buffer. Only one (1) out of six (6) overall replicates at a ratio of 6:1 (sample: EB) gave a false positive.
Discussion
The use of remnant liquid swab samples stored in UTM/VTMs can negatively impact the performance of COVID-19 rapid antigen tests. These sample types are problematic in that some manufacturers do not support the use of UTM/VTMs (e.g., Becton Dickinson, Healgen, and Abbott) whereas other manufacturers recommend specific UTM/VTMs (e.g., AccessBio, SD Biosensor, and Quidel) and some specify that certain UTM/VTM products should not be used (e.g., Quidel) [2-5]. In retrospective evaluations of these rapid antigen tests including the Healgen assay,
UTM/VTM liquid samples are being used. It should be noted that some other manufacturers use relatively high volumes (e.g., 350 - 400 µL) of VTM sample to compensate for original sample dilution thereby improving analytical sensitivity but could negatively impact the Healgen assay by potentially causing false positive results [3,5].
In fact, during an evaluation of the Healgen rapid antigen test by Charité Hospital it was noted that the assay exhibited cross reactivity with seven respiratory non-COVID-19 samples (e.g., adenovirus, entero/rhinovirus, Influenza A H1, Influenza A H3, and parainfluenza 1, 2 and 3). This was inconsistent with the internal cross reactivity data generated by the manufacturer. The authors concluded that one possibility for the false positives was due to non-specific binding. In some cases, duplicate tests of the positive results were performed which resulted in the expected negative result [8].
A study was performed to determine the impact of UTM/VTM liquid samples on the pH, surfactant concentration and ionic strength. The extraction buffer plays several key roles in assay performance and when it is mixed with stored liquid samples the composition can change. The surfactant compounds are key to lysing virus particles (and subsequently inactivating them) and releasing the nucleocapsid protein making it accessible to the antibodies used in the assay. These compounds also help to reduce non-specific interactions between the gold sol conjugates and the test and control line antibodies. The pH of the buffer is critical for maintaining a consistent overall charge on all the relevant protein species (e.g., antigen, conjugate, and test and control line antibodies). The ionic strength or salt concentration of the buffer also creates an environment where non-specific electrostatic interactions are reduced. Any disruption of these factors may introduce unwanted results. The key finding here was that it appears that ionic strength is the most important factor affecting the assay performance with respect to false positive results using stored samples.
This study was expanded to look at dilution studies with saline and water on two different reagent lots. The water dilution showed the worst-case scenario as the conductivity of water is very low (C = 0.15 mS/cm). Even at a dilution of 1:1 (water:EB) false positive results begin to occur. The observed lot differences may be due to slight variation in extraction buffer composition or preparation of the samples tested. Viral samples are not recommended to be stored in water solution for any type of testing and this condition represents an extreme. However, dilution of UTM/VTM samples with water to increase the sample volume for multiple rapid antigen test evaluations could be problematic scenario. The results of the saline dilution study were more favorable due to the higher conductivity of the 0.9% saline alone (C = 16-17 mS/cm). No false positives were observed when mixed with extraction buffer even up to 2:1 (saline: EB). Saline is a common way to store viral swabs and provides enough ionic strength to reduce false positive results. The conductivity measurements from this study have also shown that an approximate cut off point for false positive generation with this test is around 17 mS/cm.
To better understand results generated with UTM/VTM seven (7) different materials were screened and it was found that two (2) gave a false positive result at an ~66% mixture with assay extraction buffer. Interestingly, none of the materials generated false positives when tested with a 50% mixture (i.e., 300 µL of sample:300 µL of extraction buffer). The false positive results generated with a 66% mixture revealed that either dilution of the extraction buffer or material present in the UTM/VTM was the source. Both possibilities were examined thoroughly. Based on the findings from UTM/VTM screening and buffer components study above, it was suggested that there may be differences in the conductivity between UTM/VTM from different sources. The conductivity of each UTM/VTM material was testing and ranged from about 2 – 12 mS/cm. This indicates some variability in material based on their respective formulas. The objective of storing swabs in VTM is to preserve and protect the organism for future use either for cell culture or most likely in the case of SARS-CoV-2 samples molecular testing. Different UTM/VTMs have unique purposes and their formulations reflect those needs. In the case of the Healgen rapid antigen testing, the UTM/VTM’s with the lowest conductivity caused a false positive result at a 66% mixture with extraction buffer as expected based on conductivity measurements.
Another possible explanation for poor rapid test performance with UTM/VTM use is that one or more of the components in the UTM/VTMs interferes with the assay. Several different components commonly found in UTM/VTMs were tested at high concentrations (e.g., FBS, BSA, Gelatin, lactalbumin and yeast extract). These components were chosen because they are proteins or contain a mixture of proteins with the potential to interfere in an immunoassay. None of these materials was found to generate false results, further supporting the role of ionic strength in false positive results from samples stored in UTM/VTM. In relation to this approach, a lab made VTM was prepared and tested for conductivity and false positive generation, only when run in the absence of extraction did this mixture give a false positive.
Lastly, negative RT-PCR nasopharyngeal swabs tested at varying ratios to extraction buffer were tested. These results showed false positives were only generated when the samples were tested without extraction buffer in most cases. This suggests that none of the excess material collected from a swab is interfering with the assay by generating false results, but rather as shown in this paper the conductivity of the sample storage material is the likely cause.
In conclusion, the most likely reason for discrepant results with a rapid antigen test when used with stored samples is the composition of the UTM/VTM sample - extraction buffer mixture. The properties of this solution may drive non-specific electrostatic interactions at the assay test line that generate false positive results when samples are mixed improperly and tested in this manner.
Nonstandard Abbreviations: UTM, universal transport media; VTM, viral transport media; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RT-PCR, reverse transcriptase polymerase chain reaction; FBS, fetal bovine serum; HBSS, Hank’s balanced salt solution; EB, extraction buffer; BSA, bovine serum albumin.
Author Contributions: All authors contributed equally to this article.
Authors’ Disclosures or Potential Conflicts of Interest: None
Employment or Leadership: J.A. Mayfield, Siemens Healthineers; J. Oritz, Siemens Healthineers; D.J. Ledden, Siemens Healthineers.
Consultant or Advisory Role: None declared.
Stock Ownership: None declared.
Honoraria: None declared.
Research Funding: Siemens Healthineers.
Expert Testimony: None declared.
Patents: None declared.
Role of Sponsor: Not applicable.
Acknowledgements
The authors would like to acknowledge John Benco for his enthusiastic support and editorial support. The authors would like acknowledge Cassidy Yost for her technical support.
References
- Baro B, Rodo P, Ouchi D, Bordoy A, et al. Performance characteristics of five antigen-detecting rapid diagnostic test (Ag-RDT) for SARS-CoV-2 asymptomatic infection: a head-to-head benchmark comparison.
- Abbott BinaxNow™ COVID -19 Ag Card IFU Summary and Explanation of the Test Section (Page 1) - BinaxNOW COVID-19 Ag Card is a rapid lateral flow immunoassay for the qualitative detection and diagnosis of SARS-CoV-2 directly from nasal swabs, without viral transport media.
- AccessBio CareStart™ COVID-19 Antigen IFU Nasopharyngeal Swab in Viral Transport Media (VTM) Test Procedure (Page 9) - NOTE: Only BD universal transport media have been validated with the assay.
- Quidel Sofia® SARS Antigen FIA IFU Limitations Section (page 14) – Remel M4 and M4RT should not be used in the Sofia SARS Antigen FIA Assay in either the Sofia or Sofia 2. Some lots of M4 and M4RT have been shown to cause false positive results when used with the Sofia SARS Antigen FIA Assay.
- SD Biosensor Standard™ Q COVID-19 Ag Test IFU SPECIMEN COLLECTION AND PREPARATION Section (Page 2) Transport Medium Table shows 7 acceptable VTMs and recommended storage conditions.
- Kontogianni K, Edwards T, Wooding D, Buist K, et al. Limit of detection in different matrices of nineteen commercially available rapid antigen tests for the detection of SARS-CoV-2.
- Preparation of Viral Transport Medium, SOP#: DSR-052-05, Centers for Disease Control and Prevention, Atlanta, GA, USA.
- Corman V, Haage V, Bleicker T, Schmidt M, et al. Comparison of seven commercial SARS-CoV-2 rapid point-of-care antigen tests: a single-centre laboratory evaluation study. Lancet Microbe. 2021. Published online April 7, 2021.