The Environmental Carcinogen Diepoxybutane Modulates the PI3K/AKT/ mTOR and Wnt Cell Signaling Pathways in DU145 Prostate Cancer Cell Organoids
Article Information
Harriet Hammond, Keerthika Yalakaturi, Eduardo Martinez-Ceballos, Xiaoping Yi*
Department of Biological Sciences and Chemistry, Southern University and A&M College, Baton Rouge, LA 70813, USA
*Corresponding author: Xiaoping Yi, Department of Biological Sciences and Chemistry, Southern University and A&M College, Baton Rouge, LA 70813, USA.
Received: 14 February 2024; Accepted: 22 February 2024; Published: 28 March 2024
Citation: Harriet Hammond, Keerthika Yalakaturi, Eduardo Martinez-Ceballos, Xiaoping Yi. The Environmental Carcinogen Diepoxybutane Modulates the PI3K/AKT/mTOR and Wnt Cell Signaling Pathways in DU145 Prostate Cancer Cell Organoids. Archives of Clinical and Biomedical Research. 8 (2024): 112-120.
View / Download Pdf Share at FacebookAbstract
Prostate cancer is the most common cancer in men and one of the leading causes of cancer-related deaths worldwide. Multiple factors, including age, lifestyle, genetic, and environmental factors, are associated with increased risk for prostate cancer. Butadiene (BD), an industrial and environmental chemical used in synthetic rubber and plastic manufacturing, is a known human carcinogen and is associated with an increased risk of certain types of cancer in occupational settings. Studies suggest a potential link between exposure to diepoxybutane (DEB), the key carcinogenic metabolite of BD, and the development of prostate cancer. However, further research is needed to clarify the potential association and understand the underlying mechanisms, if any, between exposure to BD and/or its metabolites like DEB, and prostate cancer in humans. The PI3K/AKT/mTOR signaling pathway plays a central role in regulating various cellular processes, including cell growth, survival, and metabolism. Dysregulation of the PI3K/AKT/mTOR pathway is associated with various diseases, including cancer, where excessive activation can lead to uncontrolled cell growth and tumor formation. The main goal of this study is to determine whether DEB exposure disrupts signaling of the PI3K/AKT/mTOR pathway in prostate cancer cells, potentially promoting tumor progression. To achieve this goal, 3D organoid cultures of DU145 prostate cancer cells were treated with various concentrations of DEB. Subsequently, RNA or protein were extracted from both treated and control (untreated) DU145 cells for gene and protein expression analyses, respectively. Our results show that DEB promotes the activation of the PI3K/AKT cell signaling pathway that may occur via the upregulation of cell cycle genes such as CDK1, CDK4, CCNB1 (Cyclin B1), and CCNE1 (Cyclin E1), accompanied by the upregulation of cancer stem cell markers, including OCT3, OCT4, and NANOG. While our data suggests that DEB may promote tumor progression in DU145 prostate cancer cells, more research is needed to uncover the potential genetic factors that contribute to this progression. Results obtained from this project will increase our understanding of the role played by BD and DEB exposure on prostate cancer progression and will contribute to the improvement of prostate cancer prevention, diagnosis, treatment, and overall patient outcomes.
Keywords
Butadiene; Diepoxybutane; Prostate Cancer; Cancer Stem Cells; PI3K/AKT Pathway; Organoids; 3D Culture; Cancer
Butadiene articles; Diepoxybutane articles; Prostate Cancer articles; Cancer Stem Cells articles; PI3K/AKT Pathway articles; Organoids articles; 3D Culture articles; Cancer articles
Article Details
Introduction
Prostate cancer is the second most frequently diagnosed cancer and the second highest cancer-related cause of mortality in men [1,2]. Prostate cancer development and progression is multifaceted, resulting from a dynamic interplay between genetic predisposition and environmental factors including region, age, race, ethnicity, and lifestyle. Occupational exposures to highly reactive chemical compounds such as 1,3-butadiene (BD) have been linked to increased risk of cancers, such as prostate cancer [3] EPA, 2019. BD is primarily used in industrial processes, especially in the manufacturing of rubber products, plastics, and resins, and can also be found in automobile exhaust and cigarette smoke as a component of air pollution. BD poses significant health risks to humans upon exposure as it is metabolized to 1,2:3,4-Diepoxybutane (DEB) once in the body. In cells, DEB exhibits genotoxic properties, capable of forming DNA-DNA and DNA-protein cross-links, which can lead to mutations, cause cell cycle arrest, induce apoptosis, and may contribute to the development of cancer [4,5]. Due to its hazardous nature, regulatory agencies, such as the Environmental Protection Agency (EPA) in the United States and similar organizations worldwide, impose strict guidelines and regulations on the use, handling, and disposal of DEB to minimize human exposure and environmental contamination [3] EPA, 2019. The phosphatidylinositol 3-kinase (PI3K)/serine-threonine kinase (AKT)/mammalian target of the rapamycin (mTOR) (PI3K/AKT/mTOR) signaling pathway is implicated in the malignant transformation, growth, proliferation, and metastasis of various cancers, including prostate cancer [2,6,7]. Mutations and/or amplifications in genes encoding components of this pathway lead to increased cell survival, proliferation, and resistance to apoptosis, all frequently observed in prostate cancer [8]. Furthermore, investigations into the genotoxic properties of DEB and its potential to disrupt cellular pathways implicated in cancer, such as the PI3K/AKT/mTOR signaling cascade, raise significant concerns regarding its role in prostate carcinogenesis [8,9]. Therefore, understanding the intricate relationship between environmental exposure to BD/ DEB, dysregulated PI3K/AKT/mTOR signaling pathway, and prostate cancer development, holds immense clinical and public health significance. Unraveling these connections will shed light on the different potential mechanisms of carcinogenesis and will open new avenues for targeted interventions and preventive strategies aimed at mitigating the impact of environmental contaminants on prostate cancer initiation and progression [8,9]. We have previously reported that low concentrations of DEB (0.5 µM) induce cell migration in monolayer cultures of DU145 prostate cancer cells [10,11]. Additional preliminary data suggest that DEB may promote tumor progression in prostate cancer cells (data not shown). Herein, this study aims to elucidate the impact of DEB exposure on gene expression within the PI3K/AKT/mTOR signaling pathway using DU145 prostate cancer cells. Following exposure to various concentrations of DEB (0, 1 µM and 1.5 µM), genomic and proteomic analyses were performed on RNA and protein samples extracted from these cells, respectively. Understanding the toxic effects of DEB is crucial in assessing its potential health risks and, particularly, its role in promoting cancer development and its impact on cellular signaling pathways like the PI3K/AKT/mTOR pathway in the context of diseases like prostate cancer. Investigations into how environmental contaminants such as DEB impact this pathway can provide valuable insights into potential mechanisms contributing to prostate cancer progression and may suggest novel therapeutic avenues.
Materials and Methods
Chemicals
1,2:3,4-Diepoxybutane (DEB) was purchased from Sigma-Aldrich and was diluted in dimethyl sulfoxide (DMSO, Sigma).
Cell Culture and Treatments
DU145 prostate cancer cells (American Type Culture Collection, ATCC, Cat. #30-2001, Manassas, VA) were cultured in RPMI-1640 (ATCC) supplemented with 10% fetal bovine serum (FBS, Invitrogen Life Technologies, Inc.), 2 mM L-glutamine (Invitrogen), 1 mM sodium pyruvate (Invitrogen), and 1% penicillin- streptomycin (Sigma Aldrich). The cells were incubated at 37° C in a humidified atmosphere with 5% CO2. Culturex UltiMatrix Reduced Growth Factor Basement Membrane Extract (R&D Systems, Inc., #BME001- 05) was used as a growth scaffold for 3D organoid cultures. Briefly, 50 µL of matrix was used to form a single dome. After 15 days of culture, cells were treated with DMSO (control), 1 µM DEB, or 1.5 µM DEB for 48 h. Total RNA was extracted from these cells for cDNA synthesis and qPCR analyses. Protein was extracted for SDS-PAGE and Western blotting.
Quantitative PCR
Total RNA was extracted from the cells using PureZOL reagent (Bio-Rad #7326890), according to standard procedures. The RNA concentration was determined by NanoDrop (Thermo Scientific). Then, 1.g of RNA was used for cDNA synthesis (Quanta Bio qScript cDNA Synthesis Kit #95047). Quantitative real- time PCR (qPCR) was performed utilizing SsoAdvanced Universal SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA), Perfecta SYBR Green Fastmix (Quanta Bio), or iTaq Universal SYBR Green Supermix (Bio-Rad) and a real-time thermal cycler (Bio-Rad). Custom primers were synthesized by Integrated DNA Technologies (IDT; Coralville, IA) and were designed as follows: 18S (internal control) forward 5’ – GGCCCTGTAATTGGAATGAGTC – 3’, reverse 5’ CCAAGATCCAACTACGAGCTT – 3’; AKT forward 5’ – GAGAAGAAGCTCAGCCCACC – 3’, reverse 5’ – TCCACACACTCCATGCTGTC – 3’; β-catenin forward 5’ –CTGAGGAGCAGCTTCAGTCC – 3’, reverse 5’ – GAGTAGCCATTGTCCACGCT – 3’; Bcl-2 forward 5’ –GATAACGGAGGCTGGGATGC – 3’, reverse 5’ – TCACTTGTGGCCCAGATAGG – 3’; Bcl-xl forward 5’ –ACTCTTCCGGGATGGGGTAA – 3’, reverse 5’ – AGGTAAGTGGCCATCCAAGC – 3’; CD133 forward 5’ –CCCAAGACTCCCATAAAGCTGG – 3’, reverse 5’ CCCGACAGTGCGATGGG – 3’; CD34 forward 5’ –CGCTGCCTTGCCAAGACTAA – 3’, reverse 5’ – GCACCGAGTGGAAGACACTA 3’; CD38 forward 5’ –CAGAAGGGGAGGTGCAGTTT - 3’, reverse 5’ – TGAACTCGCAGTTGGCCATAG - 3’; CDK1 forward 5’ –CTGGGGTCAGCTCGTTACTC – 3’, reverse 5’ – GGAGTGCCCAAAGCTCTGAA – 3’; CDK4 forward 5’ – ATGGACGTCTGTGCCACATC – 3’, reverse 5’ - AACTGGCGCATCAGATCCTT – 3’; Cyclin B1 forward 5’ – CTGCTGGGTGTAGGTCCTTG – 3’, reverse 5’ – TGCCATGTTGATCTTCGCCT – 3’; Cyclin E1 forward 5’ – ATACTTGCTGCTTCGGCCTT – 3’, reverse 5’ – TCAGTTTTGAGCTCCCCGTC – 3’; E-cadherin forward 5’ –CTGATGCTGATGCCCCCAATA – 3’, reverse 5’ – CTGCATCTTGCCAGGTCCTT – 3’; fibronectin forward 5’ –AACCTTGCTCCTGACAGCTC – 3’, reverse 5’ – TTGGTGGGCTGACATTCTCC – 3’; IAP-1 forward 5’ –TGAACAGCTGCTATCCACATC – 3’, reverse 5’ – CTAAAGCCCATTTCCACGGC – 3’; JNK1 forward 5’ –CCACCACCAAAGATCCCTGA – 3’, reverse 5’ – CTAAAGCCCATTTCCACGGC – 3’; JNK2 forward 5’ –GAAGCCCCACCACCTCAAA – 3’, reverse 5’ –TGCTGCATCTGAAGGCTGAT – 3’; MCL-1 forward 5’ –CACCCTCACGCCAGACTC – 3’, reverse 5’ – GGGGCTTCCATCTCCTCAAG – 3’; mTOR forward 5’ –TCCGAGAGATGAGTCAAGAGGA – 3’, reverse 5’ – GCATTCCCACCTTCCACTCC – 3’; MYC forward 5’ –GTAGTGGAAAACCAGCAGCCT – 3’, reverse 5’ – CTGCTGGTAGAAGTTCTCCTCCT – 3’; Nanog forward 5’ –ACCTACCCCAGCCTTTACTCT – 3’; reverse 5’ – AGGACTGGATGTTCTGGGTCT – 3’; Oct3 forward 5’ –GAAAGTCACGCGCCTAGCA – 3’, reverse 5’ – ATGAACAAGTCCGAGCCTCA – 3’; Oct4 forward 5’ –GCCCGAAAGAGAAAGCGAAC – 3’, reverse 5’ – CTCGGACCACATCCTTCTCG – 3’; p21 forward 5’ –AATTCCCCTCTGCTGCTGTC – 3’, reverse 5’ – GGGTGCCCTTCTTCTTGTGT – 3’; p53 forward 5’ –CCCTGCCCTCAACAAGATGT – 3’, reverse 5’ – CTCCGTCATGTGCTGTGACT – 3’; PI3K forward 5’ –TGGTGAGGATTCAGTTGGAGTG – 3’, reverse 5’ – AGGCAACATCCGAAGATCCA – 3’; PI3Kv1 forward 5’ –GAATAGTAGCAGGCGGCGG – 3’, reverse 5’ – ACGGGGCCCTAAGAATGGT – 3’; XIAP forward 5’ –TTGGAAGCCCAGTGAAGACC – 3’, reverse 5’ – AGTTCTTACCAGACACTCCTCA – 3’. Reactions were run in triplicate from three independent experiments. Expression data were normalized to the geometric mean of the housekeeping gene 18S to control the variability in expression levels and were analyzed using the standard 2 -ΔΔCT method. Expression levels were analyzed by one-way ANOVA with Tukey's post-hoc analysis using GraphPad Prism 5.0 software.
Western Blotting
Briefly, after treatment with DEB, cells were lysed in RIPA buffer (Millipore #CS203153) supplemented with Halt Protease Inhibitor (Thermo Scientific #1860932). Protein was separated by 8% - 12% SDS-PAGE and transferred onto a polyvinylidene fluoride (PVDF; GE Healthcare Amersham Hybrid 0.2 uM PVDF #10600021) or nitrocellulose membrane (Bio-Rad #1620112). Membranes were blocked in 1% bovine serum albumin in TBST at room temperature for 1 hour and incubated with primary antibodies at a 1:1000 dilution overnight. Commercially available antibodies were purchased from Cell Signaling Technologies: Akt1 (D9R8K), pAkt (S473), mTOR (2972S), pmTOR (2971S), pPI3K (17366S), cyclin E2 (4132S), cMyc (5605S), nanog (4903S), p21 (Waf1/Cip1) (2947S), p53 (2527S), pPDK1 (3061S), XIAP (14334S), Cyclin B1 (4138S), Cyclin D1 (2978T), Cyclin E2 (4132S), and GAPDH, or from Bio-Rad: PTEN (MCA6312). After washing with TBST, the membranes were incubated with the appropriate horseradish peroxidase-conjugated secondary antibody for 1 hour. Horse anti-mouse or goat anti-rabbit antibodies (Cell Signaling Technologies #7076P2, #7074P2) were utilized at a 1:20,000 dilution. Antigen levels were detected using Signal Fire ECL Reagent (Cell Signaling Technology, Danvers, MA), Cytiva Amersham ECL Prime Western Blotting Detection Reagent (Cytiva Life Sciences, Marlborough, MA, #RPN2232), or Radiance Q Chemiluminescent Substrate (Azure Biosystems Inc., Dublin, CA) on an Azure 400 Visible Fluorescent Imager.
Results
Alterations in multiple cellular signaling pathways is a common signature of carcinogenesis and the accompanied gene changes in most metastatic and primary prostate cancer patients are related to androgen receptor, PI3K-PTEN, Wnt, DNA repair, and cell cycle signaling pathways [6,7,12]. As we previously reported that DEB treatment of monolayer DU145 prostate cancer cells promote cancer progression (Dixon and Martinez-Ceballos, 2010), for this work, we sought to investigate the effect of DEB treatment of prostate cancer cells grown as 3D cultures on the activation of cell signaling pathways known to play a role during tumorigenesis.
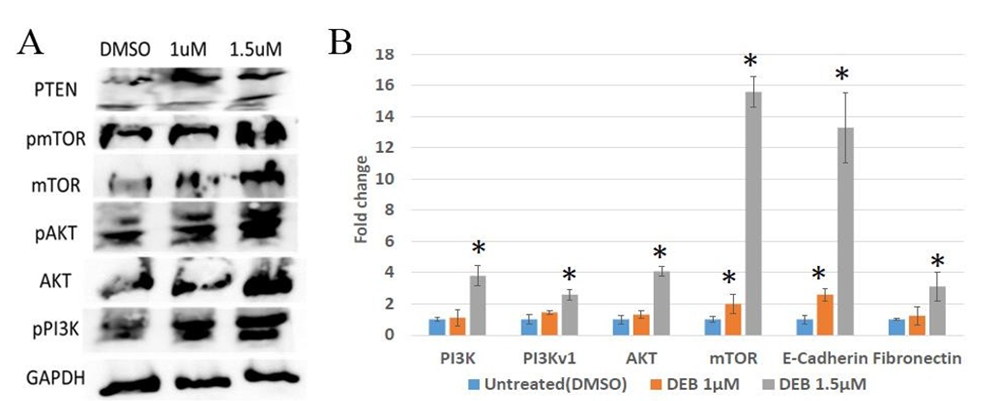
Effect of DEB treatment on the activation of the PI3K/AKT/mTOR pathway in prostate cancer organoids Aberrant PI3K/AKT/mTOR activity has been detected in multiple forms of cancer [2,6,7]. Previous research has established a link between the advancement of cancer and the activation of PI3K/AKT signaling [13]. Recent studies have indicated that the severity of prostate cancer can be attributed to an expression of markers that activate/suppress this pathway [9]. The PI3K/AKT/mTOR pathway is initiated by the activation of PI3K, which phosphorylates PIP3. PIP3 recruits AKT to the cell membrane. Once recruited to the membrane, AKT becomes activated through phosphorylation at specific sites. Subsequently, activated AKT phosphorylates a multitude of downstream targets, including mTOR, regulating various cellular processes involved in cell survival, growth, and proliferation. The tumor suppressor gene PTEN (phosphate and tensin homologue) antagonizes the PI3K/AKT signaling pathway and is implicated in the modulation of cellular proliferation [2,7,14,15]. To explore this further, we conducted Western blot analyses of key members of the PI3K/AKT/mTOR pathway, including the activators Akt and mTOR, as well as the inhibitor PTEN. Western blot analyses revealed dose-dependent increases in protein levels of mTOR, phosphorylated mTOR, AKT, phosphorylated AKT, and phosphorylated PI3K in 3D cultures of DU145 prostate cancer cells treated with 1 and 1.5 µM DEB compared to untreated controls (Figure 1A). However, PTEN levels were higher in cells treated with 1 µM DEB than with 1.5 µM DEB. Nevertheless, PTEN protein levels were higher in treated cells than in controls. These results indicate that DEB treatment increases the levels of activated mTOR, PI3K, and AKT, suggesting that exposure to this xenobiotic pollutant may over-stimulate the PI3K/AKT pathway in prostate tumors in humans.
To extend this investigation at the gene expression level, we conducted qPCR analyses on DU145 organoids under the same treatment conditions as above. The results revealed significant increases, as compared to controls, in the expression of PI3K (~4-fold change), PI3Kv1 (~2.5 fold), AKT (~4-fold), and mTOR (~15.5- fold) mRNA levels when organoids were exposed to 1.5 µM DEB (Figure 1B). For the 1 µM DEB treatments, mTOR mRNA levels were increased by ~2-fold but other pathway members were not affected. Because activation of the PI3K/AKT/mTOR pathway has been found to promote Epithelial-to-Mesenchymal Transition (EMT), we also examined the mRNA expression of two EMT markers, E-cadherin (decreases during EMT) and fibronectin (increases with EMT). E-cadherin mRNA levels increased about 2.5-fold with 1 µM DEB but significantly increased (~13-fold) with 1.5 µM DEB treatment with respect to controls. The levels of fibronectin mRNA increased with treatment, but only at the 1.5 µM concentration (Figure 1B). Taken together, these results provide additional insights into the cellular response of prostate cancer cells to DEB treatment and indicate that exposure to 3D-cultured DU145 prostate cancer cells results in the upregulation of members of the PI3K/AKT/mTOR pathway at both the mRNA and protein levels.
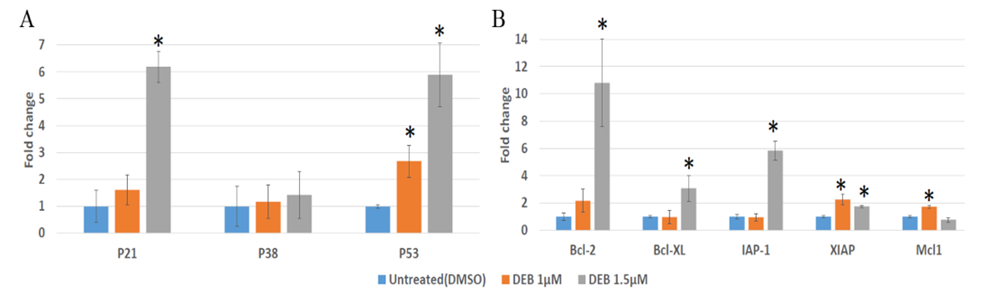
Figure 1: Differential expression of PI3K/AKT/mTOR pathway members in DU145 organoids treated with DEB. (A) Western blot. DEB treatment increased the protein expression of PI3K/AKT/mTOR members in a dose-dependent manner. Representative image from independent triplicate experiments shown. (B) Analysis of expression of pathway members by qPCR. DEB treatment resulted in upregulation of most genes assayed. DEB effect shown as fold change in mRNA expression in treated cells as compared to controls (expressed as the unit). Results from sextuplicate experiments. * = p<0.05.
DEB induces upregulation of genes involved in the Wnt/β-catenin pathway
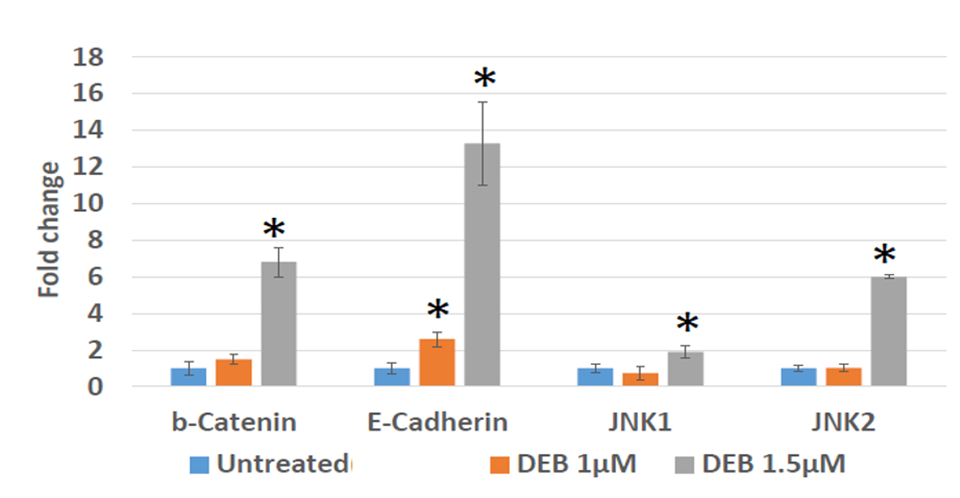
To further investigate the effect of DEB on EMT promotion, we examined the mRNA expression levels of Wnt pathway family members in treated versus control DU145 organoids. The justification for these analyses is that both the canonical and non-canonical Wnt signaling pathways play a role in the onset of EMT [16,17,18]. As compared to non-treated controls, we observed that DU145 cells treated with 1.5 µM DEB display a notable elevation in the levels of the EMT marker E-cadherin, along with significant increases in the expression of β-catenin, JNK1, and JNK2 (Figure 2). Conversely, marker mRNA levels were not evidently increased in organoids exposed to 1 µM DEB, except for E-cadherin, which increased by about 2.5-fold as compared to controls (Figure 2). Thus, these results indicate that DEB, at the 1.5 µM concentration, can activate both the canonical and the non- canonical Wnt pathways in DU145 prostate cancer organoids.
Figure 2: Differential expression by qPCR of genes associated with the Wnt/β -catenin pathway in 3D cultures of DU145 cells treated with DEB. DEB effect shown as fold change in mRNA expression in treated cells as compared to controls (expressed as the unit). Results from sextuplicate experiments. * = p<0.05.
DEB causes differential expression of genes and proteins involved in the cell cycle
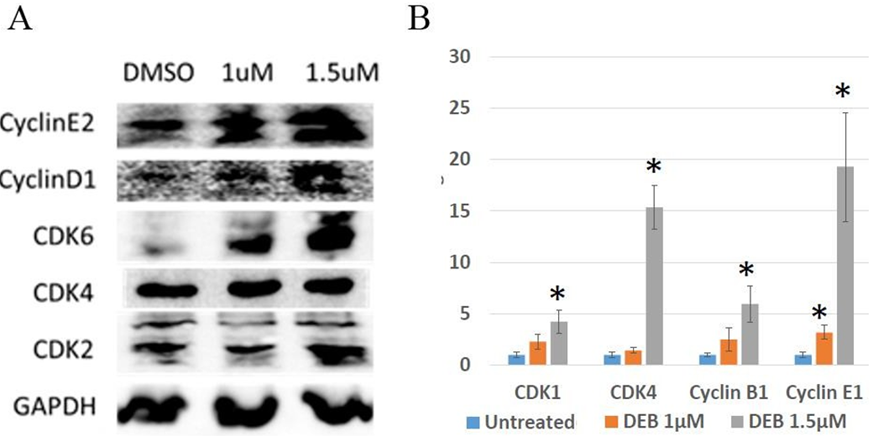
The cell cycle is one of the most important, highly organized, and tightly regulated biological processes in the human body. Progression through distinct phases of the cell cycle is regulated by cyclins and cyclin- dependent kinases (CDKs). Cyclin-CDK complexes govern the activation of several genes and transcription factors [19, 20]. Dysregulation of the cell cycle is an important characteristic involved in the initiation and progression of cancer, resulting in uncontrolled cell proliferation [21] Goel et al., 2018. In this series of experiments, we aimed to explore the expression levels of cell cycle regulators using both Western blot and qPCR in DU145 prostate cancer cells exposed to DEB. Western blot analyses indicate a correlation between DEB concentration and augmented levels of cell cycle proteins in DU145 prostate cancer cell organoids, with notable increases in the protein levels of CCNE2 (CyclinE2), CCND1 (CyclinD1), CDK6, and CDK2 with increased DEB treatment compared to unexposed controls (Figure 3A). Similarly, qPCR analyses revealed significant increases in the mRNA levels of CCNE1 (CyclinE1) after exposure to 1 µM DEB, while mRNA levels of CDK1, CDK4, and CCNB1 (Cyclin B1) were all significantly upregulated in samples treated with 1.5 µM DEB (Figure 3B). In the current study, we observed that levels of proteins involved in the cell cycle increased with both concentrations of DEB examined, while mRNA levels of cell cycle regulators only increased with the highest DEB concentration used (1.5 mM), except for Cyclin E1 that was upregulated by 1 mM DEB (~ 3-fold change). These results indicate that treatment of DU145 organoids with DEB positively affects the cell cycle.
Figure 3: Differential expression of genes associated with the cell cycle in 3D cultures of DU145 cells treated with DEB. (A) Western blot. DEB treatment increases cell cycle protein expression. Representative image from independent triplicate experiments shown. (B) Analysis of expression of cell cycle genes by qPCR. Treatment with 1.5.M DEB resulted in upregulation of most genes assayed. Results from sextuplicate experiments. * = p<0.05.
DEB up-regulates the expression of cancer stem cell markers
Armed with evidence that DEB modulates the PI3K/AKT/mTOR and Wnt signaling pathways, which are known regulate stem cell renewal, proliferation, differentiation, and apoptosis [22], we next sought to examine the effect of DEB on the expression of Cancer Stem Cell markers in DU145 organoids.
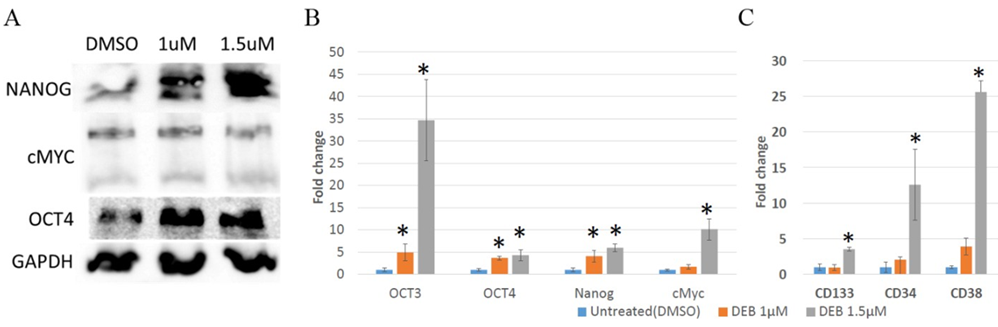
Figure 4: Differential expression of stem cell marker genes in 3D cultures of DU145 cells treated with DEB. (A) Western blot. DEB treatment increased the protein expression of NANOG and OCT4, but not of cMYC. Representative image from independent triplicate experiments is shown. (B and C) Analysis of expression of Cancer Stem Cell (CSC) marker genes by qPCR. DEB upregulated CSC genes in treated DU145 organoids. Treatment with 1 ?M DEB failed to increase the expression of cell surface markers (panel C). Results from sextuplicate experiments. * = p<0.05.
As shown in Figure 4A, Western blot analyses revealed an elevation in the protein levels of NANOG and OCT4 in cells treated with both concentrations of DEB, whereas cMYC showed no significant variation. At the mRNA levels, DEB treatment resulted in the upregulation of OCT3, OCT4, cMYC and NANOG, albeit cMYC mRNA levels only increased in cells treated with 1.5 mM but not 1 mM DEB. (Figure 4B). Remarkably, a substantial increase in the expression of OCT3 (~35-fold) was observed with the highest concentration of DEB treatment in comparison to the control group. These results suggest that DEB, especially at the 1.5 mM concentration, promotes stemness in DU145 cells cultured as 3D organoids.
DEB upregulates the expression of apoptosis-related genes in DU145 organoids
In efforts to decipher the survival mechanisms of DU145 prostate cancer organoids exposed to DEB, we investigated whether DEB treatment alters the mRNA expression of genes related to the apoptotic pathway. Previous studies have shown that DEB-induced cell death in human lymphoblasts is due to apoptosis and not necrosis, which results in a concentration-dependent elevation of p53 levels and of its downstream targets, further suggesting that p53 may play a role in mediating the apoptotic response of human lymphoblasts to DEB [23,24,25]. Similarly, in human acute myeloid leukemia (HL-60) cells, DEB was found to upregulate several genes involved in the intrinsic mitochondrial apoptosis pathway, including Bcl-2. Consistent with these reports, our results show a significant upregulation in p53 mRNA expression in DU145 prostate cancer cells following exposure to both 1 µM DEB (~2.5-fold) and 1.5 µM DEB (~6-fold, see Figure 5A). The levels of p21 levels were also significantly upregulated in samples exposed to 1.5 µM DEB (~6-fold). However, DEB did not have any effect on the expression of p38. With regards to antiapoptotic gene expression, 1 µM DEB increased the mRNA expression of XIAP and Mcl1 (Figure 5B). Bcl-2, Bcl-xl, and IAP-1 expression was not altered by 1µM DEB, but all apoptosis-related genes examined were upregulated by 1.5 µM DEB, except for p38 and Mcl1. Of all the affected genes, Bcl-2 showed the highest response to DEB as it experienced a remarkable 11-fold increase compared to the control after treatment with 1.5 µM DEB. In the present study, we show that DEB induces changes in the expression of apoptotic regulators such as the BCl-2 family, potentially disrupting the balance between pro-survival and pro-apoptotic signals. The apoptotic pathway is a tightly regulated process of programmed cell death and is crucial for maintaining tissue homeostasis and eliminating damaged or abnormal cells. When this pathway is dysregulated, it can lead to uncontrolled cell survival, a hallmark of cancer. Overall, our results demonstrate that DEB treatment of DU145 organoids upregulates the mRNA expression of anti-apoptotic genes, which suggest that this effect allows cancer cells to counteract the disruptive effect of this xenobiotic pollutant on genomic DNA integrity.
Discussion
Investigations into the impact of DEB on key signaling pathways in prostate cancer cells reveal potential mechanistic insights into how environmental contaminants may disrupt critical cellular signaling cascades, potentially contributing to and/or exacerbating the pathogenesis of prostate cancer. Our study provides evidence that DEB exposure significantly disrupts key cellular processes in DU145 prostate cancer cells, unraveling a complex network of dysregulation across multiple critical pathways implicated in cancer progression. We found that DEB dysregulates protein and mRNA expression of genes involved in apoptosis, cancer stem cell markers, the cell cycle, Wnt/β-catenin, and PI3K/AKT/mTOR cell signaling pathways, all known promoters of tumor progression and proliferation. The observed alterations in apoptosis-related genes underscore DEB’s potential to subvert the programmed cell death machinery, contributing to the evasion of regulatory mechanisms. Furthermore, the dysregulation of cancer stem cell markers highlights DEB’s impact on the acquisition of stem cell-like properties, potentially fostering tumor heterogeneity and resistance to therapies. Perturbations in the cell cycle machinery signify DEB-induced disruptions in the temporal control of cell division, likely promoting uncontrolled proliferation. Aberrant modulation of the Wnt/β-catenin pathway suggests a role for DEB in enhancing pro-tumorigenic signals associated with cellular growth and migration. Wnts are powerful regulators of cell proliferation and differentiation. One of the main players in this pathway is the transcription factor β-catenin [14,16,18]. The canonical Wnt/β-catenin signaling pathway plays a pivotal role in tumor progression, particularly through its influence on epithelial-mesenchymal transition (EMT) [16-18]. When Wnt signaling is active, the proteasomal degradation of β-catenin is inhibited. This stabilization of β-catenin facilitates the activation of Wnt target gene transcription, leading to the promotion of cellular proliferation and survival, which are critical factors in tumor development [14,16,26]. Moreover, Wnt/β-catenin signaling interacts synergistically with the JNK signaling cascade, a process integral to tumorigenesis. The JNK pathway not only contributes to elevated β-catenin levels, influenced by various growth factors, but also plays a role in modulating the Wnt signaling pathway, particularly in the context of the E-cadherin/β-catenin complex. The disruption of this complex is closely linked to the advancement of cancer [14]. Moreover, Wnt/β-catenin signaling can lead to AKT activation, highlighting the complex interplay between multiple signaling pathways in the context of cancer biology [26]. In the present study, we cultivated DU145 3D cell organoids with the aim of mimicking the microenvironment of prostate cancer tissue. We assessed the Wnt/β-catenin signaling pathway markers at m-RNA level by RT-PCR analysis focusing on the expression of cell survival genes, specifically β-catenin and E-Cadherin, as well as the JNK pathway genes, JNK1 and JNK2. Compared to non-treated controls, we showed that DU145 cells treated with DEB display a notable elevation in the levels of the EMT markers, along with significant increases in the expression of β-catenin, Jnk1, and Jnk2 (Figure 2). These findings align with known roles of Wnt family members as positive regulators of cell survival (anti-apoptotic) and cell proliferation [14]. Perhaps most notably, the profound dysregulation of the PI3K/AKT/mTOR signaling pathway, a central regulator of cell growth and survival, unveils a potential mechanistic link between DEB exposure and the promotion of prostate cancer progression.
With regards to the observed effect of DEB on cell cycle regulation, our observations align with previous research that has revealed a dose-dependent upregulation of CCNB1 (CyclinB1) protein expression in mouse germ cells following DEB treatment [27]. Similarly, we observed significant increases in CCNB1 (CyclinB1) mRNA levels following treatment with 1.5 µM DEB in DU145 prostate cancer cells (Figure 3B). Although we observed an increase on the levels of CDK4 at the mRNA but not at the protein levels (Figure 3), we found that CDK6 protein levels were highly increased by DEB. As CDK4 and CDK6 are homologous serine/threonine kinases, controlled by D-type cyclins (D1, D2, and D3), that critical mediators of cellular transition into S phase [20], our results indicate that DEB exposure may promote cell division in DU145 organoids. Taken together, these data suggest that DEB exposure may increase the malignancy of established tumors in humans. Concurrent with this hypothesis, we observed that all the markers examined were upregulated at the protein or mRNA levels by either 1 or 1.5 µM DEB, except for cMYC. NANOG and OCT4 were both upregulated by DEB as compared to untreated controls. In cells, the OCT family of transcription factors plays important roles in determining and maintaining cellular identity. Its family members are involved in regulating cellular reprogramming, establishing stem cells, and promoting tumorigenesis [28,29]. The expression level of OCT3/4 is related to the tumorigenesis of multiple cancers, including prostate cancer [28]. Furthermore, OCT3/4, along with cMYC, play important roles in the creation of induced pluripotent stem cells [29]. OCT 3/4 also enhances transcription and protein expression of NANOG, a transcription factor involved in self-renewal and maintenance of pluripotency [28]. With regards to cell-surface CSC markers, we examined their mRNA expression in control versus DEB-treated cells, and found that they were upregulated by 1.5 mM but not by 1.M DEB. From the literature, the expression of cluster of differentiation cell surface antigens, such as CD133, CD34, and CD38, are often used to characterize stem cell populations [30,31], and has been found that the functions of these cell surface antigens vary and that they are implicated in many biological processes, including but not limited to signal transduction, EMT, cell cycle regulation, as well as cellular differentiation, adhesion, migration, and proliferation [30]. Because in the present study, we saw notable increases in the mRNA levels of CD133, CD34, and CD38 in DU145 cells exposed to 1.5 µM DEB (Figure 4C), it is possible that only the highest DEB concentration used promotes the generation of true stem cell-like cells in our 3D cultures.
In addition to aberrant signaling of genes involved in the cell cycle, we also observed a significant increase in the mRNA levels of p21 and p53 in DU145 prostate cancer organoids following DEB exposure (Figure 5A). Both p21 and p53 are CDK inhibitors involved in regulating various checkpoints of the cell cycle [20]. Furthermore, multiple components of the cyclin-dependent kinase pathway are commonly mutated in human cancers [19,20]. Thus, our results may indicate that an initial response of cells to DEB is to halt the cell cycle. However, as shown in Figure 3, DU145 cells in the organoids exposed to DEB may counteract this cell cycle-inhibitory effect by activating cell survival pathways (see Figure 5) that result in continuous cell proliferation in the presence of DNA-DEB adducts. Taken together, our data suggests that DEB exposure exacerbates the pre-existing aberrant cell cycle in DU145 prostate cancer cells, suggesting that this xenobiotic may increase the malignancy of prostate cancer cells grown as organoids. Some limitations of traditional prostate cancer research include the potential for low reproducibility due to the cellular and molecular heterogenic nature of prostate cancer. To help overcome this limitation, we cultured DU145 cells, a castration-resistant, androgen receptor negative prostate cancer cell line, derived from brain metastasis, as 3D organoids. Spatial organization in a 3D environment mimics the structure of living tissue and supports the complex interplay of cell-cell interaction. Studies have demonstrated the advantage of using 3D cell culture models to understand the molecular biology of prostate cancer [32,33]. For example, studies have demonstrated significant differences in the percentage of viable versus apoptotic cells in response to treatment with the chemotherapeutic drug docetaxel, as well as significant differences in the expression of key EMT markers in 2D monolayers versus 3D spheroid cell cultures of prostate cancer cells, including DU145 cells, suggesting significant differences in the phenotype of prostate cancer cells cultured in different conditions [32,33]. Future studies should include the use of additional prostate cancer cell lines, such as the immortalized, benign prostate cell line PNT1A or the normal prostatic epithelial cell line RWPE-1, to examine the impact of DEB-induced signaling on normal prostate cells. Research into the mechanisms by which DEB alters cellular processes is essential for developing strategies to mitigate its harmful effects on human health and the environment.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Funding
Research reported in this publication was supported by an award from the National Institute of General Medical Sciences of the National Institutes of Health under award number 5P20GM135000-02.
References
- Centers for Disease Control and Prevention. U.S. Cancer Statistics Prostate Cancer Stat Bite. US Department of Health and Human Services (2023).
- Roudsari NM, et al. Inhibitors of the PI3K/Akt/mTOR pathway in prostate cancer chemoprevention and intervention. Pharmaceutics 13 (2021): 1195.
- Chen WQ, XY Zhang. 1, 3-Butadiene: a ubiquitous environmental mutagen and its associations with diseases. Genes and Environment 44 (2022): 1-22.
- Gherezghiher TB, et al. 1, 2, 3, 4-Diepoxybutane-induced DNA–protein cross-linking in human fibrosarcoma (HT1080) cells. Journal of proteome research 12 (2013): 2151-2164.
- Michaelson-Richie ED, et al. DNA−protein cross-linking by 1, 2, 3, 4-diepoxybutane. Journal of proteome research 9 (2010): 4356-4367.
- Cancer Genome Atlas Research Network. The Molecular Taxonomy of Primary Prostate Cancer. Cell 163 (2015): 1011-1025.
- Robinson, D., et al., Integrative clinical genomics of advanced prostate cancer. Cell 161 (2015): 1215-1228.
- Morgan TM, TD Koreckij, E Corey. Targeted therapy for advanced prostate cancer: inhibition of the PI3K/Akt/mTOR pathway. Current cancer drug targets 9 (2009): 237-249.
- Yang, J., et al., Targeting PI3K in cancer: mechanisms and advances in clinical trials. Molecular cancer 18 (2019): 1-28.
- Dixon LA, E Martinez-Ceballos. Does Diexpoxybutane (DEB) Increase Cancer Stem Cells and Allow Resistance to Anti-Tumor Therapy? in International Journal Of Toxicology (2010).
- Dixon L, E Martinez-Ceballos. Role of DEB-Induced ROS Production During the Activation of Cell Survival Pathways in Prostate Cancer Cells. In International Journal Of Toxicology (2011).
- Chen Y, et al. ETS factors reprogram the androgen receptor cistrome and prime prostate tumorigenesis in response to PTEN loss. Nature medicine 19 (2013): 1023-1029.
- Vara JÁF, et al. PI3K/Akt signalling pathway and cancer. Cancer treatment reviews 30 (2004): 193-204.
- Centelles JJ. General Aspects of Colorectal Cancer. ISRN Oncology (2012) 2012.
- Crumbaker M, L Khoja, AM Joshua. AR signaling and the PI3K pathway in prostate cancer. Cancers 9 (2017): 34.
- MacDonald BT, K Tamai, X He. Wnt/β-catenin signaling: components, mechanisms, and diseases. Developmental cell 17 (2009): 9-26.
- Morin PJ. β-catenin signaling and cancer. Bioessays 21 (1999): 1021-1030.
- Shang S, F Hua, ZW Hu. The regulation of β-catenin activity and function in cancer: therapeutic opportunities. Oncotarget 8 (2017): 33972.
- Dalton S. Linking the cell cycle to cell fate decisions. Trends in cell biology 25 (2015): 592-600.
- Otto T, P Sicinski. Cell cycle proteins as promising targets in cancer therapy. Nature Reviews Cancer 17 (2017): 93-115.
- MacLachlan TK, N Sang, A Giordano. Cyclins, cyclin-dependent kinases and cdk inhibitors: implications in cell cycle control and cancer. Critical Reviews™ in Eukaryotic Gene Expression 5 (1995).
- Mohammed MK, et al, Wnt/β-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance. Genes & diseases 3 (2016): 11-40.
- Ewunkem AJ, et al. Diepoxybutane induces the p53-dependent transactivation of the CCL4 gene that mediates apoptosis in exposed human lymphoblasts. Journal of Biochemical and Molecular Toxicology (2023): e23316.
- Yadavilli S, PM Muganda. Diepoxybutane induces caspase and p53-mediated apoptosis in human lymphoblasts. Toxicology and applied pharmacology 195 (2004): 154-165.
- Yadavilli S, et al. Diepoxybutane activates the mitochondrial apoptotic pathway and mediates apoptosis in human lymphoblasts through oxidative stress. Toxicology in Vitro 21 (2007): 1429-1441.
- Stamos JL, Weis WI. The beta-catenin destruction complex. Cold Spring Harbor Perspectives in Biology 5 (2013): a007898.
- Dong J, et al. Induction of DNA damage and G2 cell cycle arrest by diepoxybutane through the activation of the Chk1-dependent pathway in mouse germ cells. Chemical Research in Toxicology 28 (2015): 518-531.
- Baek KH, J Choi, CZ Pei. Cellular functions of OCT-3/4 regulated by ubiquitination in proliferating cells. Cancers 12(2020): 663.
- Kim KP, et al. Biological importance of OCT transcription factors in reprogramming and development. Experimental & Molecular Medicine 53 (2021): 1018-1028.
- Anjos-Afonso F, D Bonnet. Human CD34+ hematopoietic stem cell hierarchy: how far are we with its delineation at the most primitive level? Blood Journal (2023): 2022018071.
- Su CY, et al. Analyzing the expression of biomarkers in prostate cancer cell lines. In vivo 35 (2021): 1545-1548.
- Fontana F, et al. Epithelial-to-mesenchymal transition markers and CD44 isoforms are differently expressed in 2D and 3D cell cultures of prostate cancer cells. Cells 8 (2019): 143.
- Fujiike AY, et al. Effects of docetaxel on metastatic prostate (DU-145) carcinoma cells cultured as 2D monolayers and 3D multicellular tumor spheroids. Journal of Toxicology and Environmental Health, Part A 87 (2024): 227-244.
- Geske F, et al. Early stages of p53-induced apoptosis are reversible. Cell Death & Differentiation 8 (2001): 182-191.
- Huang K, et al. Elevated p53 expression levels correlate with tumor progression and poor prognosis in patients exhibiting esophageal squamous cell carcinoma. Oncology letters 8 (2014): 1441-1446.
- Le PM, et al. Cross-linking by epichlorohydrin and diepoxybutane correlates with cytotoxicity and leads to apoptosis in human leukemia (HL-60) cells. Toxicology and Applied Pharmacology 352 (2018): 19-27.
- Proposed Designation of 1,3-Butadiene (CASRN 106-99-0) as a High-Priority Substance for Risk Evaluation. United States Environmental Protection Agency. Office of Chemical Safety and Pollution Prevention (2019) Regulations.gov.
- Wilson T, P Johnston, D Longley. Anti-apoptotic mechanisms of drug resistance in cancer. Current cancer drug targets 9 (2009): 307-319.