The Impact of Bone Metastasis Location in the Clinical Outcome of Patients with Metastatic Renal Cell Carcinoma (mRCC): An Analysis from the Latin American Renal Cancer Group (LARCG)
Article Information
Diego Abreu1*, Antonia Angeli Gazola2, Alessandra Borba Anton de Souza2, Felipe Pizzolo2, Guillermo Gueglio3, Alberto Jurado3, Luis Meza4, Carlos Scorticati5, Maximiliano Lopez5, Stenio de Cassio Zequi6, Walter Henriques da Costa6, Alice Scalzilli Becker2, Júlia Elisa Hübner2, Rodrigo Pellegrini2, Juan Yandian7, Luis Ubillos8, Noelia Ferreira7, Marcos Tobias Machado9, Oscar Rodriguez10, Carlos Ameri11, Alejandro Nolazco12, Pablo Martinez12, Gustavo Carvalhal13, Pablo M. Barrios13, Ruben Bengio14, Leandro Arribillaga14, Diego Muguruza15, José Gadu16, Edgar Bravo16, Ricardo Castillejos17, Francisco Rodríguez-Covarrubias17, Pablo Mingote18, Nicolás Ginastar18, Rafael Alonso19, Roberto Puente7, Ricardo Decia1, Gustavo Cardoso Guimarães6, Joan Palou10, André P. Fay2,20
1Department of Urology - Hospital Pasteur - Montevideo, Uruguay
2 Oncology Research Group-CNPq, PUCRS, Porto Alegre, Brazil
3Hospital Italiano, Buenos Aires, Argentina
4Instituto Nacional de Enfermedades Neoplásicas, Lima, Peru
5Hospital de Clinicas, Buenos Aires, Argentina
6A.C. Camargo Cancer Center, São Paulo, Brazil
7Department of Urology - Hospital de Clínicas, Montevideo, Uruguay
8Department of Medical Oncology - Hospital de Clinicas, Montevideo, Uruguay
9Escuela de Medicina ABC, San Pablo, Brazil
10Fundación Puigvert, Barcelona, Spain
11Hospital Alemán, Buenos Aires, Argentina
12Hospital Británico, Buenos Aires, Argentina
13PUCRS School of Medicine, Porto Alegre, Brazil
14Clinica Profesor Bengio, Cordoba, Argentina
15COMEPA, Paysandu, Uruguay
16Hospital Militar, Ciudad de México, México
17Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCNSZ), Ciudad de México, México
18Policlinico Neuquén, Neuquén, Argentina
19Department Quantitative Methods, Faculty of Medicine, Montevideo, Uruguay
20Department of Medical Oncology - Hospital Sao Lucas da PUCRS, Porto Alegre, Brazil
*Corresponding Author: Dr. Diego Abreu, Department of Urology - Hospital Pasteur - Montevideo, Uruguay
Received: 29 September 2020; Accepted: 12 October 2020; Published: 27 October 2020
Citation: Diego Abreu, Antonia Angeli Gazola, Alessandra Borba Anton de Souza, Felipe Pizzolo, Guillermo Gueglio, Alberto Jurado, Luis Meza, Carlos Scorticati, Maximiliano Lopez, Stenio de Cassio Zequi, Walter Henriques da Costa, Alice Scalzilli Becker, Júlia Elisa Hübner, Rodrigo Pellegrini, Juan Yandian, Luis Ubillos, Noelia Ferreira, Marcos Tobias Machado, Oscar Rodriguez, Carlos Ameri, Alejandro Nolazco, Pablo Martinez, Gustavo Carvalhal, Pablo M. Barrios, Ruben Bengio, Leandro Arribillaga, Diego Muguruza, José Gadu, Edgar Bravo, Ricardo Castillejos, Francisco Rodríguez-Covarrubias, Pablo Mingote, Nicolás Ginastar, Rafael Alonso, Roberto Puente, Ricardo Decia, Gustavo Cardoso Guimarães, Joan Palou, André P. Fay. The Impact of Bone Metastasis Location in the Clinical Outcome of Patients with Metastatic Renal Cell Carcinoma (mRCC): An Analysis from the Latin American Renal Cancer Group (LARCG). Journal of Cancer Science and Clinical Therapeutics 4 (2020): 526-537.
View / Download Pdf Share at FacebookAbstract
Background: Tumor burden and metastatic disease sites are well-established prognostic factors in many malignancies, including metastatic Renal Cell Carcinoma (mRCC).
Objective: We aimed to evaluate the impact of bone metastasis (BM) location on clinical outcome of mRCC patients.
Methods: This study is a retrospective analysis of 4060 mRCC patients from the Latin American Renal Cancer Group (LARCG) database. Clinico-pathological characteristics, 24-months-survival, overall survival (OS), and BM sites were collected. To estimate the association between BM location and clinical outcomes we used Cox regression method.
Results: Out of 4060 patients, 530 (14.5%) had metastatic disease. Among those, we analyzed the fifty-six that had only BM. The median follow-up was 20.8 months (range from 0 to 188 months). Non-spinal BM (NSBM) were identified in 33 (58.9%) patients and spinal BM (SBM) in 23 (41.1%) patients. Median OS was 35 months, and 24-months OS was 76% for patients with NSBM and 46% with SBM (HR: 2.22). In multivariable analysis SBM (HR: 3.08), ASA classification 3-4 (HR: 2.37), non-cc histology (HR: 5.11), and age (HR 1.06) were independent prognostic factors for OS.
Conclusions: Our study showed that SBM predicted shorter OS, suggesting that the location of BM may impact the clinical outcome of patients with mRCC.
Keywords
<p>Renal Cell Carcinoma; Spine metastasis; Vertebral metastasis; Kidney Cancer; Metastasis site</p>
Renal Cell Carcinoma articles; Spine metastasis articles; Vertebral metastasis articles; Kidney Cancer articles; Metastasis site articles
Renal Cell Carcinoma articles Renal Cell Carcinoma Research articles Renal Cell Carcinoma review articles Renal Cell Carcinoma PubMed articles Renal Cell Carcinoma PubMed Central articles Renal Cell Carcinoma 2023 articles Renal Cell Carcinoma 2024 articles Renal Cell Carcinoma Scopus articles Renal Cell Carcinoma impact factor journals Renal Cell Carcinoma Scopus journals Renal Cell Carcinoma PubMed journals Renal Cell Carcinoma medical journals Renal Cell Carcinoma free journals Renal Cell Carcinoma best journals Renal Cell Carcinoma top journals Renal Cell Carcinoma free medical journals Renal Cell Carcinoma famous journals Renal Cell Carcinoma Google Scholar indexed journals Spine metastasis articles Spine metastasis Research articles Spine metastasis review articles Spine metastasis PubMed articles Spine metastasis PubMed Central articles Spine metastasis 2023 articles Spine metastasis 2024 articles Spine metastasis Scopus articles Spine metastasis impact factor journals Spine metastasis Scopus journals Spine metastasis PubMed journals Spine metastasis medical journals Spine metastasis free journals Spine metastasis best journals Spine metastasis top journals Spine metastasis free medical journals Spine metastasis famous journals Spine metastasis Google Scholar indexed journals Vertebral metastasis articles Vertebral metastasis Research articles Vertebral metastasis review articles Vertebral metastasis PubMed articles Vertebral metastasis PubMed Central articles Vertebral metastasis 2023 articles Vertebral metastasis 2024 articles Vertebral metastasis Scopus articles Vertebral metastasis impact factor journals Vertebral metastasis Scopus journals Vertebral metastasis PubMed journals Vertebral metastasis medical journals Vertebral metastasis free journals Vertebral metastasis best journals Vertebral metastasis top journals Vertebral metastasis free medical journals Vertebral metastasis famous journals Vertebral metastasis Google Scholar indexed journals Kidney Cancer articles Kidney Cancer Research articles Kidney Cancer review articles Kidney Cancer PubMed articles Kidney Cancer PubMed Central articles Kidney Cancer 2023 articles Kidney Cancer 2024 articles Kidney Cancer Scopus articles Kidney Cancer impact factor journals Kidney Cancer Scopus journals Kidney Cancer PubMed journals Kidney Cancer medical journals Kidney Cancer free journals Kidney Cancer best journals Kidney Cancer top journals Kidney Cancer free medical journals Kidney Cancer famous journals Kidney Cancer Google Scholar indexed journals Metastasis site articles Metastasis site Research articles Metastasis site review articles Metastasis site PubMed articles Metastasis site PubMed Central articles Metastasis site 2023 articles Metastasis site 2024 articles Metastasis site Scopus articles Metastasis site impact factor journals Metastasis site Scopus journals Metastasis site PubMed journals Metastasis site medical journals Metastasis site free journals Metastasis site best journals Metastasis site top journals Metastasis site free medical journals Metastasis site famous journals Metastasis site Google Scholar indexed journals Bone Metastasis articles Bone Metastasis Research articles Bone Metastasis review articles Bone Metastasis PubMed articles Bone Metastasis PubMed Central articles Bone Metastasis 2023 articles Bone Metastasis 2024 articles Bone Metastasis Scopus articles Bone Metastasis impact factor journals Bone Metastasis Scopus journals Bone Metastasis PubMed journals Bone Metastasis medical journals Bone Metastasis free journals Bone Metastasis best journals Bone Metastasis top journals Bone Metastasis free medical journals Bone Metastasis famous journals Bone Metastasis Google Scholar indexed journals Spinal Bone Metastasis articles Spinal Bone Metastasis Research articles Spinal Bone Metastasis review articles Spinal Bone Metastasis PubMed articles Spinal Bone Metastasis PubMed Central articles Spinal Bone Metastasis 2023 articles Spinal Bone Metastasis 2024 articles Spinal Bone Metastasis Scopus articles Spinal Bone Metastasis impact factor journals Spinal Bone Metastasis Scopus journals Spinal Bone Metastasis PubMed journals Spinal Bone Metastasis medical journals Spinal Bone Metastasis free journals Spinal Bone Metastasis best journals Spinal Bone Metastasis top journals Spinal Bone Metastasis free medical journals Spinal Bone Metastasis famous journals Spinal Bone Metastasis Google Scholar indexed journals Vascular Endothelial Growth Factor articles Vascular Endothelial Growth Factor Research articles Vascular Endothelial Growth Factor review articles Vascular Endothelial Growth Factor PubMed articles Vascular Endothelial Growth Factor PubMed Central articles Vascular Endothelial Growth Factor 2023 articles Vascular Endothelial Growth Factor 2024 articles Vascular Endothelial Growth Factor Scopus articles Vascular Endothelial Growth Factor impact factor journals Vascular Endothelial Growth Factor Scopus journals Vascular Endothelial Growth Factor PubMed journals Vascular Endothelial Growth Factor medical journals Vascular Endothelial Growth Factor free journals Vascular Endothelial Growth Factor best journals Vascular Endothelial Growth Factor top journals Vascular Endothelial Growth Factor free medical journals Vascular Endothelial Growth Factor famous journals Vascular Endothelial Growth Factor Google Scholar indexed journals Body Mass Index articles Body Mass Index Research articles Body Mass Index review articles Body Mass Index PubMed articles Body Mass Index PubMed Central articles Body Mass Index 2023 articles Body Mass Index 2024 articles Body Mass Index Scopus articles Body Mass Index impact factor journals Body Mass Index Scopus journals Body Mass Index PubMed journals Body Mass Index medical journals Body Mass Index free journals Body Mass Index best journals Body Mass Index top journals Body Mass Index free medical journals Body Mass Index famous journals Body Mass Index Google Scholar indexed journals non-Clear Cell articles non-Clear Cell Research articles non-Clear Cell review articles non-Clear Cell PubMed articles non-Clear Cell PubMed Central articles non-Clear Cell 2023 articles non-Clear Cell 2024 articles non-Clear Cell Scopus articles non-Clear Cell impact factor journals non-Clear Cell Scopus journals non-Clear Cell PubMed journals non-Clear Cell medical journals non-Clear Cell free journals non-Clear Cell best journals non-Clear Cell top journals non-Clear Cell free medical journals non-Clear Cell famous journals non-Clear Cell Google Scholar indexed journals
Article Details
Abbreviations:
mRCC- Metastatic Renal Cell Carcinoma; RCC- Renal Cell Carcinoma; BM- Bone Metastasis; SBM- Spinal Bone Metastasis; NSBM- Non-spinal Bone Metastasis; SRE- Skeletal Related Events; OS- Overall Survival; IMDC- International Metastatic Renal Cell Carcinoma; MSKCC- Memorial Sloan-Kettering Cancer Center (MSKCC/Motzer) Score; LARCG- Latin American Renal Cell Group; VEGF- Vascular Endothelial Growth Factor; BMI- Body Mass Index; ECOG- Eastern Cooperative Oncology Group; ASA-American Society of Anesthesiologists; cc- Clear Cell; non-cc- non-Clear Cell; mTOR- mammalian target of rapamycin; KPS- Karnofsky Performance; LDH- High Lactate Dehydrogenase; CSS- Cancer-specific Survival
1. Introduction
Of all renal cancers, RCC is the most common subtype, corresponding to 85% of all RCC cases, and being responsible for 15,000 of Americans diagnosed annually according to the American Cancer Society [1, 2]. Approximately 30% of patients treated with a curative-intent surgical resection of a localized renal tumor will develop metastatic disease [3]. Tumor burden and metastatic disease sites are well-established prognostic factors in many malignancies, including metastatic renal cell carcinoma (mRCC).
Approximately 30% of patients with mRCC have bone metastasis (BM) which is associated with significant morbidity and high rates of skeletal complications, resulting in a shorter overall survival [4]. There is increasing evidence that the presence of BM harms mRCC prognosis, being a predictor of poor progression-free survival (PFS) and overall survival (OS) among this population [5]. It is estimated that nearly 70% of patients with mRCC will develop at least one skeletal-related event (SRE) during the disease course when BM is present [6]. Close to 5% of patients with BM have exclusively BM at diagnosis and spinal metastasis is associated with poor prognosis [7, 8]. A recent systematic review published by Goodwin et al. including 807 patients showed that from the time of spinal metastasis diagnosis the median survival was 11,7 months [9].
The identification of prognostic factors is important to tailor treatment options, to stratify risks, and to offer counseling for mRCC patients [10]. International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) and MSKCC prognostic risk scores are widely used to define treatment strategies in mRCC [1]. In this analysis, we sought to evaluate the impact of BM location in the clinical outcome of mRCC patients in a large multicentric cohort from a Latin American population.
2. Methods
The Latin American Renal Cancer Group (LARCG) is a multi-institutional and multidisciplinary group that established a database on Renal Cancer [11]. LARCG involves 45 centers from 8 countries including Uruguay, Brazil, Argentina, Mexico, Peru, Chile, Bolivia, and Spain. Data was collected using medical records, and pathological reports from each institution with a total of 4,060 renal cancer cases.. The primary objective of this analysis was to evaluate the impact of BM location in the prognosis of mRCC patients who have exclusively bone metastasis. Clinico-pathological characteristics such as age, sex, body mass index (BMI), Eastern Cooperative Oncology Group (ECOG) Performance Status, American Society of Anesthesiologists (ASA) and Karnowski performance status, symptoms at presentation, tumor size, lymph node staging, nuclear Fuhrman grade, perirenal fat invasion, necrosis, histological subtype, sarcomatoid differentiation of the BM, the realization of cytoreductive nephrectomy, the primary form of treatment of the metastasis, administration of systemic therapy, survival status and location of BM were collected using standard templates.
Chi-square and Fisher’s exact tests were used to evaluate the relationships between clinical and pathologic variables between spinal and non-spinal. The significance level of the tests was fixed at 0.05. Kaplan-Meier product-limit method was used to estimate OS and CSS at 60 months, and differences in the curves were assessed using log-rank tests (Figure 1). Survival time was calculated as the difference between the date of surgery and the date of last follow-up or death. Univariate and multivariate Cox proportional hazards regression models were used to evaluate the relationship between clinical and pathologic variables with OS and CSS. For the Cox model, variables with less than 15% of missing values were considered. After that, multiple imputations were used to replace missing values. The mi and ice library of Stata software was used for the multiple imputations.
Variables associated with survival in univariate analysis with (p<0.2) were included for multivariate modeling. The proportional risk assumption was evaluated graphically and using Schoenfeld residuals. The Statistical Package for Social Sciences software v. 24 (SPSS) and Stata software were used for the calculation.
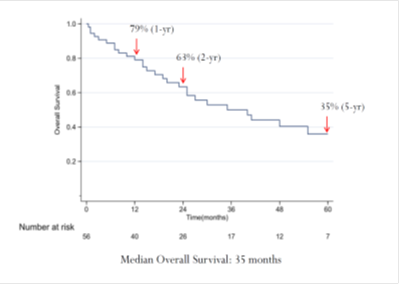
Figure 1: Overall Survival for the entire patient cohort.
3. Results
We identified 530 (14.5%) patients with metastatic disease out of 4060 from the LARCG dataset. From 530, 56 (10.5%) had exclusively BM. The median follow-up was 20.8 months (0-188 range). Our population was mostly under 65 years (66.1%), and male (64.3%), where the median age was 59.5 (40-85), and the number of male participants was 36 (64.3%). Most patients presented symptoms at diagnosis (90.6%). We examined histological subtypes and found that 42 (85.7%) patients had clear cell histology, and 7 (14.3%) patients had non-clear cell histology (1 (2%) had papillary histology, 2 (4.1%) had chromophobe histology, 1 (2%) had unclassified histology, and 3 (6.1%) had other histological subtypes). Sarcomatoid differentiation was identified in 8 (19%) patients. Cytoreductive nephrectomy was performed in 46 patients (82.1%). 35 (68.6%) patients received anti-VEGF/ mTOR systemic therapy for kidney cancer, 1 (2%) received cytokines (interferon and interleukin-2) and 15 (29.4%) had no systemic therapy (Table 1 and 2).
|
Bone Metastasis (BM) |
||||
|
Variable |
All Cases (n=56) |
Non-Spinal (n=33) |
Spinal (n=23) |
P |
|
Age (years) |
||||
|
Median |
59.5 |
60 |
58 |
0.585 |
|
Range |
(40-85) |
(40-84) |
(43-85) |
|
|
Age |
||||
|
< 65 |
37 (66.1) |
22 (66.7) |
15 (65.2) |
0.910 |
|
≥ 65 |
19 (33.9) |
11 (33.3) |
8 (34.8) |
|
|
Gender |
||||
|
Male |
36 (64.3) |
17 (51.5) |
19 (82.6) |
0.017 |
|
Female |
20 (35.7) |
16 (48.5) |
4 (17.4) |
|
|
BMI |
||||
|
< 25 |
14 (35) |
8 (36.4) |
6 (33.3) |
0.842 |
|
≥ 25 |
26 (65) |
14 (63,6) |
12 (66.7) |
|
|
ECOG PS |
||||
|
0 |
11 (20) |
7 (21,2) |
4 (18.2) |
0.904 |
|
1 |
33 (60) |
20 (60.6) |
13 (59.1) |
|
|
≥ 2 |
11 (20) |
6 (18.2) |
5 (22.7) |
|
|
ASA |
||||
|
1-2 |
33 (61.1) |
21 (65,6) |
12 (54.5) |
0.412 |
|
3-4 |
48 (90.6) |
11 (34,4) |
10 (45.5) |
|
|
Symptoms at Presentation |
||||
|
No |
5 (9.4) |
3 (9.4) |
2 (9.5) |
0.986 |
|
Yes |
48 (90.6) |
29 (90.6) |
19 (90.5) |
|
|
Size (pT) |
||||
|
≤ 7 cm |
27 (58.7) |
16 (59.3) |
11 (57.9) |
0.926 |
|
> 7 cm |
19 (41.3) |
11 (40.7) |
8 (42.1) |
|
Table 1: Sociodemographic and Clinical characterization of this Study´s Patients-Part I.
This table represents the sociodemographic and clinical characterization of the study, differentiating our patients between SBM or NSBM. Our population was primarily under 65 years of age and male, and there was significant (p=0.017) correlation between sex and spinal metastasis.
|
Bone Metastasis (BM) |
||||
|
Variable |
All Cases (n=56) |
Non-Spinal (n=33) |
Spinal (n=23) |
P |
|
Stage (pN) |
||||
|
pN0 |
25 (86.2) |
15 (88.2 |
10 (83.3) |
0.706 |
|
pN1 |
4 (13.8) |
2 (11.8) |
2 (16.7) |
|
|
Fuhrman |
||||
|
1-2 |
13 (29.5) |
8 (32) |
5 (26.3) |
0.682 |
|
3-4 |
31 (70.5) |
17 (68) |
14 (73.7) |
|
|
Perirenal Fat Invasion |
||||
|
No |
26 (63.4) |
15 (62.5 |
11 (64.7) |
0.885 |
|
Yes |
15 (36.6) |
9 (37.5) |
6 (35.3) |
|
|
Necrosis |
||||
|
No |
17 (42.5) |
8 (34.8) |
9 (52.9) |
0.251 |
|
Yes |
23 (57.5) |
15 (65.2) |
8 (47.1) |
|
|
Subtype istological |
||||
|
Clear cell |
42 (85.7) |
25 (89.3) |
17 (81) |
0.572 |
|
Papillary |
1 (2) |
1 (3.6) |
0 (0) |
|
|
Chromophobe |
2 (4.1) |
1 (3.6) |
1 (4.8) |
|
|
Unclassified |
1 (2) |
0 (0) |
1 (4.8) |
|
|
Others |
3 (6.1) |
1 (3.6) |
2 (9.5) |
|
|
Sarcomatoid differentiation |
||||
|
No |
34 (81) |
22 (91.7) |
12 (66.7) |
0.041 |
|
Yes |
8 (19) |
2 (8.3) |
6 (33.3) |
|
|
Citoreductive Nephrectomy |
||||
|
No |
10 (17.9) |
5 (15.2) |
5 (21.7) |
0.822 |
|
Yes |
46 (82.1) |
28 (84.8) |
18 (78.3) |
|
|
Treatment of Metastasis |
||||
|
Surgery |
7 (35) |
4 (36.3) |
3 (33.3) |
|
|
Radiotherapy |
8 (40) |
4 (36.3) |
4 (44.4) |
|
|
Others |
5 (25) |
3 (27.3) |
2 (22.2) |
|
|
Systemic Therapy |
||||
|
Anti-VEGF/mTOR |
35 (68.6) |
20 (66.7) |
14 (70) |
|
|
Cytokines IFN/IL2 |
1 (2) |
1 (3.3) |
0 (0) |
|
|
No ST |
15 (29.4) |
9 (30) |
6 (30) |
|
|
Status Survival |
||||
|
Alive |
24 (43.6) |
16 (50) |
8 (34.8) |
|
|
Dead |
31 (56.4) |
16 (50) |
15 (65.2) |
|
|
Dead by Cancer |
29 (93.6) |
15 (93.8) |
14 (93.3) |
|
Table 2: Sociodemographic and Clinical characterization of this Study´s Patients-Part II.
This table represents the sociodemographic and clinical characterization of the study, differentiating our patients between SBM or NSBM. Most of our patients presented with clear cell carcinoma and had received cytoreductive nephrectomy as the primary surgical approach and anti-VEGF/mTor as principal systemic therapy. There was a significant (p=0.041) correlation between having sarcomatoid differentiation of the tumor and spinal metastasis. Among the 56 patients with exclusively BM, 33 (58.9%) had NSBM and 23 (41.1%) had SBM. 18 patients (32.1%) presented with a single BM, while 38 (67.9%) had multiple metastases (Table 3).
|
Bone Metastasis (BM) |
|||
|
Location |
All Cases (n=56) |
Single (n=18) |
Multiple (n=38) |
|
Upper Limbs |
5 (8.9) |
3 (60) |
2 (40%) |
|
Lower Limbs |
8 (14.3) |
5 (62.5) |
3 (37.5) |
|
Pelvic |
5 (8.9) |
3 (60) |
2 (40) |
|
Spine |
13 (23.2) |
5 (38.5) |
8 (61.5) |
|
Thorax |
4 (7.1) |
1 (25) |
3 (75) |
|
Skull |
2 (3.6) |
1 (50) |
1 (50) |
|
Limbs (both) |
1 (1.8) |
0 (0) |
1 (100) |
|
Spine + Limbs |
3 (5.4) |
0 (0) |
3 (100) |
|
Spine + Pelvic |
3 (5.4) |
0 (0) |
3 (100) |
|
Spine + Thorax |
4 (7.1) |
0 (0) |
4 (100) |
|
Pelvic + Limbs |
3 (5.4) |
0 (0) |
3 (100) |
|
Thorax + Limbs |
5 (8.9) |
0 (0) |
5 (100) |
Table 3: Bone Metastasis Locations among all Analysed Patients.
We separated our patients per location and number of metastasis. Most of our population did not have spinal metastasis (n=33) and had multiple bone metastases (n=38).
Factors positively associated with SBM were Sarcomatoid differentiation (p=0.041) and male gender (p=0.017). The 24-month OS for the entire patient cohort was 63%, and the median OS was 35 months (Figure 1). Univariable analysis showed associations between OS and the presence ASA classification 3-4 (HR 2.38, 95% CI 1.04 - 5.43, p<0.04) and SBM (HR 2.22, 95% CI 1.03 - 4.77, p=0.041). The 24-month OS for patients with NSBM was 76% and, for patients with SBM, 46%, and median OS for NSBM and SBM was 55 and 19 months, respectively (Figure 2).
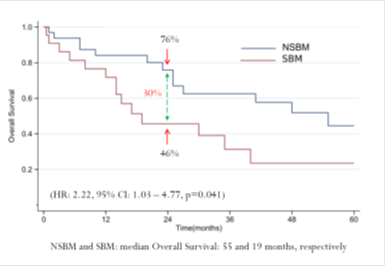
Figure 2: Overall Survival: a Comparison Between Groups with Spinal Bone Metastasis and Non-spinal Bone Metastasis.
In multivariate analysis were independent prognostic factors of 5-year OS, SBM (HR 3.08, 95% CI 1.31-7.23, p=0.010), ASA 3-4 (HR 2.37, 95% CI 1.00 -5.61, p=0.05), non-cc histology (HR 5.11, 95% CI 1.66 -15.71, p<0.004) and AGE (HR 1.06, 95% CI 1.01-1.11, p=0.012) (Table 4).
|
5-year Overall Survival |
|||||||
|
Variable |
Univariable |
Multivariable |
|||||
|
HR |
[95% CI] |
P |
HR |
[95% CI] |
P |
||
|
BM (SBM vs NSBM) |
2.22 |
1.03-4.77 |
0.041 |
3.08 |
1.31 |
7.23 |
0.01 |
|
AGE |
1.04 |
1.00-1.08 |
0.071 |
1.06 |
1.01 |
1.11 |
0.012 |
|
ASA (3-4 vs 1-2) |
2.38 |
1.04-5.43 |
0.040 |
2.37 |
1.00 |
5.61 |
0.05 |
|
Histology (non-cc vs cc) |
2.19 |
0.84-5.75 |
0.110 |
5.11 |
1.66 |
15.71 |
0.004 |
|
Metastases (multiple vs single) |
1.96 |
0.79-4.86 |
0.148 |
- |
- |
- |
- |
|
Gender (male vs female) |
1.29 |
1.06-2.80 |
0.513 |
- |
- |
- |
- |
|
ECOG (≥ 1 vs 0) |
1.68 |
0.58-4.85 |
0.339 |
- |
- |
- |
- |
Table 4: Cox regression analysis for overall survival (OS).
In the left, we separated the variables, distinguishing between univariable and multivariable analysis. We reported the Hazard Ratio (HR), Confidence Interval (CI) and statistical significance (p) of each variable.
4. Discussion
Data concerning the natural history and treatment-related outcomes amongst mRCC patinets in Latin America are lacking. Bone metastasis has been associated with poor clinical outcome in patients with mRCC. In our series of 530 mRCC patients, 10.5% had exclusively bone metastasis, comparable to the study by Bianchi et al (12%), from an extensive database of 11.157 cases of mRCC [12]. Another study, performed by Woodward et al structured a cohort of RCC patients and analyzed the development of bone metastases. Patient demographics were mostly of fair-skinned man (71% male). Their cohort also detected the predominance of clear cell histology (83%), corroborating our data (85.7%) [4].
The median OS in our cohort was 35 months, and survival rates at 1 and 5 years were 79% and 35%, respectively. Similar results were reported by Szendroi et al (1 and 5 years OS, 75%, and 35%, respectively [13], Althausen (84% and 55%, respectively), and Tobisu et al (77% and 45%, respectively) [14, 15]. Survival outcomes in mRCC significantly increased from approximately 9 months in the immunotherapy era (2002-2005) to 30 months in the target therapies era [16]. However, in our cohort, the high survival rate may have been influenced by the number of patients who underwent cytoreductive nephrectomy (82%) and systemic treatment (68.6%). Against this, a small number of patients (12.5%) underwent surgery for metastasis, and isolated metastasectomy or associated systemic treatment has shown a longer OS [13, 17].
Other findings from our cohort were the high percentage of cases with Fuhrman grade 3-4 (70%), and the presence of sarcomatoid differentiation in 19% (8/56) of the cases. Regarding this last characteristic, our analysis could indicate that spinal metastasis is more likely to happen in RCC with sarcomatoid differentiation. Nevertheless, our study did not find a statistically significant association in the univariate analysis, which was reported in previous studies, being able to influence the high number of lost data [14, 18]. YueJun et al described similar results to ours in the Fuhrman grade 3-4 percentage (78.2%), with an increased sarcomatoid differentiation in their cohort (84.2%), which could be the cause of the lower median OS (7.6%) in the non-metastasectomies patients' group [18].
The location of BM and its impact on the clinical outcome were the main purposes of our analysis. Many studies have sought this analysis, comparing mainly the appendicular versus axial topography. The results have been contradictory, once appendicular BM had a better prognosis [14, 19] and others could not demonstrate the same [20, 21]. We found that the prognosis is worse when the BM topography is of spinal location, compared to the non-spinal. Patients with SBM had 24 months OS 30% lower than the ones with NSBM. In multivariate analyses, SBM, ASA 3-4, non-cc histology, and age were an independent prognostic factor of 5-year OS. Having SBM implies a 3-fold increase in the risk of death in our multivariate model, which is consistent with previous literature results.
Kume et al reported SBM as an independent prognostic value for poor OS. Also, sarcomatoid differentiation, extraosseous metastasis, alkaline phosphatase increased to 1.5 times the upper limit of normal, and C-reactive protein increased to greater than 0.3 mg/dl were shown to be significant risk factors [8]. Our group previously reported that ASA 3-4 is a prognostic variable for OS, both in mRCC and in localized RCC [22, 23]. This classification can be a very useful tool, considering it is simple and reproducible.
When it comes to assessing prognosis for mRCC, two systems commonly used are the MSKCC model and the IMDC model. The MSKCC model was developed in the era of immunotherapy, and its adverse prognostic factors are low Karnofsky performance status (KPS), high lactate dehydrogenase (LDH), low serum hemoglobin, high corrected serum calcium, and interval from diagnosis to treatment of less than 1 year [24]. With the advent of targeted therapy, it became necessary to validate the prognosis criteria in the setting of these new treatments, and the IMDC model was developed. This system adds neutrophil and platelet count to four factors already used in the MSKCC model [25]. Recently, a study was published by Massari et al. which aim was to evaluate if the addition of a new independent variable could improve IMDC prognosis prediction and reduce heterogeneity within the risk category [26]. Besides other variables included in the IMDC score, the presence of brain, bone, and/or liver as the first site of metastatic disease was significantly associated with OS, since 15% of patients modified their initial risk category [26]. This seems to be a good example to follow, analyzing and including variables, such as the topography of BM, may allow us to use more accurate prognostic models that help make a better therapeutic decision.
We reported for the first time the role of prognostic factors in a small international cohort of BM in Latin America. The main limitations of this study are inherent to its retrospective nature and the absence of IMDC or MSKCC prognostic scores for all patients. Additionally, the absence of a central pathology review limits our assessment of certain elements such as sarcomatoid histology and other non-clear cell subtypes. Treatment discrepancies among different centers may have led to variations in clinical presentation, surgical technique, and patient adherence to follow-up.
5. Conclusions
Our study showed that SBM predicted shorter OS, suggesting that the location of BM may impact the clinical outcome of patients with mRCC. ASA 3-4, non-cc histology, and age were an independent prognostic factor of OS. External validation of this data could lead to a simple and straightforward prognostic tool for patients with BM of renal cell carcinoma.
Acknowledgements
The authors recognize the participation of the following LARCG members in providing data or input for the study: Patricio Garcia, Ana Maria Autran, Lucas Nogueira, Fernando Secin, Jose Vazquez, Deusdedit Vieira Cortez da Silva Neto, Daniel Beltrame Ferreira, Agustin Rovegno, Raul Langenhin, Jorge Clavijo, Omar Clark, Miguel Sánchez, Carmen González Antonio Carlos Lima Pompeo, Martin Varela, Pablo Bouza, Ana Vilas and Alvaro Zuñiga.
Conflicts of Interest
The authors have no disclosures or conflicts of interest regarding this research and manuscript.
References
- Langdon J, Way A, Heaton S, et al. The management of spinal metastases from renal cell carcinoma. Ann R Coll Surg Engl 91 (2009): 649-652.
- David A. Swanson, William L. Orovan, Douglas E. Johnson, et al. Osseous metastases secondary to renal cell carcinoma. Urology 18 (1981): 556-561.
- Taunk NK, Spratt DE, Bilsky M, et al. Spine radiosurgery in the management of renal cell carcinoma metastases. JNCCN J Natl Compr Cancer Netw 13 (2015): 801-809.
- Woodward E, Jagdev S, McParland L, et al. Skeletal complications and survival in renal cancer patients with bone metastases. Bone 48 (2011): 160-166.
- Beuselinck B, Oudard S, Rixe O, et al. Negative impact of bone metastasis on outcome in clear-cell renal cell carcinoma treated with sunitinib. Ann Oncol 22 (2011): 794-800.
- Santini D, Procopio G, Porta C, et al. Natural history of malignant bone disease in renal cancer: Final results of an italian bone metastasis survey. PLoS One 8 (2013): 6-12.
- Santoni M, Conti A, Procopio G, et al. Bone metastases in patients with metastatic renal cell carcinoma: Are they always associated with poor prognosis? J Exp Clin Cancer Res 34 (2015): 1-9.
- Kume H, Kakutani S, Yamada Y, et al. Prognostic factors for renal cell carcinoma with bone metastasis: Who are the long-term survivors? J Urol 185 (2011): 1611-1614.
- Goodwin CR, Ahmed AK, Boone C, et al. The Challenges of Renal Cell Carcinoma Metastatic to the Spine: A Systematic Review of Survival and Treatment. Glob Spine J 8 (2018): 517-526.
- Li H, Samawi H, Heng DYC. The use of prognostic factors in metastatic renal cell carcinoma. Urol Oncol Semin Orig Investig 33 (2015): 509-516.
- Zequi S de C, Abreu D, Nolazco A, et al. The creation, development and diffusion of the larcglatin american renal cancer group. Int Braz J Urol 43 (2017): 3-6.
- Bianchi M, Sun M, Jeldres C, et al. Distribution of metastatic sites in renal cell carcinoma: A population-based analysis. Ann Oncol 23 (2012): 973-980.
- Szendroi A, Dinya E, Kardos M, et al. Prognostic factors and survival of renal clear cell carcinoma patients with bone metastases. Pathol Oncol Res 16 (2010): 29-38.
- Althausen P, Althausen A, Jennings LC, et al. Prognostic factors and surgical treatment of osseous metastases secondary to renal cell carcinoma. Cancer 80 (1997): 1103-1109.
- Tobisu K, Kakizoe T, Takai K TY. Prognosis in renal cell carcinoma: analysis of clinical course following nephrectomy. Jpn J Clin Oncol 19 (1989): 142-148.
- Judith Manola, Patrick Royston, Paul Elson, et al. Prognostic Model for Survival in Patients with Metastatic Renal Cell Carcinoma: Results from the International Kidney Cancer Working Group. Clin Cancer Res 17 (2011): 5443-5450.
- Vallet S, Pahernik S, Höfner T, et al. Efficacy of targeted treatment beyond third-line therapy in metastatic kidney cancer: Retrospective analysis from a large-volume cancer center. Clin Genitourin Cancer 13 (2015): e145-e152.
- Du YJ, Pahernik S, Hadaschik B, et al. Survival and prognostic factors of patients with renal cell cancer with bone metastasis in the era of targeted therapy: A single-institution analysis. Urol Oncol Semin Orig Investig 34 (2016): 433.e1-433.e8.
- Jung ST, Ghert MA, Harrelson JM SS. Treatment of osseous metastases in patients with renal cell carcinoma. Clin Orthop Relat Res (2003): 223-231.
- Han KR, Pantuck AJ, Bui MHT, et al. Number of metastatic sites rather than location dictates overall survival of patients with node-negative metastatic renal cell carcinoma. Urology 61 (2003): 314-319.
- Fottner A, Szalantzy M, Wirthmann L, et al. Fottner, Andreas Szalant (2010): 2-7.
- Mourão TC, Abreu D, Carvalhal GF, et al. Small renal masses in Latin-American population: characteristics and prognostic factors for survival, recurrence and metastasis - a multi-institutional study from LARCG database. BMC Urol 20 (2020): 85.
- de Cássio Zequi S, de Campos ECR, Guimarães GC, et al. The Use of the American Society of Anesthesiology Classification as a Prognostic Factor in Patients with Renal Cell Carcinoma. Urol Int (2010): 67-72.
- Motzer BRJ, Bacik J, Murphy BA, et al. Interferon-Alfa as a Comparative Treatment for Clinical 20 (2002): 289-296.
- Heng DYC, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter study. J Clin Oncol 27 (2009): 5794-5799.
- Massari F, Di Nunno V, Guida A, et al. Addition of Primary Metastatic Site on Bone, Brain, and Liver to IMDC Criteria in Patients With Metastatic Renal Cell Carcinoma: A Validation Study. Clin Genitourin Cancer (2020): 1-9.
