The Efficacy of Combination Therapies Including Antiviral Drugs, Methylprednisolone and Daily Proning in 20 Patients with COVID-19 Requiring Invasive Mechanical Ventilation-Case Series in A Single Critical Care Center in Osaka, Japan
Article Information
Haruka Shimazu1*, Kazuhisa Yoshiya1, Shuhei Maruyama1, Shuji Kanayama1, Shuhei Matsunami1, Hiromu Iwamura1, Daiki Wada1, Tomoyuki Yoshihara1, Fukuki Saito1, Yasushi Nakamori1, and Yasuyuki Kuwagata2
1Department of Emergency and Critical Care Medicine, Kansai Medical University Medical Center, 10-15 Fumizono-cho, Moriguchi, Osaka, Japan
2Department of Emergency and Critical Care Medicine, Kansai Medical University Hospital, 2-3-1 Shinmachi, Hirakata, Osaka, Japan
*Corresponding Author: Dr. Haruka Shimazu, Department of Emergency and Critical Care Medicine, Kansai Medical University Medical Center, 10-15 Fumizono-cho, Moriguchi, Osaka 570-8507, Japan
Received: 03 August 2020; Accepted: 14 August 2020; Published: 14 September 2020
Citation: Haruka Shimazu, Kazuhisa Yoshiya, Shuhei Maruyama, Shuji Kanayama, Shuhei Matsunami, Hiromu Iwamura, Daiki Wada, Tomoyuki Yoshihara, Fukuki Saito, Yasushi Nakamori, Yasuyuki Kuwagata. The Efficacy of Combination Therapies Including Antiviral Drugs, Methylprednisolone and Daily Proning in 20 Patients with COVID-19 Requiring Invasive Mechanical Ventilation-Case Series in A Single Critical Care Center in Osaka, Japan. Archives of Clinical and Medical Case Reports 4 (2020): 867-882.
View / Download Pdf Share at FacebookAbstract
Background: Since December 2019, the COVID-19 infection has drastically spread across China and the world, including Japan. Few reports so far have clarified the prognosis and treatment of critically ill patients managed with invasive mechanical ventilation. We describe the clinical courses and detail treatments of 20 critically ill patients with invasive mechanical ventilation, which may be valuable for determining future therapies and intensive care of critically ill patients with COVID-19.
Methods: We carried out descriptive analysis for 20 critically ill patients with laboratory-confirmed SARS-CoV-2 infection who were admitted to our hospital ICU and required invasive mechanical ventilation. The patients’ general characteristics, laboratory data, treatments, and outcomes were assessed between survivors and non-survivors.
Results: Among 20 patients, 14 patients survived and 6 patients died. The lowest lymphocyte cunt (93 vs 279/μL, p<0.01) and platelet count (12 vs 152 × 103/μL, p<0.01) were significantly lower, and the highest KL-6 value (1584 vs 546 U/mL, p=0.02) was significantly higher, in non-survivors during ICU stay. In addition to antiviral treatments and daily proning of the patients, methylprednisolone was administered to all patients to control cytokine storm syndrome following the virus infection. Six patients died from complications, but no patients died of respiratory failure. As a result, none of the patients required ECMO.
Conclusion: We conducted multidisciplinary treatments using a single protocol, including antiviral drugs, methylprednisolone and daily proning, for critically ill COVID-19 patients who required invasive mechanical ventilation. Out protocol was effective in our ICU and may be valuable for determining future therapies of COVID-19.
Keywords
COVID-19; Favipiravir; Methylprednisolone; Mycosis; Proning; Invasive mechanical ventilation
COVID-19 articles, Favipiravir articles, Methylprednisolone articles, Mycosis articles, Proning articles, Invasive mechanical ventilation articles
COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Favipiravir articles Favipiravir Research articles Favipiravir review articles Favipiravir PubMed articles Favipiravir PubMed Central articles Favipiravir 2023 articles Favipiravir 2024 articles Favipiravir Scopus articles Favipiravir impact factor journals Favipiravir Scopus journals Favipiravir PubMed journals Favipiravir medical journals Favipiravir free journals Favipiravir best journals Favipiravir top journals Favipiravir free medical journals Favipiravir famous journals Favipiravir Google Scholar indexed journals Methylprednisolone articles Methylprednisolone Research articles Methylprednisolone review articles Methylprednisolone PubMed articles Methylprednisolone PubMed Central articles Methylprednisolone 2023 articles Methylprednisolone 2024 articles Methylprednisolone Scopus articles Methylprednisolone impact factor journals Methylprednisolone Scopus journals Methylprednisolone PubMed journals Methylprednisolone medical journals Methylprednisolone free journals Methylprednisolone best journals Methylprednisolone top journals Methylprednisolone free medical journals Methylprednisolone famous journals Methylprednisolone Google Scholar indexed journals tumor articles tumor Research articles tumor review articles tumor PubMed articles tumor PubMed Central articles tumor 2023 articles tumor 2024 articles tumor Scopus articles tumor impact factor journals tumor Scopus journals tumor PubMed journals tumor medical journals tumor free journals tumor best journals tumor top journals tumor free medical journals tumor famous journals tumor Google Scholar indexed journals Mycosis articles Mycosis Research articles Mycosis review articles Mycosis PubMed articles Mycosis PubMed Central articles Mycosis 2023 articles Mycosis 2024 articles Mycosis Scopus articles Mycosis impact factor journals Mycosis Scopus journals Mycosis PubMed journals Mycosis medical journals Mycosis free journals Mycosis best journals Mycosis top journals Mycosis free medical journals Mycosis famous journals Mycosis Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals laparoscopy articles laparoscopy Research articles laparoscopy review articles laparoscopy PubMed articles laparoscopy PubMed Central articles laparoscopy 2023 articles laparoscopy 2024 articles laparoscopy Scopus articles laparoscopy impact factor journals laparoscopy Scopus journals laparoscopy PubMed journals laparoscopy medical journals laparoscopy free journals laparoscopy best journals laparoscopy top journals laparoscopy free medical journals laparoscopy famous journals laparoscopy Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals Proning articles Proning Research articles Proning review articles Proning PubMed articles Proning PubMed Central articles Proning 2023 articles Proning 2024 articles Proning Scopus articles Proning impact factor journals Proning Scopus journals Proning PubMed journals Proning medical journals Proning free journals Proning best journals Proning top journals Proning free medical journals Proning famous journals Proning Google Scholar indexed journals Invasive mechanical ventilation articles Invasive mechanical ventilation Research articles Invasive mechanical ventilation review articles Invasive mechanical ventilation PubMed articles Invasive mechanical ventilation PubMed Central articles Invasive mechanical ventilation 2023 articles Invasive mechanical ventilation 2024 articles Invasive mechanical ventilation Scopus articles Invasive mechanical ventilation impact factor journals Invasive mechanical ventilation Scopus journals Invasive mechanical ventilation PubMed journals Invasive mechanical ventilation medical journals Invasive mechanical ventilation free journals Invasive mechanical ventilation best journals Invasive mechanical ventilation top journals Invasive mechanical ventilation free medical journals Invasive mechanical ventilation famous journals Invasive mechanical ventilation Google Scholar indexed journals
Article Details
Abbreviations:
ACE2- Angiotensin converting enzyme 2; APACHE II- Acute Physiology and Chronic Health Evaluation II; ARDS- Acute respiratory distress syndrome; COVID-19- Coronavirus disease 2019; CT- Computed tomography; ECMO- Extracorporeal membrane oxygenation; IQR- interquartile range; KL- Krebs von den Lungen; RT-PCR- Reverse transcriptase polymerase chain reaction; SARS-CoV-2- Severe acute respiratory syndrome coronavirus 2; SOFA- Sequential Organ Failure Assessment; WHO- World Health Organization
1. Introduction
In December 2019, an outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection began in Wuhan city, the capital of Hubei province in China. Since then, the virus has drastically spread across China and the world, and this infection was later designated coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO) [1]. As of May 31, 2020, a total of 5,939,234 cases of SARS-CoV-2 infection have been confirmed, and 367,255 people have died around the world according to WHO [2]. In Japan, the Ministry of Health, Labor and Welfare reported that 16,884 cases of SARS-CoV-2 infection had been detected by May 31, 2020, and 892 patients had died [3].
SARS-CoV-2 infection starts with an interaction with angiotensin converting enzyme 2 (ACE2). The virus binds to and invades cells that express ACE2, such as type II alveolar cells of lung, kidney proximal tubule cells, myocardial cells and enterocytes of the ileum and colon, and causes various organ dysfunctions including lung injury and acute respiratory distress syndrome (ARDS) [4]. Infection of SARS-CoV-2 is thought to secondarily cause macrophage activation syndrome and hemophagocytic lymphohistiocytosis, which are characterized by cytokine storm syndrome accompanied by multiple organ failure [5]. Thus far, there are no specific therapeutic agents for COVID-19. Therefore, to treat severely ill patients with COVID-19, in addition to antiviral therapies, control of hypercytokinemia due to macrophage activation syndrome and hemophagocytic lymphohistiocytosis is also important.
The clinical courses of COVID-19 range from asymptomatic to critically ill, and most patients appear to have a favorable prognosis. However, the prognosis of critically ill patients, especially mechanically ventilated patients, is unclear. Studies from China [6, 7], Italy [8], the US [9] and Canada [10] showed mortality rates ranging from 15% to 62% in critically ill patients. In these studies, many patients were still in the ICUs, and the exact mortality may actually be worse than has been reported. There are no reports, to our knowledge, on the prognosis of patients with invasive mechanical ventilation. It is important not only to prevent the patients’ condition from worsening but also to determine how to treat and save critically ill patients who require invasive mechanical ventilation. We describe the clinical courses of 20 critically ill patients with COVID-19 treated with invasive mechanical ventilation. We believe that the data from this study may be valuable for determining future therapies and intensive care of critically ill patients with COVID-19.
2. Methods
2.1 Participants
Between February 1 and May 31, 2020, at a single urban, academic, critical care center in Osaka, Japan, we provided medical care for severely ill patients with laboratory-confirmed SARS-CoV-2 infection who were admitted to our hospital ICU and required invasive mechanical ventilation. We excluded patients with DNR code status and cardiopulmonary arrest on admission. SARS-CoV-2 infection was defined by a positive result of reverse transcriptase polymerase chain reaction (RT-PCR) detection of a specimen collected on a nasal/oropharyngeal swab or sputum. Our analysis was approved by the ethics committee of Kansai Medical University General Medical Center and followed the principles of the Declaration of Helsinki. No informed consent was required, and researchers analyzed only deidentified data.
2.2 Procedures
We conducted the treatments according to our intensive care COVID-19 management algorithm as follows: 1) Invasive mechanical ventilation (pressure control ventilation mode with target tidal volume of 6-8 mL.kg−1, positive end-expiratory pressure of 5-20 cmH2O, and plateau pressure of less than 30 cmH2O); 2) daily proning as possible; 3) therapeutic agents for COVID-19 (favipiravir, ciclesonide, nafamostat mesilate, and methylprednisolone); 4) prophylactic antibacterial drug; 5) antifungal drug if necessary; 6) catecholamine support if necessary; 7) hemodialysis if necessary; and 8) extracorporeal membrane oxygenation (ECMO) when the patients’ ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (P/F ratio) is continuously below 100.
Twenty patients who required invasive mechanical ventilation were identified (Table 1). The patients were divided into 2 groups (survivors group and non-survivors group), and a comparative review of the two groups was made statistically as described below. General data, including patient characteristics, were recorded retrospectively from the patients’ electronic medical records. Coexisting disorders were obtained from physician’s documentation, and the Acute Physiology and Chronic Health Evaluation II (APACHE II) score and Sequential Organ Failure Assessment (SOFA) score were calculated based on the disorders recorded.
Patients were assessed on admission and during ICU hospitalization. Laboratory data and the P/F ratio were recorded. Laboratory data included complete blood counts and values of plasma D-dimer, serum ferritin, sialylated carbohydrate antigen Krebs von den Lungen (KL-6), hepatic transaminase, creatine kinase, lactate dehydrogenase, and lactate. Treatment outcomes were length of the ICU stay, duration of invasive mechanical ventilation, and complications during hospitalization. All radiological assessments and findings, including chest radiography and computed tomography (CT), were performed at the discretion of the treating physicians.
|
Survivors (n=14) |
Non-survivors (n=6) |
|
|
Hospital transfer, n (%) |
13 (92) |
6 (100) |
|
Age*, median (year, IQR) |
59 (54-66) |
76 (73-77) |
|
Male sex, n (%) |
12 (85) |
4 (66) |
|
Coexisting disease, n (%) |
||
|
Cancer |
2 (14) |
1 (16) |
|
Chronic kidney disease |
1 (7) |
1 (16) |
|
Chronic obstructive pulmonary disease |
2 (14) |
1 (16) |
|
Collagen disease |
0 |
1 (16) |
|
Diabetes mellitus |
9 (64) |
5 (71) |
|
Hyperlipidemia |
3 (21) |
2 (33) |
|
Hypertension |
8 (57) |
1 (16) |
|
Myocardial infarction |
0 |
2 (33) |
|
Parkinson’s disease |
0 |
1 (16) |
|
Stroke |
2 (14) |
0 |
|
No disorder |
2 (14) |
0 |
|
Imaging of computed tomography, n (%) |
14 (100) |
6 (100) |
|
Intubation prior to admission, n (%) |
5 (35) |
2 (33) |
|
Days from onset to intubation, median (IQR) |
9 (8-9) |
9 (5-9) |
|
APACHE II, median (IQR) |
19 (15-21) |
18 (16-20) |
|
SOFA, median (IQR) |
5 (4-7) |
5 (3-6) |
Variables with asterisk indicate significant difference (P value < 0.05) between the 2 groups; APACHE II Acute Physiology and Chronic Health Evaluation II, IQR interquartile range, SOFA Sequential Organ Failure Assessment
Table 1: Baseline characteristics of the patients with invasive mechanical ventilation.
2.3 Statistical analysis
Descriptive statistics were used to summarize the data. Continuous variables are reported as the median and interquartile range (IQR), and categorical variables are summarized as counts and percentages. The Mann-Whitney U test and Fisher’s exact test were used, as appropriate, to compare differences between the 2 groups. A p-value of <0.05 was considered to indicate statistical significance. Statistical analyses were performed with SPSS for Windows version 22.0 software (SPSS, Inc., Chicago, IL, USA).
2.4 Role of the funding source
The funder had no role in study design, data collection, data analysis, data interpretation, or writing of this report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1 Patient characteristics
From March 24 to May 10, 2020, 42 patients with moderate or severe COVID-19 were transported to our ICU, of whom 21 required invasive mechanical ventilation. Seven patients were transferred to our hospital after being intubated at another hospital. Fourteen patients with suspected or confirmed COVID-19 were admitted to our hospital and intubated in our ICU because of worsening oxygenation and severe findings on chest CT. One patient was excluded from this study because he tested positive for SARS-CoV-2 IgG antibodies but negative for SARS-CoV-2 by RT-PCR test. Thus, 20 patients were included in this study who required invasive mechanical ventilation and had laboratory-confirmed COVID-19. All patients were observed until they recovered and left the ICU, were transferred to another hospital without the need for mechanical ventilation, or died. Fourteen patients were alive and recovered from COVID-19 (survivor group), and 6 patients died (non-survivor group) in our hospital. The characteristics of the 20 patients are shown in Table 1. There is no significant difference in each variable except age is younger in survivor group than in non-survivor group.
3.2 Laboratory examinations
We compared the laboratory data of the 14 survivors and 6 non-survivors (Table 2). There was no significant difference between the two groups in the laboratory data on admission, including APACHE II and SOFA scores. However, the lowest lymphocyte count (93 vs 279/μL, p<0.01, Mann-Whitney U test) and the lowest platelet count (12 vs 152 × 103/μL, p<0.01) were significantly lower, and the highest β-D glucan value (368 vs 4.5 pg/mL, p<0.01) and the highest KL-6 value (1584 vs 546 U/mL, p=0.02) were significantly higher, in the non-survivors versus survivors during their ICU stay.
|
Survivors (n=14) |
Non-survivors (n=6) |
|
|
On admission |
||
|
White-cell count (/μL) |
7600 (5925-10850) |
6500 (4650-8200) |
|
Lymphocyte count (/μL) |
605 (471-797) |
455 (145-939) |
|
Platelet count (x103/μL) |
177 (152-210) |
154 (144-163) |
|
Ferritin (ng/mL) |
1635 (812-2572) |
1554 (759-2237) |
|
Sialylated carbohydrate antigen KL-6 (U/mL) |
311 (203-413) |
388 (356-521) |
|
D-dimer (μg/dL) |
1.0 (0.8-2.3) |
2.3 (1.4-4.9) |
|
Aspartate aminotransferase (U/L) |
53 (40-73) |
60 (46-75) |
|
Alanine aminotransferase (U/L) |
36 (20-61) |
24 (20-46) |
|
Creatinine kinase (U/L) |
9.5 (6.0-15.6) |
0.9 (0.7-1.7) |
|
C-reactive protein (mg/dL) |
9.4 (5.9-15.5) |
15.1 (4.5-18.2) |
|
Lactate dehydrogenase (U/L) |
408 (355-561) |
418 (312-516) |
|
Lactate (mg/dL) |
8 (6-10) |
8 (7-8) |
|
During ICU stay |
||
|
Lowest lymphocyte count* (/μL) |
279 (174-387) |
93 (64-106) |
|
Lowest platelet count* (x103/μL) |
152 (133-198) |
12 (11-13) |
|
Highest β-D glucan* (pg/mL) |
4.5 (4.5-8.7) |
368 (77-563) |
|
Highest D-dimer (μg/dL) |
24.7 (7.4-42.6) |
35.1 (27.6-51.8) |
|
Highest lactate dehydrogenase (U/L) |
558 (410-675) |
673 (478-961) |
|
Highest sialylated carbohydrate antigen KL-6* (U/mL) |
546(481-1038) |
1584 (996-2866) |
Results are expressed as median (interquartile range) and analyzed by Mann-Whitney U-test; Variables with asterisk indicate significant difference (P value < 0.05) between the 2 groups; KL-6 Krebs von den Lungen-6
Table 2: Laboratory data.
3.3 Antiviral treatments
We performed treatments for COVID-19 following our own algorithms. Favipiravir, ciclesonide, azithromycin, and nafamostat mesilate, which are expected to have antiviral effects, were administered to all patients. One patient received lopinavir/ritonavir only on the first day because we could not prepare favipiravir, but from the second day, we started favipiravir instead of lopinavir/ritonavir. Hydroxychloroquine sulfate was administered to one patient before transfer to our hospital.
3.4 Anti-inflammatory treatments
We used methylprednisolone to control cytokine storm syndrome following the virus infection. With reference to chest radiographic findings and the severity of hypoxemia, methylprednisolone was started at 125 mg per day or 1000 mg per day. In the non-survivors, the administration period of methylprednisolone was prolonged and the cumulative doses were increased, as shown in Table 3. We also regard tocilizumab as a candidate drug to control cytokine storm syndrome, but we could not start using it in our hospital until later in the study period. Accordingly, tocilizumab was administered to 3 patients before intubation, and 2 of them received tocilizumab before transfer to our hospital.
|
Therapies |
Survivors (n=14) |
Non-survivors (n=6) |
|
Antiviral agents, n (%) |
||
|
Favipiravir |
14 (100) |
6 (100) |
|
Lopinavir, Ritonavir |
1 (7) |
0 |
|
Antibacterial agents, n (%) |
||
|
Azithromycin |
14 (100) |
6 (100) |
|
Ceftriaxone |
5 (35) |
3 (50) |
|
Linezolid |
12 (85) |
6 (100) |
|
Meropenem |
3 (21) |
5 (83) |
|
Sulbactam/Ampicillin |
3 (21) |
1 (16) |
|
Tazobactam/Piperacillin |
14 (100) |
6 (100) |
|
Vancomycin |
0 |
4 (66) |
|
Antimycotic agents, n (%) |
3 (21) |
4 (66) |
|
Catecholamines, n (%) |
9 (64) |
6 (100) |
|
Steroids |
||
|
Ciclesonide, n (%) |
14 (100) |
6 (100) |
|
Methylprednisolone (initial dose of 125 mg), n (%) |
4 (28) |
1 (16) |
|
Methylprednisolone (initial dose of 1000 mg), n (%) |
12 (85) |
5 (83) |
|
Duration of methylprednisolone therapy*, median (days, IQR) |
9 (8-13) |
18 (13-21) |
|
Cumulative dose of methylprednisolone, median (mg, IQR) |
3495 (2718-3866) |
6995 (3933-8773) |
|
Other drugs, number (%) |
||
|
Nafamostat mesilate |
14 (100) |
6 (100) |
|
Hydroxychloroquine sulfate |
1 (7) |
0 |
|
Tocilizumab |
2 (14) |
1 (16) |
|
Respiratory physical therapy |
||
|
Prone position, n (%) |
13 (92.9) |
6 (100) |
|
Duration of prone position*, median (days, IQR) |
7 (5-9) |
21 (17-25) |
|
Outcomes, median (days, IQR) |
||
|
Duration of mechanical ventilation* |
10 (7-12) |
24 (18-26) |
|
Duration of stay in ICU* |
14 (10-16) |
28 (24-30) |
|
Complications, n (%) |
||
|
Acute renal failure* (requiring hemodialysis) |
2 (14) |
6 (100) |
|
Bacteremia |
1 (7) |
1 (16) |
|
Bleeding |
1 (7) |
0 |
|
Liver dysfunction (AST>40 or ALT>40) |
13 (92) |
6 (100) |
|
Mycosis* |
1 (7) |
4 (66) |
|
Transient cardiac myopathy |
1 (7) |
1 (16) |
|
Stroke |
1 (7) |
2 (33) |
Variables with asterisk indicate significant difference (P value < 0.05) between the 2 groups; ALT alanine aminotransferase, AST aspartate aminotransferase, IQR interquartile range.
Table 3: Therapies, outcomes and complications.
3.5 Respiratory therapies
We treated the 20 critically ill patients with therapeutic agents and invasive mechanical ventilation with daily prone positioning according to our own algorithm. Six of the 20 patients died from complications, but no patients died of respiratory failure. Our algorithm includes ECMO when the patient’s P/F ratio is continuously below 100, but daily proning and methylprednisolone therapy increased the P/F ratio above 100 and accordingly, we did not use ECMO in any of the patients.
The transition of mechanical ventilation parameters (lowest P/F ratio, plateau pressure, and positive end-expiratory pressure) from day 1 to 10 is shown in Figure 1. There were no significant differences in each parameter between the survivor group and non-survivor group. Nineteen patients were placed in a complete prone position for 16 consecutive hours every day until their oxygenation improved. As Table 3 shows, the intubation period of the survivors was 10 (IQR: 7-12) days, duration of stay in ICU was 14 (IQR: 10-16) days, and proning was performed for 7 (IQR: 5-9) days. Each period above was significantly shorter than that of the non-survivors (p<0.01). No patients received neuromuscular blockade except at the time of intubation. Tracheostomy was performed in only one survivor.
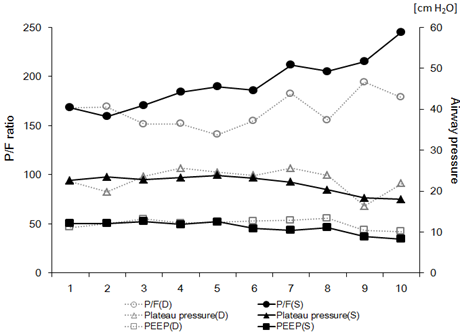
Figure 1: Daily trends of PaO2/FIO2 (P/F) ratio (circle), plateau pressure (triangle), and positive end-expiratory pressure (PEEP, square) during mechanical ventilation are indicated as sequential line graphs. Solid lines indicated data from the survival (S) group, and dotted lines indicate data from the non-survivor (D) group. The P/F ratio is scaled on the left vertical axis, and plateau pressure and PEEP are scaled on the right vertical axis.
3.6 Antibiotic treatments
Antibiotics were given prophylactically in all cases. To prevent nosocomial infection of the virus, bacterial culture tests other than blood culture were avoided in our hospital. Therefore, broad-spectrum antimicrobial (e.g., tazobactam/piperacillin) and anti-MRSA (e.g., linezolid) drugs were used. The incidence of fungal infection, which is considered to be a catheter-related bloodstream infection, was significantly higher in the non-survivors. Antifungal drugs were administered to 4 of the 6 non-survivors diagnosed as having mycosis by blood culture or increasing serum levels of β-D glucan. (66% vs 7%, p<0.01, Fisher’s exact test).
3.7 Complications
Complications are listed in Table 3. Acute renal failure requiring hemodialysis (100% vs 14%, p<0.01, Fisher’s exact test) and mycosis (66% vs 7%, p=0.01) occurred significantly more frequently in the non-survivors. Mycosis affected the prognosis of the non-survivors.
3.8 Representative case
The treatment course of a representative patient is illustrated in Figure 2.
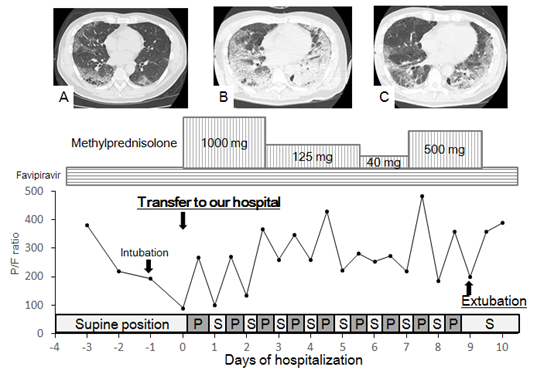
Figure 2: Treatment course in a 56 year-old man with pulmonary emphysema. His chief complaints were high fever, rhinorrhea, and fatigue. Polymerase chain reaction testing at a public health department was positive for COVID-19. He was treated for pneumonia caused by COVID-19 in a local hospital for 4 days. Administration of favipiravir did not stop the deterioration of his respiratory insufficiency, and he was subsequently intubated and placed on mechanical ventilation. He was then transferred to our hospital for treatment of severe acute respiratory distress syndrome. On admission, his P/F ratio was 89.5, and thoracic CT revealed a diffuse ground-glass pattern with multiple bulla and blebs (A). Methylprednisolone pulse therapy and prone positioning began on day 1 in addition to administration of favipiravir and antibiotics. During the prone position, his P/F ratio tended to improve. On day 5, his P/F ratio transiently worsened with expansion of ground-glass consolidations on CT (B). After additional half-pulse therapy with methylprednisolone for 3 days, his P/F ratio rose to >400. Finally, he could be extubated on day 10, and the diffuse ground-glass pattern on thoracic CT began to fade away (C). P prone, S supine.
4. Discussion
This single-center case series described 20 consecutive patients admitted to ICU during the first wave of the SARS-CoV-2 infection in Japan who required invasive mechanical ventilation. Our hospital is located in Osaka, Japan, with a population of 8.8 million. In Osaka, we expected to have 300 intubated patients during the peak period, and our hospital had prepared 20 beds for severe COVID-19 in the ICU. In actuality, the maximum number of intubated patients treated at the same time was 10, and we were able to provide sufficient intensive care in all cases without the collapse of medical services. A treatment protocol was prepared for critically ill patients after referring to the papers based on experiences with Wuhan patients and those from the cruise ship Diamond Princess. We used favipiravir, nafamostat mesilate, ciclesonide, and azithromycin in combination as antiviral drugs. If oxygenation could not be maintained by usual invasive mechanical ventilation and antiviral therapy, we administered methylprednisolone according to ARDS treatment. We aggressively performed daily proning because the COVID-19 pneumonia tended to have dorsal peripheral and subpleural lesions.
The mortality rate of patients with COVID-19 who require invasive mechanical ventilation is not clear. In an early report from Wuhan, all 25 patients who required invasive mechanical ventilation died [11]. The mortality rates of patients requiring treatment in an ICU were reported from Washington State in the US [9], Wuhan in China [7], and the Lombardy region of Italy [8], however, these reports did not mention the exact mortality rate of patients received invasive mechanical ventilation. A large multicenter study reported on 5700 patients requiring hospitalization for COVID-19 in New York [12]. Among 2634 patients who were discharged or died at the study endpoint, 88.1% of 320 patients who received invasive mechanical ventilation died. However, 831 intubated patients remained in hospital, and the prognosis of the intubated patients cannot be determined. In our small series, the mortality rate of the patients receiving invasive mechanical ventilation was 30%. The reason for the lower mortality rate than those in the past reports is that medical services did not collapse in Japan, and the combination of antiviral, methylprednisolone, and proning therapies was effective. Especially, methylprednisolone and proning therapies effectively improved the patients’ respiratory condition.
Our most anticipated antiviral drug is favipiravir, which is an RNA polymerase inhibitor developed as an anti-influenza drug and has been reported to have growth inhibitory activity against SARS-CoV-2 [13]. In a non-randomized study conducted in China, patients receiving favipiravir and interferon α had a faster negative PCR result for SARS-CoV-2 and faster improvement of CT findings than those receiving lopinavir/ritonavir and interferon α [14]. We administered favipiravir from the first day in all patients at a dose of 1800 mg on the first day and 800 mg on the second and subsequent days. We used favipiravir for 2 weeks or until the patients received a negative PCR result for SARS-CoV-2 or serious side effects occurred. In Japan, an observational study on COVID-19 patients treated with favipiravir was started in February 2020, and the results are anticipated.
Nafamostat mesylate is one of the serine protease inhibitors and has been used in Japan as an anticoagulant during hemodialysis and as an agent for disseminated intravascular coagulation. It inhibits the process of membrane fusion of SARS-CoV-2 and cells in vitro and is expected to have an antiviral effect on SARS-CoV-2 [5]. In addition, COVID-19 is reported to be associated with thrombosis, and prophylactic administration of anticoagulants is recommended in all hospitalized patients [15]. In anticipation of its antiviral and anticoagulant effects, we administered nafamostat mesylate for 1 week in all patients. Ciclesonide is a safe drug widely used as an inhaled steroid for bronchial asthma and is expected to have local anti-inflammatory and viral replication inhibitory effects on COVID-19 [16]. We administered ciclesonide 800 μg daily from a reservoir connected to the ventilator circuit for 2 weeks in all patients.
Some patients struck severely by SARS-CoV-2 might suffer cytokine storm syndrome that can lead to multi-organ failure including ARDS [5]. Conquering the cytokine storm is one of the essential strategies to save severely ill patients with ARDS from SARS-CoV-2 infection, and corticosteroid is a candidate drug to control cytokine storm syndrome after SARS-CoV-2 infection. However, there is insufficient evidence to recommend corticosteroid treatment according to the current interim guidance from WHO [17]. They concluded that administration of corticosteroid for acute severe lung injury from SARS-CoV and MARS-CoV inhibits immune response and virus clearance, making the patient vulnerable to side effects such as psychosis, viremia, diabetes, avascular necrosis and secondary bacterial or fungal infection. Meanwhile, Shang et al. offered a negative perspective on use of corticosteroids for lung injury caused by SARS-CoV-2 because the evidence supporting the effectiveness of corticosteroids came mostly from observational studies and was insufficient to conclude its usefulness [18]. Chen et al. supportively reported that proper use of corticosteroids reduced mortality and shortened the length of the hospital stay in critically ill patients without secondary infection and other complications [19]. Wu et al. reported that administration of methylprednisolone to patients with severe COVID-19 was effective and reduced the risk of death from ARDS [20]. In reference to the standard therapy of methylprednisolone for interstitial pneumonia [21], we used a new protocol of methylprednisolone therapy as described above. With the use of this protocol, all patients under the age of 70 survived from ARDS. Unfortunately, our protocol had a limited effect on elderly patients. More than half of the patients over age 70 died from complications due to long-term use of methylprednisolone including brain infarction, upper GI bleeding and secondary bacterial and fungal infections. Although the administration of methylprednisolone for severe pneumonia caused by SARS-CoV-2 is controversial, our protocol exhibited a certain effect for limited cases.
Tocilizumab, an anti-IL-6 receptor antibody, has been approved for the cytokine release syndrome associated with CAR-T (chimeric antigen receptor-modified T cell) therapy [22]. Tocilizumab, along with steroids, is expected to control cytokine storm syndrome in COVID-19 [23]. In our case series, tocilizumab was administered to two patients prior to intubation, but it failed to improve oxygenation, and these patients required additional methylprednisolone administration. Thus, it was difficult to assess the effect of tocilizumab in the present study.
In intubated patients with severe ARDS, early and prolonged proning improves oxygenation and decreases mortality [24, 25]. In adult patients with severe ARDS, prone ventilation for 12-16 hours per day is recommended also in COVID-19 [26]. Among the treatment methods introduced for the management of ARDS patients, proning can be used as an adjuvant therapy for improving respiratory function in these patients. Proning should not be a desperate final attempt but should be considered in the early stages of respiratory therapy [27] as the available evidence suggests that the early application of prolonged ventilation in the prone position decreases 28- and 90-day mortality in patients with severe ARDS [17]. In our case series, 19 of the 20 patients were placed in a completely prone position with mechanical ventilation for 16 consecutive hours every day, which continued until improvement of respiratory condition or a life-threatening reason caused its cessation. The time from intubation to the first proning procedure was a median 1.8 (range 1-9) days, and the median number of procedures per person was 8.9 (range 0-24). Patients ventilated in the prone position face risks such as accidental removal of the tracheal tube, bending or pulling of catheters, pressure sores, facial edema, gastroesophageal reflux, and hypersalivation [28]. Several of our patients developed facial pressure sores, but no life-threatening complications occurred.
Although the number of cases is small, the combination of antiviral and anti-inflammatory therapies and respiratory physiotherapy according to a single protocol were effective and resulted in a mortality rate of 30%. Especially, combination of methylprednisolone and proning was most effective treatment in our protocol and no patients needed ECMO. It will be necessary to analyze more cases and develop standard treatments that improve the prognosis of severely ill patients with COVID-19.
5. Conclusion
We conducted multidisciplinary treatments using a single protocol, including antiviral drugs, methylprednisolone and daily proning, for critically ill COVID-19 patients who required invasive mechanical ventilation. Out protocol was effective in our ICU and may be valuable for determining future therapies of COVID-19.
Acknowledgements
Keisuke Tamagaki, Yasutaka Okamoto, Hitoshi Nakano, Hiroto Okubata and Yumiko Miyano contributed to patients treatment and data collection.
Authors’ Contributions
HS and KY conceived, designed, and coordinated the study. HS wrote the first draft of this manuscript. SM, SK, SM, and HI contributed to the collection, analysis, and interpretation of the data and assisted in the preparation of the manuscript. DW, TY, FS and YN helped to draft the manuscript and revised it critically for important intellectual content. YK contributed to the final approval of the version to be published. All authors read and approved the final manuscript.
Funding
There are no sources of funding for this case report.
Availability of Data and Materials
This case report only contains clinical data from the medical records of the patient reported herein. The data will be made available upon request.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Written informed consent was waived by the Ethics Committee due to the retrospective nature of this study and emergency circumstances of this infectious disease
Competing Interests
The authors declare that they have competing interests.
References
- World Health Organization. Novel coronavirus-China. http://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/ (2020).
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/ (2020).
- Ministry of Health, Labour and Welfare. https://www.mhlw.go.jp/stf/newpage_11621.html (2020).
- Zou X, Chen K, Zou J, et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med 14 (2020): 185-192.
- Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395 (2020): 1033-1034.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395 (2020): 497-506.
- Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 8 (2020): 475-481.
- Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 323 (2020): 1574-1581.
- Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 323 (2020): 1612-1614.
- Mitra AR, Fergusson NA, Lloyd-Smith E, et al. Baseline characteristics and outcomes of patients with COVID-19 admitted to intensive care units in Vancouver, Canada: a case series. CMAJ (2020).
- Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 46 (2020): 846-848.
- Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 323 (2020): 2052-2059.
- Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30 (2020): 269-271.
- Cai Q, Yang M, Liu D, et al. experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering (Beijing). https://doi.org/10.1016/j.eng.2020.03.007. (2020).
- Barnes GD, Burnett A, Allen A, et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis 50 (2020): 72-81.
- Iwabuchi K, Yoshie K, Kurakami Y, et al. Therapeutic potential of ciclesonide inhalation for COVID-19 pneumonia: report of three cases. J Infect Chemother 26 (2020): 625-632.
- Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 395 (2020): 473-475.
- Shang L, Zhao J, Hu Y, et al. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet 395 (2020): 683-684.
- Chen RC, Tang XP, Tan SY, et al. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest 129 (2006): 1441-1452.
- Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med (2020).
- Arai T, Tachibana K, Sugimoto C, et al. High-dose prednisolone after intravenous methylprednisolone improves prognosis of acute exacerbation in idiopathic interstitial pneumonias. Respirology 22 (2017): 1363-1370.
- Maude SL, Barrett D, Teachey DT, et al. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J 20 (2014): 119-122.
- Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA 117 (2020): 10970-10975.
- Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone position for acute respiratory distress syndrome. a systematic review and meta-analysis. Ann Am Thorac Soc 14 (2017): S280-S288.
- Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 368 (2013): 2159-2168.
- World Health Organization. Clinical management of COVID-19. https://www.who.int/publications/i/item/clinical-management-of-covid-19. (2020).
- Xie H, Zhou ZG, Jin W, et al. Ventilator management for acute respiratory distress syndrome associated with avian influenza A (H7N9) virus infection: A case series. World J Emerg Med 9 (2018): 118-124.
- McCormick J, Blackwood B. Nursing the ARDS patient in the prone position: the experience of qualified ICU nurses. Intensive Crit Care Nurs 17 (2001): 331-340.
