The Hereditary Angioedema Triggered by Estrogen: Systematic Review
Article Information
Jullyana Egito Peixoto da Costa1#, João Pedro de Oliveira Lopes1#, Bruno Debona Souto2, Denis Henrique de Oliveira1, Severino Correia do Prado Neto1,2, Neire Moura de Gouveia1*
1Faculty Morgana Potrich (FAMP), Mineiros – GO, Brazil.
2University Center of Mineiros (UNIFIMES), Mineiros – GO, Brazil.
*Corresponding author: Neire Moura de Gouveia, Avenida Antônio Carlos Paniago, 65, Setor Mundinho-Centro, Mineiros-GO, 75.830-000, Brazil
# Equal contributed
Received: 25 September 2019; Accepted: 05 October 2019; Published: 09 October 2019
Citation: Jullyana Egito Peixoto da Costa, João Pedro de Oliveira Lopes , Bruno Debona Souto, Denis Henrique de Oliveira, Severino Correia do Prado Neto, Neire Moura de Gouveia. The Hereditary Angioedema Triggered by Estrogen: Systematic Review. Archives of Clinical and Biomedical Research 3 (2019): 343-356.
View / Download Pdf Share at FacebookAbstract
Background: Hereditary angioedema (HAE) triggered by estrogen related to women and their life milestones. Objective: This study aimed to establish a relationship between hereditary angioedema and estrogen in women at puberty, pregnancy or on contraceptives use, their mean age at onset of manifestations, age at diagnosis, and signs and symptoms associated with the precipitating factors for HAE. Data source: Systematic review of articles in English and Portuguese using the Virtual Health Library, Google Scholar, PubMed, and Springer from 2009 to 2019. The descriptors were “hereditary angioedema,” “female,” and “contraceptive”, combined by the boolean operator “AND”. Methods: Case reports and clinical studies on HAE in women were selected. The articles were selected by reading the title and abstract, followed by followed by integral reading, where theses, dissertations, book chapters, grey literature, and review articles were excluded. Information on age, number of people, symptomatology, life stages, and precipitating factors for HAE crises were collated in a table. Results: The mean age of the women was 28.63 years; no postmenopausal case related to hormone replacement therapy was observed. Pregnancy was majorly affected, followed by the phase with use of oral contraceptives, puberty, and eventually menstrual periods. The most evident signs and symptoms were facial edema, abdominal pain, and subcutaneous manifestations, with cases of airway involvement, specifically during pregnancy. Conclusion: The HAE symptoms initially appear, mostly during puberty, and worsen during the menstrual periods. The worst edemigenic attacks were noticed during pregnancy, confirming that estrogen is an important precipitating and aggravating factor triggering HAE.
Keywords
Hereditary angioedema; Women; Estrogen; Edemigenic attacks
Article Details
1. Introduction
In nineteenth century, a physician named Heinrich Quincke was the first to report the clinical occurrence of hereditary angioedema (HAE) [1-3]. After six years, the Canadian physician and researcher, Willian Osler, revealed the genetic association of the disease as autosomal dominant [4-8]. In the term angioedema, “angio” originates from the Greek word aggeion, which expresses the idea of a blood vessel or lymphatic vessel, whereas “edema” originates from the Greek word oidema that indicates swelling [9].
This disease occurs due to the deficiency in the C1 esterase inhibitor gene (C1-INH), which binds directly to the four pillars of body function (i.e., complement, coagulation, kinin, and fibrinolysis). The symptoms vary based on whether bradykinin is in excess, ranging from mild mucosal edema to abdominal discomfort and eventually to edema crisis, with multiple organs involved varying with the edema intensity [10,11], and in certain cases, leading to the obstruction of upper airways [11].
There is no cure reported for angioedema; however, in the last guideline for the diagnosis and treatment of HAE 2017 [12], prophylaxis was emphasized to comprise mild manifestations of the disease with the use of androgens and antifibrinolytics. During emergencies, the use of plasma-derived C1-INH concentrate (cpdC1-INH), Ecallantide, and Icatibanto is used; however, only Ecallantide is not commercialized in the Federative Republic of Brazil and in the absence of the aforementioned three compounds, fresh frozen plasma (FCC) [12] is used.
Notably, in any kind of pathology, developmental triggers commonly related to the onset of edemigenic sensations, such as emotions, sex hormones (estrogen, progesterone, and testosterone), climatic changes, trauma and infections, can be identified. Regarding prevalence, there are discrepant data that fluctuate in the sphere of 1 per 50,000 inhabitants to 1 per 150,000 [14]. The association of estrogen with certain traits of this condition remains unclear. Nevertheless, such assessment is indispensable in diagnostic evaluation and has a high theoretical value, since it is based on case reports and reviews indicating that women are more affected by this disorder than men.
When a new case is diagnosed, the patient is encouraged to perform genetic tests to rule out the histaminergic reactions or to relate the illness-person-stimulus to close the case. In particular, women are the reference who at different stages of life (puberty, combined oral contraceptive (COC) use, contraceptive phase, pregnancy, and menopausal hormone replacement), at which times they were identified with HAE. It is evident that all the aforementioned phases have estrogen in common.
Data on the pathophysiology of estrogen activating HAE is unclear; however, information will be revealed to the general public and also to patients who have not yet been diagnosed with HAE, but rather to anaphylaxis, and that on several occasions the hormonal factor triggered the attacks. Therefore, the review provides additional data to the health professionals and the general public about the reduced estrogen-angioedema ratio in clinical settings where the primary suspect was an allergy.
Hence, the main objective is to mention the life stages in which the estrogen influenced larger edemigenic attacks in women. Moreover, to describe which are they the symptoms according to age, number of people involved in the research (n) of several studies; consequently, cooperating with patients on information regarding HAE.
2. Material and Methods
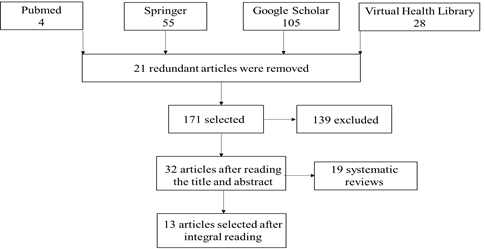
We searched PubMed, Springer, Google Scholar, and Virtual Health Library (VHL) databases. The descriptors used were “hereditary angioedema”, “female”, “oral contraceptive” or “contraceptive” and “C1 inhibitor”, combined by the Boolean operator AND. We selected articles with HAE clinical studies or case reports in women at puberty, during contraceptive use, gestation, or hormone replacement. The studies were selected
regardless of the presence of men in the same study. Theses, dissertations, grey literature, book chapters, and review articles were excluded. The publication deadline was between 2009 and 2019.
The articles were selected by reading the title and abstract, followed by a thorough reading of the entire article. The following information was extracted from the selected articles: number of people, age, symptoms, life stages, and precipitating factor of HAE.
In total, 192 articles were selected, of which 21 redundant articles were removed. Next, 139 articles were excluded after reading the title and abstract. After a thorough reading, 19 articles were removed as they were systematic reviews. About 13 reports were found with predominant information about women and the use of estrogen (Flow Chart I).
3. Results
About 76.92% of the studies reported only on women using exogenous estrogen or endogenous precipitation such as pregnancy and menstruation. The remaining 23.08% have evaluation of both men and women, there is no focus in the specific life stage, but they emphasize in detail precipitating factors. In general, 38.41% were clinical studies and 61.53% were case reports.
On analyzing all the studies, 463 patients were investigated, aged between 9 and 76 years, with a mean age of 28.63 years. As presented in Table 1, we noticed no postmenopausal cases, despite the studies reporting women older than 50 years, and the correlation between HAE and hormone replacement was not defined. The major phase when HAE occurred in women was pregnancy (seven reports), followed by six cases with COC use, and five reports of puberty and periods of menstruation. Only one case revealed a combination of COC use and worsening of HAE during menstruation.
The clinical signs and symptoms are presented in Table 1; these included facial edema affecting the lips, neck, and causing some dyspnea in 100% of cases, followed by abdominal cramps and subcutaneous manifestations of angioedema.
The life stage did not determine any specific sign or symptom in the patients; however, notably, the edemigenic attacks in pregnancy are linked directly to the airways. The main precipitating factor is related to the exogenous use of estrogen; however, any factor that alters the body homeostasis and also elevates the estrogen receptors, acts as a trigger for HAE crises.
COC: oral contraceptive; X: without reports in the article
Table 1: Information extracted from selected studies
The study by Zotter et al. [11] included 140 individuals, 134 of which revealed subjective symptoms and 92 were evaluated in stage I (SI). During SI (2004–2010), 56 women and 36 men were followed up. The age group evaluated was 12–48 years, with a mean of 38.35 years. In stage II (SII), only 27 individuals participated (22 women and 5 men), aged between 9 and 58 years (mean of 36.26 years). This study did not report the life stage in which the patients were; however, more female edemigenic attacks were observed compared to males. In SI, of the 3176 total edemigenic attacks, 2256 were on women. Consider that there was an n sample of 20 women, which was larger than the n sample of men. Moreover, the syndrome manifestations of angioedema were higher in women. The attacks are manifested by episodes of abdominal pain, subcutaneous edema, dysphagia, and upper airway edema (UAE). The precipitating factors presented included menstruation that reflected in symptomatology in 63% of cases, and infections in second place causing 38% of complaints; mental stress in third with 26% of cases, and the triggers of reactions were physical exercise (25%), climatic changes (21%), and mechanical traumas (small percentage not reported in the base article).
The evaluation of Garro et al. [15] refers to a patient with 33 years of oral contraceptive use (COC), wherein in his clinical history, no other medication has been reported. After exclusion of other diagnoses, COC caused facial edema associated with sensation of suffocation, dyspnea, dysphonia, and subcutaneous edema.
Similarly, a report by Fu and Silviu-Dan [16], narrated a 41-year old patient with severe edema in the face and tongue. No description was available regarding the life stage, no use of exogenous estrogen was mentioned, but the precipitating factor diagnosed was an ovarian cyst.
Bork et al. [17] conducted a survey of 147 women, out of which 63 had never used COC, 42 had no symptomatic change in COC use, 15 had moderate symptoms, and 22 had severe symptoms. Five patients had with mutation in coagulation factor XII and four patients had attacks during pregnancy. The phase of life related to edemigenic symptoms was COC use, and in gestation, no age intervals were detailed. As the occurrences of the disease became more pronounced and attacked VAS, causing edema and were confused with anaphylaxis.
The description presented by Miranda et al. (2013), detailed three cases, respectively, aged: 35 (case I), 35 (case II), and 26 (case III) years. These patients had the life stage regarding use of COC and places of affection in common; in contrast, the precipitating factors diverged, because patient I, when using only COC, expressed facial edema, abdominal pain, and respiratory changes, whereas patient II had worsening situations of emotional stress and menstruation, with severe edema of upper lip and face, and eventually, for patient III, seizures were noted only after the discontinuation of COC. Such a fact could be explained by the fluctuating levels of estrogen leading to crises with facial edema and severe abdominal pain.
Saule et al. [19] reported a study with 55 women, of which 14 had the first symptoms at puberty due to menstruation, 27 patients became pregnant during this period, and 46 used COC. No reports were available on the age of patients, and this study in particular, discriminated the symptoms by HAE type, that is, the occurrence of abdominal pain was 92.8% in type I, 100% in type II, and 68.4% in type III. Subcutaneous occurrences were 92.8% in HAE type I, 50% in type II, and 94.7% in type III. Laryngeal edema had 50.7% outbreak in HAE type I, 50% in type II, 58.1% in type III.
A study by Firinu et al. [20], evaluated 27 symptomatic people of the same family, men and women, of which surprisingly 13 had HAE and 14 acquired angioedema. The women had an edemigenic event during pregnancy; from every 11 pregnant women, 8 had abrupt and severe symptoms. The onset of edema in these women was around 20.5 years, because the age range was 9 to 76 years old. Furthermore, it was observed that face edema is predominant with involvement of tongue, larynx, abdomen, and periphery.
Satomura et al. [21] reported the case of a 38-year-old female patient, diagnosed at 7 years with HAE type I, thus comparing the frequency of edemigenic attacks before and during her four pregnancies. In the first gestation, there were few episodes of edema; however, in the other three subsequent pregnancies, there was a need for abortion due to worsening episodes of edema. At age 25, she had neck, limb, and abdomen involvement, but the patient never used COC. The mean values of edemigenic attacks per month before and during her first gestation were 0.8 and 0.3, respectively. In the second gestation at age 32, the frequency changed to 1 and 1.5 attacks/month. In the third gestation at age 34, attacks increased to 2.4 and 3.5 attacks per month. In the fourth gestation, at age 35, the attacks passed to 2.6 and 4.2, respectively. Signs and symptoms prior to gestation were edema (62%), isolated abdominal attacks (38%), and both occurring simultaneously (24%). The crises during pregnancy manifested at the extremities and in the rest of the abdomen, with all attacks being isolated. Notably, the period of pregnancy and breastfeeding is strongly linked to the higher number of attacks and HAE severity.
In another report by Iahn-Aun et al. [22], two cases were described with two patients suffering from polycystic ovary syndrome (PCOS). On using COC, they presented facial edema and abdominal pain, and both had a family history of HAE type III. When the medication was discontinued, the patients remained asymptomatic. Patient I had irregular menstrual cycle, menarche at age 13, hirsutism, and oligomenorrhea, with periods of amenorrhea up to 4 months. The ultrasonography revealed PCOS and the use of combined COC was introduced. After use, she presented recurrent attacks of facial and tongue edema, without papules. Also, presented also abdominal symptoms lasting more than 3 days, in addition, the patient had a family history positive for HAE.
Patient II, with an irregular menstrual cycle since menarche at 10 years, reported amenorrhea of up to 3 months. The patient was diagnosed with PCOS and started treatment with combined COC, followed by attacks of facial edema, extremities, genital, and larynx, without papules that lasted more than 3 days, and also presented symptomatic complementary, abdominal pain, nausea, and vomiting. She was intubated at age 19 for laryngeal edema, and was thus diagnosed with HAE. The patient has a positive family history for HAE.
Feray et al. [23] describe a 32-year-old female diagnosed at age 27 with type III HAE in her third pregnancy, with mutated factor XII. She used combined COC during puberty, during which she presented transient edemigenic attacks on the upper and lower limbs, face, and abdominal pain. She underwent laparoscopic surgery without any findings. The first gestation proceeded without edemigenic attacks, whereas the second gestation affected her with edema of lower limbs and abdominal pains. In the third gestation, she presented edema of hands and feet, abdominal edema, and facial edema, which resolved spontaneously in 48 h. In the postpartum period, there was a decrease in the edema.
Maxfield et al. [24] reported a 21-year-old African American female patient with an episode of facial edema along with airways. She denied previous events of edema and absence of family history. During clinical examination, she had a weak, stridor-free, tachypneic voice, saturating at 100% with supplemental oxygen. Physical examination revealed pain, hypersensitivity, and edema in the facial and neck areas. Medical intervention was required to protect VAS and to configure emergency cesarean delivery, and she had to be intubated, and 14 days later she was extubated, due to severe edema crises.
In the clinical study reported by Deroux et al. [25], of the 57 participants, 46 were female, belonging to 24 distinct families. They were diagnosed with HAE linked to the mutated factor XII. Patients had the first attack on average at 21 years of age, and at age 31, they were diagnosed with HAE. The representativeness of the symptomatic cases was 36 in 38 cases, representing 98% of the cases of attacks. Ninety-one percent of the patients reported facial edema, 87% reported edema in the extremities and/or abdominal region (80%), another 74% reported edema involving ears, nose, and throat. Three patients in the study presented with a greater number of edemigenic crises, since they were pregnant. Twenty-one percent of women during pregnancy or with use of estrogen contraceptives had edema only during these phases, whereas 67% had attacks even outside these referred conditions.
Ferreira et al. [26] reported about a 26-year-old female patient with severe colic type abdominal pain, sudden, recurrent, and diffuse onset associated with nausea, softened stools, edema of hands, feet, and eyelids. The attacks lasted for 3–5 days, reporting improvement with opioid use, with no fever, urinary, and respiratory complaints. During the remission intervals of the attacks, the abdominal pain persisted, but at low intensity and diffused. Patient reported use of COC from 2008 to 2017. Due to this situation, the patient underwent six laparoscopies, free fluid was found in the peritoneal cavity, and in one of the surgeries, intestinal adherence was observed. The patient underwent appendectomy and cholecystectomy, wherein only the organs were swollen. In addition, the increase in stress level led to an increase in the edemigenic crises.
4 Discussion
Among the clinical studies analyzed, most referred to the involvement of edematous crises in women. In accordance with our results, Caballero et al. [27] reported that, although the symptoms vary widely between the individuals (men and women), women are affected with greater severity.
There is evidence that female sex hormones are part of the cascade regulation that promotes HAE. The mechanism of action is due to estrogen affecting the kallikrein-kinin system and the coagulation system, increasing factor XII levels and function; the levels of pre-kallikrein, kallikrein, and high molecular weight kininogen are also elevated; all of these factors are linked to bradykinin formation. Estrogens positively regulate the expression and enhance B2 bradykinin receptor activity [28].
Saule et al. [19] reported 55 patients, of which 14 had symptoms started at puberty, and Iahn-Aun et al. [22] reported the case of two patients who also presented symptoms in this same phase. In some of these women, the attacks became more frequent with menstruation. Similarly, the study by Bouillet et al. [10] confirmed that the symptoms of higher severity occur during puberty and certain edemigenic attacks are reported after or during menstruation [29].
The hormonal regulation of the disorder for HAE was supervised in the European PREHEEAT [29] (new methods for prevention and treatment of attacks in patients with HAE), where 150 patients with secondary HAE to C1 inhibitory serine protease deficiency (C1-INH), were studied [29]. The estrogenic influence of puberty, menstruation, and menopause was examined. In 62% of cases, puberty was harmful to the patient, followed by menstruation in 35% of cases, and ovulation in 14% of the patients. Due to the relevance of manifestations, when the patients used combined COC, attacks occurred in 80% of cases, and those who used COC with progestogen alone presented attacks in 64% of the cases [29].
Four clinical studies by Bork et al. [17], Saule et al. [19], Firinu et al. [20], and Deroux et al. [25] reported that during pregnancy, an increase was observed in the edematous manifestations. Several facts may influence the HAE course during pregnancy. First to analyze is the beginning of the symptomatology, because when it is early, it suggests greater severity and increase of attacks during pregnancy [30]. When woman with HAE related to the decrease of C1-INH generates a fetus, it means full predisposition to edemigenic crises during pregnancy [27]. This mechanism that facilitates this phenomenon occurs because C1-INH crosses the placental barrier reaching the fetal circulation, causing the fetus to consume serinoprotease [30].
For explaining the ratification of edemigenic attacks in pregnancy by C1-INH that crosses the placental barrier, thus reaching the fetal circulation and estrogenic increase, the European study PREHAEAT ??correlated the triggering of menstruation symptoms with the symptoms during pregnancy. They mentioned 37 women who reported menstrual edemigenic attacks, of which 59% had attacks during pregnancy [29]. Of the 70 women who reported no change with the menstrual period, only 24% reported attacks during pregnancy [29]. Briefly, women who had no edema symptoms during menstruation, mostly revealed edema during pregnancy. Other women who did not have symptoms during menstruation also presented edema that bothered them, that is, the number of manifestations in pregnancy far outweighs the episodes in the menstrual period. Thus, noteworthily, peculiarities existing between individual-disease and the manifestations often present themselves with greater intensity in some individuals than in others.
Eventually, the PREHAEAT study also demonstrated that HAE symptoms are worse with subsequent pregnancies; hence, multiparous women are more likely to have severe symptoms when they are affected by edemigenic episodes [29]. The report described by Feray et al. (2018) in Table 1 reveals that during gestations, patients’ symptoms worsened to the point where they required airway intervention, laparoscopy, and investigation in the third pregnancy due to intense edemigenic crisis [31].
Considering the symptomatology due to postmenopausal hormone replacement, we did not obtain conclusive data in the research and no specific report in this age group. As for validating this fact, we found COC information inferring the contraindication to the use in HAE patients, studies on estrogen use, and also reviews about the effects of androgenic medications.
The clinical course and HAE symptoms follow a pattern that closely resembles patients. Most cases are diagnosed in childhood or adolescence, and worsening of edema occurs with puberty and persists throughout the patient’s life [32]. In all studies, edema was described as a symptom, affecting only the facial area and/or any part of the body; in eight of these cases, a VAS involvement was observed requiring intervention. In 9 of the 13 patients, abdominal pain was reported.
According to Bork et al. [32], subcutaneous edema are the most common and can affect extremities in most patients, accompanied by the face and genitals, and more rarely trunk and neck [32,33]; however, abdominal pain in this same analysis is recurrent, and edema of the gastrointestinal tract was reported in 70%–90% of patients with AHF, [33] when they became intermittent, with severe cramps followed by vomiting and diarrhea, which was reported by Ferreira et al. [26].
Eventually, the laryngeal symptoms, according to Bork et al. [32] are rare and potentially fatal. Less than 1% of all edema will involve the larynx, with approximately half of all patients with HAE having an edematous attack at some point in their lives. In accordance to the aforementioned results, notably, even though the laryngeal symptoms are rare, the present study demonstrated that 100% of the patients were affected in several degrees, some with greater and others with lesser severity.
5. Conclusions
The present study demonstrated that mostly the edemigenic symptomatology begins in women at puberty and may worsen during menstrual periods. The phase of the woman consuming COC surpassed the number of manifestations and diagnosis. During pregnancy, the worst edemigenic crises were observed, requiring emergency intervention. The most prevalent symptoms in patients are face and VAS edema, abdominal pain, and subcutaneous edema, respectively. Finally, it is verified when taking into account the sum of all the studied patients, in some clinical studies estrogen is an important precipitating and aggravating factor that triggers HAE attacks.
6. Conflict of Interest
All authors declare no conflict of interest.
References
- Giavina-Bianchi P, Aun MV, Motta AA, Kalil J, Castells M. Classification of angioedema by endotypes. Clin Exp Allergy 45 (2015): 1142-1143.
- Quincke HI. Über akutes umschriebenes Hautödem. Monatsh Prakt Dermatol 1 (1982): 3.
- Giavina-Bianchi P, Aun MV, Jares EJ, Kalil J. Angioedema associated with nonsteroidal anti-inflammatory drugs. Curr Opin Allergy Clin Immunol 16 (2016): 323-332.
- Aun MV, Blanca M, Garro LS, Ribeiro MR, Kalil J, Motta AA, et al. Nonsteroidal AntiInflammatory Drugs are Major Causes of Drug-Induced Anaphylaxis. J Allergy Clin Immunol Pract 2 (2014): 414?420.
- Osler W. Hereditary angioneurotic edema. Am J Med Sci 95 (1888): 6.
- Gomes E, Cardoso MF, Praca F, Gomes L, Marino E, Demoly P. Self-reported drug allergy in a general adult Portuguese population. Clin Exp Allergy 34 (2004): 1597-1601.
- Stevenson DD, Sanchez-Borges M, Szczeklik A. Classification of allergic and pseudoallergic reactions to drugs that inhibit cyclooxygenase enzymes. Ann Allergy Asthma Immunol 87 (2001): 177-180.
- Kowalski ML, Asero R, Bavbek S, Blanca M, Blanca-Lopez N, Bochenek G, et al. Classification and practical approach to the diagnosis and management of hypersensitivity to nonsteroidal anti-inflammatory drugs. Allergy 68 (2013): 1219-1232.
- Ferreira ABH. Miniaurélio: o dicionário da língua portuguesa. 8. ed. Curitiba: Positivo, 2010. p.89/365.
- Bouillet L. Hereditary angioedema in women. Allergy Asthma Clin Immunol 6 (2010): 17.
- Zotter Z, Csuka D, Szabó E, Czaller I, Nébenführer Z, Temesszentandrási G, Fust G, Varga L, Farkas H. The influence of trigger factors on hereditary angioedema due to C1-inhibitor deficiency. Orphanet Journal of Rare Diseases 9 (2014): 44.
- Giavina-Bianchi P, Arruda LK, Aun MV, Campos RA, Herberto J. Chong-Neto. Diretrizes para o diagnóstico e tratamento do AEH 2017. Arq Asma Alerg Imunol 1 (2017): 23- 48.
- Aurora Talavera, José-Luis Larraona, José Luis Ramos, et al. Hereditary Angioedema: An Infrequent Cause of Abdominal Pain with Ascites. Am J Gastrenterology 90 (1995): 471-474.
- Goldsby, RA, Kindt TJ, Osborne BA, Kuby JI. Kuby Immunology, 4th Ed, W.H.Freeman and Company, 2000.
- Garro LS, Porter MHM, Komaroff F, Adachi CT, Malheiros MT, Mello Y, Ribeiro MR. Therapeutic trial in hereditary angioedema type III: Icatibant. World Allergy Organization Journal 8 (2015): A220.
- Fu and Silviu-Dan. Type-III hereditary angioedema resolved by surgery. Allergy, Asthma and Clinical Immunology 10 (2014): A10.
- Bork. K, Wulff K, Witzke G, Stanger C, Lohse P, Hardt J. Antihistamine-resistant Angioedema in Women with Negative Family History: Estrogens and F12 Gene Mutations. The American Journal of Medicine 126 (2013): 1142.e9-1142.e14.
- Miranda AR, Sabbag DV, Ue APF de, Furlani WJ, Souza PK de, Rotta O. Hereditary angioedema type III (estrogen-dependent) report of three cases and literature review. An Bras Dermatol 88 (2013): 578-584.
- Saule C, Boccon-Gibod I, Fain O, Kanny G, Plu-Bureau G, et al. Benefits of progestin contraception in non-allergic angioedema. Clinical & Experimental Allergy 43 (2018): 475–482.
- Firinu D, Bafunnob V, Vecchione G, Barca MP, Manconia PE, et al. Characterization of patients with angioedema without wheals: The importance of F12 gene screening. Clinical Immunology 2015.
- Satomura A, Fujita T, Nakayama T. The Japanese Society of Internal Medicine. Intern Med 57 (2018): 751-755.
- Iahn-Aun M, Aun MV, Motta AA, Kalil J, Giavina-Bianchi P, et al. The Complex Interaction Between Polycystic Ovary Syndrome and Hereditary Angioedema: Case Reports and Review of the Literature Obstetrical and Gynecological Survey 72 (2017): 417-424.
- Feray S, Fain O, Kayem G, Sabourdin N, Constant I, Rigouzzo A. Repeated attacks of Type III hereditary angioedema with Factor XII mutation during pregnancy. International Journal of Obstetric Anesthesia (2018).
- Maxfield L, Jackson JR, Julie Decesare J. The Gravid Patient Presenting with Laryngeal Angioedema. Obstetrics and Gynaecology Cases - Reviews 2 (2015): 3.
- Deroux A, Boccon-Gibod I, Fain O, Pralong P, Ollivier Y, et al. Hereditary angioedema with normal C1 inhibitor and factor XII mutation: a series of 57 patients from the French National Center of Reference for Angioedema. Clinical and Experimental Immunology 185 (2016): 332–337.
- Ferreira ZMCC, ilva AGS, Morel FDM, Freitas MAV, Araújo-Neto I, et al. Intestinal Angioedema: Case Report And Literature Review. Research Journal of Pharmaceutical, Biological and Chemical Sciences RJPBCS 9 (2018): 1453.
- Caballero T, Farkas H, Bouillet L, Bowen T, Gompel A, et al. C-1-INH Deficiency Working Group. International consensus and practical guidelines on the gynecologic and obstetric management of female patients with hereditary angioedema caused by C1 inhibitor deficiency. J Allergy Clin Immunol 129 (2012): 308 –320.
- Geng B, RiedlMA. HAE update: special considerations in the female patient with hereditary angioedema. Allergy Asthma Proc 34 (2013): 13–18.
- Bouillet L, Longhurst H, Boccon-Gibod I, Bork K, Bucher C, et al. Disease expression. in women with hereditary angioedema. Am J Obstet Gynecol 199 (2008): 484.e1–4.
- Czaller I, Visy B, Csuka D, Füst G, Tóth F, Farkas H. The natural history of hereditary angioedema and the impact of treatment with human C1-inhibitor concentrate during pregnancy: A long-term survey. Eur J Obstet Gynecol Reprod Bio 152 (2010): 44–49.
- Feray S, Fain O, Kayem G, Sabourdin N, Constant I, Rigouzzo A. Repeated attacks of Type III hereditary angioedema with Factor XII mutation during pregnancy, International Journal of Obstetric Anesthesia (2018).
- Bork K, Meng G, Staubach P, Hardt J. Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med 119 (2006): 267-274.
- Agostoni A, Aygören-Pürsün E, Brinkley KE, Blanch A, Bork K, et al. Hereditary and acquired angioedema: problems and progress: proceedings of the 22 third C1 esterase inhibitor deficiency workshop and beyond. J Allergy Clin Immunol 114 (2004): S51-S131.