The Effect of Early Mild Permissive Hyperlactatemia Strategy on Patients with Acute Intracranial Hypertension Induced by Spontaneous Intracerebral Hemorrhage
Article Information
Chen Sheng-long1, Wang Zhong-hua2, Liu Xin-qiang1, Yang Ren-qiang1, Zeng Hong-ke1, Chai Yun-fei3*
1Department of Critical Care Medicine, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, Guangdong, P. R. China
2Department of gerontological critical care medicine, Guangdong Provincial People’s Hospital/Guangdong Academy of Medical Sciences/Guangdong Provincial Geriatrics Institute, Guangzhou, Guangdong, China
3Anesthesiology Department of Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, Guangdong, P. R. China
*Corresponding Authors: Chai Yun-fei, Anesthesiology Department of Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, 510080, Guangdong, P. R. China
Received: 27 September 2019; Accepted: 14 October 2019; Published: 21 October 2019
Citation:
Chen Sheng-long, Wang Zhong-hua, Liu Xin-qiang, Yang Ren-qiang, Zeng, Hong-ke, Chai, Yun-fei. The Effect of Early Mild Permissive Hyperlactatemia Strategy on Patients with Acute Intracranial Hypertension Induced by Spontaneous Intracerebral Hemorrhage. Journal of Surgery and Research 2 (2019): 249-257.
View / Download Pdf Share at FacebookAbstract
Background: Dehydrating therapy to relieve brain edema is one of main methods to treat acute intracranial hypertension (ICH) after spontaneous cerebral hemorrhage. However, little is known regarding how much the negative fluid balance should be and indications of optimal fluid status. This study was to investigate whether mild permissive hyperlactatemia within initial 3-day is an available indicator guiding fluid management in patients with acute ICH.
Methods: Adult patients of spontaneous cerebral hemorrhage with ICH admitted to the ICU and receiving dehydrating agents were retrospectively analyzed. The daily serum lactate concentration was collected over the first 3 days after admission. According to level of serum lactate, patients were divided into three groups (Normal-lactate group: <2 mmol/L, Mild-lactate group: 2-3.5 mmol/L, High-lactate group: >3.5 mmol/L respectively). And the main endpoint of this study was the 30-day mortality following ICU admission.
Results: The mortality rate at 30 days in the high-lactate group patients was significantly higher than that in the other two groups (normal-group and mild-lactate group), (26.4% vs (16.5% and 10.9%), P<0.01). Furthermore, compared with the normal-lactate group, the 30-day mortality rate was significantly lower (10.9% vs 16.5%, P<0.01), the Glasgow Coma (GCS) Score at 7-day was better (10.8 ± 3.8 vs 8.3 ± 2.4, P=0.021), and the length of ICU stay was shorter (14.2 ± 7.3 vs 16.7 ± 8.6 d, P=0.015) in the mild-lactate group.
Conclusions: In this retrospective cohort, mild permissive hyperlactatemia (2-3.5 mmol/L) over the first 3 days as a reference range that can be used to optimize fluid management in patients with ICH who accept dehydrating therapy.
Keywords
Intracerebral Hemorrhage, Intracranial Hypertension, Hyperlactatemia
Intracerebral Hemorrhage articles, Intracranial Hypertension articles, Hyperlactatemia articles
Intracerebral Hemorrhage articles Intracerebral Hemorrhage Research articles Intracerebral Hemorrhage review articles Intracerebral Hemorrhage PubMed articles Intracerebral Hemorrhage PubMed Central articles Intracerebral Hemorrhage 2023 articles Intracerebral Hemorrhage 2024 articles Intracerebral Hemorrhage Scopus articles Intracerebral Hemorrhage impact factor journals Intracerebral Hemorrhage Scopus journals Intracerebral Hemorrhage PubMed journals Intracerebral Hemorrhage medical journals Intracerebral Hemorrhage free journals Intracerebral Hemorrhage best journals Intracerebral Hemorrhage top journals Intracerebral Hemorrhage free medical journals Intracerebral Hemorrhage famous journals Intracerebral Hemorrhage Google Scholar indexed journals Intracranial Hypertension articles Intracranial Hypertension Research articles Intracranial Hypertension review articles Intracranial Hypertension PubMed articles Intracranial Hypertension PubMed Central articles Intracranial Hypertension 2023 articles Intracranial Hypertension 2024 articles Intracranial Hypertension Scopus articles Intracranial Hypertension impact factor journals Intracranial Hypertension Scopus journals Intracranial Hypertension PubMed journals Intracranial Hypertension medical journals Intracranial Hypertension free journals Intracranial Hypertension best journals Intracranial Hypertension top journals Intracranial Hypertension free medical journals Intracranial Hypertension famous journals Intracranial Hypertension Google Scholar indexed journals Hyperlactatemia articles Hyperlactatemia Research articles Hyperlactatemia review articles Hyperlactatemia PubMed articles Hyperlactatemia PubMed Central articles Hyperlactatemia 2023 articles Hyperlactatemia 2024 articles Hyperlactatemia Scopus articles Hyperlactatemia impact factor journals Hyperlactatemia Scopus journals Hyperlactatemia PubMed journals Hyperlactatemia medical journals Hyperlactatemia free journals Hyperlactatemia best journals Hyperlactatemia top journals Hyperlactatemia free medical journals Hyperlactatemia famous journals Hyperlactatemia Google Scholar indexed journals dehydrating therapy articles dehydrating therapy Research articles dehydrating therapy review articles dehydrating therapy PubMed articles dehydrating therapy PubMed Central articles dehydrating therapy 2023 articles dehydrating therapy 2024 articles dehydrating therapy Scopus articles dehydrating therapy impact factor journals dehydrating therapy Scopus journals dehydrating therapy PubMed journals dehydrating therapy medical journals dehydrating therapy free journals dehydrating therapy best journals dehydrating therapy top journals dehydrating therapy free medical journals dehydrating therapy famous journals dehydrating therapy Google Scholar indexed journals hyperlactatemia articles hyperlactatemia Research articles hyperlactatemia review articles hyperlactatemia PubMed articles hyperlactatemia PubMed Central articles hyperlactatemia 2023 articles hyperlactatemia 2024 articles hyperlactatemia Scopus articles hyperlactatemia impact factor journals hyperlactatemia Scopus journals hyperlactatemia PubMed journals hyperlactatemia medical journals hyperlactatemia free journals hyperlactatemia best journals hyperlactatemia top journals hyperlactatemia free medical journals hyperlactatemia famous journals hyperlactatemia Google Scholar indexed journals acute intracranial hypertension articles acute intracranial hypertension Research articles acute intracranial hypertension review articles acute intracranial hypertension PubMed articles acute intracranial hypertension PubMed Central articles acute intracranial hypertension 2023 articles acute intracranial hypertension 2024 articles acute intracranial hypertension Scopus articles acute intracranial hypertension impact factor journals acute intracranial hypertension Scopus journals acute intracranial hypertension PubMed journals acute intracranial hypertension medical journals acute intracranial hypertension free journals acute intracranial hypertension best journals acute intracranial hypertension top journals acute intracranial hypertension free medical journals acute intracranial hypertension famous journals acute intracranial hypertension Google Scholar indexed journals optimize fluid management articles optimize fluid management Research articles optimize fluid management review articles optimize fluid management PubMed articles optimize fluid management PubMed Central articles optimize fluid management 2023 articles optimize fluid management 2024 articles optimize fluid management Scopus articles optimize fluid management impact factor journals optimize fluid management Scopus journals optimize fluid management PubMed journals optimize fluid management medical journals optimize fluid management free journals optimize fluid management best journals optimize fluid management top journals optimize fluid management free medical journals optimize fluid management famous journals optimize fluid management Google Scholar indexed journals systemic hypoperfusion articles systemic hypoperfusion Research articles systemic hypoperfusion review articles systemic hypoperfusion PubMed articles systemic hypoperfusion PubMed Central articles systemic hypoperfusion 2023 articles systemic hypoperfusion 2024 articles systemic hypoperfusion Scopus articles systemic hypoperfusion impact factor journals systemic hypoperfusion Scopus journals systemic hypoperfusion PubMed journals systemic hypoperfusion medical journals systemic hypoperfusion free journals systemic hypoperfusion best journals systemic hypoperfusion top journals systemic hypoperfusion free medical journals systemic hypoperfusion famous journals systemic hypoperfusion Google Scholar indexed journals serum lactate articles serum lactate Research articles serum lactate review articles serum lactate PubMed articles serum lactate PubMed Central articles serum lactate 2023 articles serum lactate 2024 articles serum lactate Scopus articles serum lactate impact factor journals serum lactate Scopus journals serum lactate PubMed journals serum lactate medical journals serum lactate free journals serum lactate best journals serum lactate top journals serum lactate free medical journals serum lactate famous journals serum lactate Google Scholar indexed journals inadequate tissue perfusion articles inadequate tissue perfusion Research articles inadequate tissue perfusion review articles inadequate tissue perfusion PubMed articles inadequate tissue perfusion PubMed Central articles inadequate tissue perfusion 2023 articles inadequate tissue perfusion 2024 articles inadequate tissue perfusion Scopus articles inadequate tissue perfusion impact factor journals inadequate tissue perfusion Scopus journals inadequate tissue perfusion PubMed journals inadequate tissue perfusion medical journals inadequate tissue perfusion free journals inadequate tissue perfusion best journals inadequate tissue perfusion top journals inadequate tissue perfusion free medical journals inadequate tissue perfusion famous journals inadequate tissue perfusion Google Scholar indexed journals
Article Details
1. Introduction
Acute intracranial hypertension is a life-threatening complication in patients with spontaneous intracerebral hemorrhage [1]. One of the main methods to treat acute intracranial hypertension (ICH) is dehydrating therapy, a method that actually reduces blood volume appropriately and maintains minimum fluid status to decrease intracranial pressure as much as possible [1, 2]. However, little is known about how much the negative fluid balance should be and the indicators for optimizing fluid management of ICH treatment. Lactate, a substrate of carbohydrate metabolism, is formed from pyruvate in the glycolysis pathway [3]. When the oxygen supply is scarce or interrupted, anaerobic metabolism predominates and serum lactate levels rise [4]. Elevation of serum lactate has been considered to be strong indicator of hypovolemia [5]. However, recent studies have shown that hyperlactatemia appeared in 40%-62% patients after neurosurgery, but didn’t correlate to systemic hypoperfusion in craniotomy patients [6]. Of those patients, elevated lactate was not associated with systemic complications such as myocardial infarction or mortality. Higher metabolic rate and oxygen demand of the brain may be explained for elevation of serum lactate in patients with spontaneous intracerebral hemorrhage [6]. Moreover, recent evidence suggests that the neurons may utilize lactate as an energy substrate [7]. Thus, we believe that the mild elevation of serum lactate is reasonable and beneficial for the patients with ICH who undergoing dehydration treatment. Our hypothesis is that early permissive mild elevation of serum lactate in those patients receiving dehydrating therapy could be a guiding goal to optimize fluid management.
2. Methods
2.1 Study design
We performed a retrospective cohort analysis of consecutive patients admitted to the Department of Critical Care Medicine, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, P.R. China, between January 2016 and July 2019. The study protocol was approved by the Institute ethics committee and informed consent was waived as stated by the application. Exclusion criteria were: age below18, Comorbid conditions included preexisting liver disease, heart failure, kidney disease, and sepsis. Clinical and laboratory data at ICU were extracted, including age, sex, Acute Physiology and Chronic Health Evaluation (APACHEII) score, Glasgow Coma Score (GCS), pre-existing diseases, arterial pH, PaO2, creatinine, total bilirubin and lactate values. In addition, data of treatment during the initial 3 days of ICU stay were obtained, including types of dehydrating agent (including albumin), fluid balance (input and output), decompressive craniectomy and mechanical ventilation. We also collected the length of ICU stay and 30-day mortality. All the patients were divided into three groups (normal-lactate group: <2 mmol/L, mild-lactate group: 2-3.5 mmol/L, high-lactate group: >3.5 mmol/L respectively) according to the daily serum lactate level over the first 3-day after admission.
2.2 Statistical analysis
Data are the mean ± standard deviation, percentages, or medians (interquartile range). The unpaired t-test or Mann-Whitney test was used for unpaired comparisons as appropriate. The Fisher’s exact or chi-square test was used to examine differences between categorical variables. The cumulative incidence of 30-day mortality was estimated according to the Kaplan-Meier method, and the log-rank statistic was used for comparisons. The Cox proportional hazards regression model was used to determine which variables were related significantly to death during the follow-up. All statistical data were analysed by licensed IBM SPSS Statistics version 24 for Windows (SPSS Inc, Chicago, IL, USA).
3. Results
3.1 Cohort description
Figure 1 demonstrates how cases were selected. A total of 390 patients were identified using the screening criteria. There were 41 patients death over the first 3-day after admission. Patients with sepsis (n=11), cirrhosis (n=6), hepatitis (n=12), end stage renal disease (ESRD) or chronic kidney disease (CKD) (n=34), missing documentation (n=6), no lactate labs drawn (n=4) were excluded, and final 273 patients were analyzed. Table 1 shows the baseline characteristics of the three group patients. Although there was no significant difference of the fluid balance among the three groups, the amount of daily fluid input in the Mil-lactate group was the least (P<0.01), and the Normal-lactate and High-lactate group was similar. The serum lactate, creatinine, APACHEIIscore, GCS score, and types of dehydrating agents at admission among the three group patients were not significant differences.
3.2 Outcomes in patients with different goals of daily serum lactate level over the first 3-day
Table 2 compares clinical outcomes in the three groups with different goals of daily serum lactate level over the first 3-day. The total 30-day mortality rate of this cohort study was 17.4% (48/273). Compared with the High-lactate group patients, the 30-day mortality of patients in both Normal-lactate and Mild-lactate groups were significantly lower (26.4% vs (16.5% and 10.9%), P<0.01). Similar results of the length of ICU stay were shown among the three group patients (19.8 ± 9.1 vs (16.7 ± 8.6 and 14.2 ± 7.3), P=0.015). Moreover, the 30-day mortality in Mild-lactate group was further deceased when compared with the Normal-lactate group patients (10.9% vs 16.5%, P<0.01). Similar results of the length of ICU stay among patients in the Normal-lactate group, Mild-lactate group, and High-lactate group were displayed respectively (16.9 ± 10.6, 16.7 ± 8.6, and 4.2 ± 7.3, P=0.015). The improvement of GCS score at day-7 was the highest in the Mild-lactate group (Mild-lactate:10.8 ± 3.8, Normal-lactate: 8.3 ± 2.4, High-lactate: 6.6 ± 3.6, P=0.021 ).
3.3 Predictors associated with 30-day mortality
In the univariate Cox hazard analysis of 30-day mortality, age (p=0.032), decompressive craniectomy (P<0.001), serum Na+ level (P=0.011), mechanical ventilation (P<0.001), serum lactate (P<0.001), APACHEIIscore (P=0.002), GCS score (P=0.001), daily positive fluid balance (P<0.001), were found to be significant factors. The multivariate Cox hazard analysis of these factors revealed that decompressive craniectomy (hazard ratio (HR)=0.866, 95% confidence interval (CI) 0.236-2.300, P<0.001), serum Na+ level (HR=3.55, 95%CI 2.161-7.910, P=0.011), serum lactate (HR=2.866, 95%CI 1.382-10.227, P<0.001), APACHEIIscore (HR=4.266, 95%CI 3.161-15.910, P=0.002), GCS score (HR=4.34, 95%CI 2.019-12.981, P=0.001), and daily positive fluid balance (HR=6.886, 95%CI 3.161-16.910, P<0.001) were significant predictive factors for the occurrence of 30-day death.
3.4 Kaplan-Meier survival analysis
Figure 2 shows that there were 15, 10 and 15 patients death in the Normal-lactate, Mild-lactate and High-lactate group, respectively during 30-day after admission. Of those deaths, 8 (53.3%) patients in Normal-lactate group, 3 (30%) patients in the Mild-lactate group and 13 (56.5%) in the High-lactate group were focused within the first 9 days. Kaplan-Meier analysis indicated that the 30-day survival rates were significantly lower in patients in the Mild-lactate group than in those in both Normal-lactate and High-lactate groups (P=0.010, log-rank test).
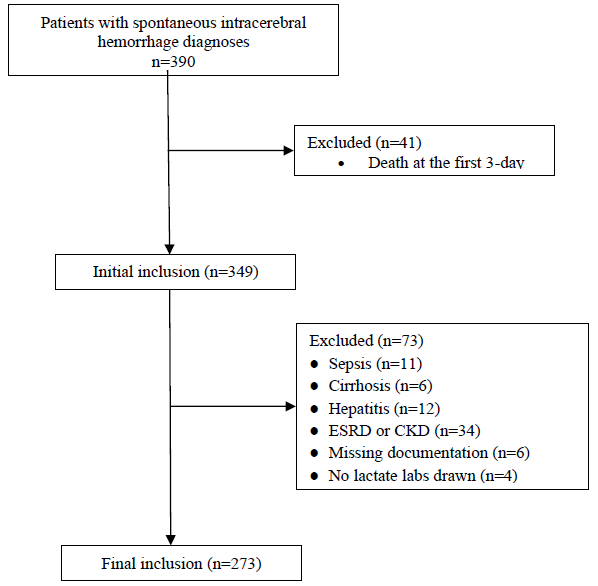
Figure 1: Retrospective case selection. ESRD indicates endstage renal disease; CKD-chronic kidney disease.
|
Variable |
All patients N=276 |
Normal-lactatea n=91 |
Mild-lactatea n=98 |
High-lactatea group n=87 |
P valueb |
|
Age (y) |
53.9 ± 10.6 |
53.8 ± 11.7 |
54.8 ± 12.1 |
52.9 ± 10.7 |
0.23 |
|
Female sex?n (%) |
135 (48.9) |
45 (49.4) |
47 (47.9) |
43 (49.4) |
0.21 |
|
APACHEIIscore |
23.7 ± 10.9 |
22.6 ± 8.9 |
24.6 ± 9.7 |
23.9 ± 9.5 |
0.19 |
|
GCS score |
6.9 ± 3.1 |
7.1 ± 2.9 |
6.8 ± 2.4 |
6.9 ± 2.6 |
0.09 |
|
Diabetes, n (%) |
68 (24.6) |
26 (28.6) |
19 (19.4) |
23 (26.4) |
0.02 |
|
Hypertension, n (%) |
120 (43.4) |
39 (42.8) |
42 (42.8) |
39 (44.8) |
0.06 |
|
Initial MAP, mmHg, |
179.1 ± 43.7 |
178.6 ± 42.9 |
180.6 ± 33.7 |
176.6 ± 38.9 |
0.42 |
|
Mechanical ventilation, n (%) |
164 (59.4) |
56 (61.5) |
59 (60.2) |
49 (56.32) |
0.04 |
|
decompressive craniectomy n (%) |
71 (25.1) |
23 (25.3) |
26 (26.5) |
22 (25.3) |
0.91 |
|
Baseline laboratory values |
|||||
|
Arterial pH |
7.40 ± 2.4 |
7.39 ± 2.1 |
7.37 ± 2.1 |
7.41 ± 1.8 |
0.52 |
|
PaO2/FiO2 |
309.3 ± 64.2 |
298.9 ± 54.1 |
321.2 ± 59.9 |
309.4 ± 60.1 |
0.39 |
|
Serum Na+, (mmol/l) |
142 ± 29.3 |
139.2 ± 24.1 |
140.3 ± 27.2 |
147.6 ± 25.9 |
0.01 |
|
Haemoglobin, g/L |
107.7 ± 26.9 |
110.6 ± 22.3 |
109.6 ± 21.9 |
102.6 ± 23.6 |
0.23 |
|
Creatinine, μmol/L |
100.6 ± 39.9 |
98.9 ± 29.3 |
101.6 ± 30.9 |
103.6 ± 32.4 |
0.65 |
|
Total bilirubin, μmol/L |
20.3 ± 10.4 |
20.1 ± 9.3 |
19.1 ± 8.4 |
22.1 ± 9.8 |
0.78 |
|
Lactate, mmol/L |
1.8 (0.8-4.0) |
1.9 (1.1-3.5) |
2.0 (0.9-3.6) |
2.1 (1.0-3.9) |
0.76 |
|
Fluid data over the first 3-day, L |
|||||
|
daily fluid input |
3.6 (2.0-7.6) |
3.9 (2.1-5.6) |
2.9 (2.0-4.2) |
4.1 (3.0-6.2) |
<0.01 |
|
daily fluid output |
4.2 (2.9-7.8) |
4.1 (2.3-5.9) |
3.5 (2.3-5.2) |
4.9 (3.1-6.1) |
<0.01 |
|
Dehydrating agent |
|||||
|
Albumin use, n (%) |
100 (36.2) |
31 (34.1) |
35 (35.7) |
34 (39.1) |
0.07 |
|
Mannitol use, n (%) |
240 (87.9) |
80 (87.9) |
84 (85.7) |
76 (87.3) |
0.87 |
|
Hypertonic saline, n (%) |
36 (13.1) |
12 (13.2) |
14 (12.3) |
10 (11.5) |
0.91 |
|
Furosemide, n (%) |
103 (37.7) |
34 (37.4) |
36 (36.7) |
33 (37.9) |
0.02 |
Continuous variables were expressed as mean ± SD or median (25th percentile-75th percentile). Categorical variables were expressed as n (%). APACHEII, acute physiology and chronic health evaluationIIscore; GCS, Glasgow Coma Score. aNormal-lactate group was defined as daily serum lactate level <2 mmol/l within 3 days after admission, and Mild-lactate group was 2-3.5 mmol/l and High-lactate group was >3.5mmol/l respectively.) according to the serum lactate level after admission. bP for global comparisons among groups by Kruskal-Wallis and chi-squared tests for continuous and categorical variables, respectively.
Table 1: Characteristics of Patient Cohort, Grouped by Different Level of Daily Serum Lactate within the first 3-day.
|
Outcome |
All patients N=276 |
Normal-lactate n=91 |
Mild-lactate n=98 |
High-lactate n=87 |
P value |
|
30-day mortality, n(%) |
48 (17.4) |
15 (16.5) |
10 (10.9) |
23 (26.4) |
<0.001 |
|
GCS score at day-7 |
8.6 ± 3.7 |
8.3 ± 2.4 |
10.8 ± 3.8 |
6.6 ± 3.6 |
0.021 |
|
Length of ICU stay, d |
16.9 ± 10.6 |
16.7 ± 8.6 |
14.2 ± 7.3 |
19.8 ± 9.1 |
0.015 |
Count variables shown with group percentage as n (%); continuous variables were expressed as mean ± SD.
Table 2: Clinical Outcomes in patients with Different Goals of Daily Serum Lactate Level over the first 3-day.
|
Decompressive craniectomy |
HR |
95% CI |
P value |
|
0.866 |
0.236-2.300 |
<0.001 |
|
|
Serum Na+ level |
3.55 |
2.161-7.910 |
0.011 |
|
Serum Lactate |
2.866 |
1.382-10.227 |
<0.001 |
|
APACHEIIscore |
4.266 |
3.161-15.910 |
0.002 |
|
GCS score |
4.34 |
2.019-12.981 |
0.01 |
|
Daily positive fluid balance |
6.886 |
3.161-16.910 |
<0.001 |
Table 3: Multivariate Cox Hazard Regression Analysis of 30-day mortality.
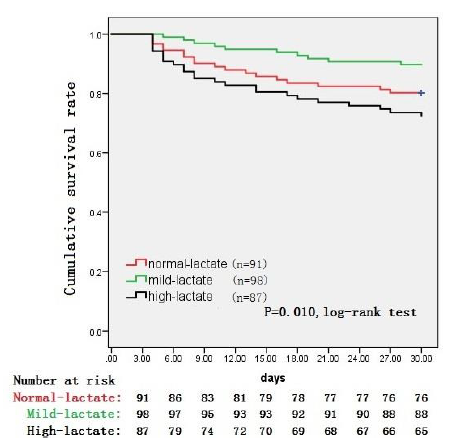
Figure 2: Kaplan-Meier survival curves comparing survival probability during 30 d following ICU admission among three groups of patients with acute intracranial hypertension induced by spontaneous intracerebral hemorrhage.
4. Discussion
Dehydrating therapy to relieve brain edema is one of crucial approaches to treat ICH after spontaneous cerebral hemorrhage [1]. Nevertheless, how much the dehydration should be? To date, little evidence in the way of laboratory values to the brain evident on CT scans that can be used to guide the optimal dehydration amount. We retrospectively analyzed data and found that permissive mild hyperlactatemia with a range of 2.0-3.5 mmol/l within the first 3-day after admission correlating with lower 30-day mortality, which could be a reference range to optimize fluid management for those acute intracranial hypertensive patients.Previous studies have established that serum lactate elevation was associated with inadequate tissue perfusion, particular the heart, liver and kidney [5, 8]. The correlation between elevated lactate at presentation and increased mortality has been known for decades and traditionally a threshold of 4 mmol/L has been used [9-11], other studies have showed that even modest increases in serum lactate with a threshold of 2.5 mmol/L are associated with poor outcome for septic patients [12, 13]. In the present study, we set a threshold of 3.5 mmol/L as remarkable high lactate and found that the 30-day mortality of those patients was significant higher than that in patients with serum lactate less than 3.5mmol/l, which is consistent with other findings [11].
As mentioned above, elevation of lactate reveals poor outcome and our cohort study indicated that lactate level and other factors, including serum Na+ level, APACHEIIscore, GCS score, and daily positive fluid balance were significant predictive factors for 30-day death. Surprisingly, our study showed that mild serum lactate elevation with a range of 2-3.5 mmol/L displayed better outcomes, including 30-day mortality, GCS score and the length of ICU stay when compared with the normal lactate patients within the first 3 days after admission. Why does mild elevated lactate reduce the mortality of patients with ICH induced by spontaneous cerebral hemorrhage in the present study? Routinely, fluid infusion may be conducted when the serum lactate elevated [14], but it’s not always right for the patients with brain damaged. Compared with normal brain, the course of the glycolytic pathway from glucose to lactate differs after injury, and the injured brain can utilize lactate as an energy substrate [15-17]. Moreover, the whole body doesn’t exist hypovolemia in this condition. And brain edema could be more severe if large amount fluid infusing for the purpose of decreasing serum lactate level. That is, mild serum lactate elevation is beneficial for the patients receiving dehydrating therapy. Furthermore, Keri L.H.Carpenter [18] concluded that the concentration of lactate in the brain depends not only on lactate production, but also lactate consumption, which meant that serum lactate would transport to the injured brain. Interestingly, a recent study by Bouzat et al. [19] showed evidence for beneficial effects (judged by surrogate markers) resulting from intravenous lactate administration in 15 TBI patients. Thus, combined literature reports and our findings, maintaining mild lactate elevation in the patients with ICH with the first 3 days can be used to optimize fluid management in patients with ICH who accept dehydrating therapy.
There are, however, limitations, mainly inherent to the single-center retrospective study design with limited sample size. In addition, the serum lactate level may not optimal as it’s not calculated by large sample. Moreover, these results only apply to intensive care units with a similar patient profile. A prospective design needed to strengthen the reliability of results.
5. Conclusions
For ICU patients with ICH induced by spontaneous cerebral hemorrhage who accept dehydrating treatment, we found an optimal range from 2-3.5mmol/L of serum lactate with the first 3 days after admission. Less or more of this range was associated with an increased risk of 30-day mortality. This study shows that the data obtained from point-of-care arterial blood gas analyser, which are frequently and easily checked at the bedside, allow for the determination of short time, which can be preferable when monitoring acute responses to treatment changes. To further improve the validity of these results, an external validation in other centers including an expansion of sample size is suggested.
Funding
This study was supported by National Natural Science Foundation of China (Grant No. 81701875) and Science and Technology Program of Guangzhou, China (Grant No. 201904010039).
References
- Morgenstern LB, Hemphill JC, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 41 (2010): 2108-2129.
- Claude Hemphill J, Lam A. Emergency Neurological Life Support: Intracerebral Hemorrhage. Neurocrit Care 27 (2017): 89-101.
- Allen M. Lactate and acid base as a hemodynamic monitor and markers of cellular perfusion. Pediatr Crit Care Med 12 (2011): 43-49.
- Ben-Yoseph O, Boxer PA, Ross BD. Assessment of the role of the glutathione and pentose phosphate pathways in the protection of primary cerebrocortical cultures from oxidative stress. J Neurochem 66 (1996): 2329-2337.
- Garcia-Alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactatemia. Crit Care 18 (2014): 503.
- Jess W Brallier, Pavan J Dalal, Patrick J McCormick, et al. Elevated intraoperative serum lactate during craniotomy is associated with new neurological deficit and longer length of stay. J Neurosurg Anesthesiol 29 (2017): 388-392.
- Carpenter KLH, Jalloh I, Hutchinson PJ. Glycolysis and the significance of lactate in traumatic brain injury. Front Neurosci 9 (2015): 112.
- Li S, Peng K, Liu F, et al. Changes in blood lactate levels after major elective abdominal surgery and the association with outcomes: a prospective observational study. J Surg Res 184 (2013): 1059-1069.
- Shin TG, Jo IJ, Hwang SY, et al. Comprehensive interpretation of central venous oxygen saturation and blood lactate levels during resuscitation of patients with severe sepsis and septic shock in the emergency department. Shock 45 (2016): 4-9.
- Moran JL, Santamaria J. Reconsidering lactate as a sepsis risk biomarker. PLoS One 12 (2017): e0185320.
- Casserly B, Phillips GS, Schorr C, et al. Lactate measurements in sepsis-induced tissue hypoperfusion: results from the Surviving Sepsis Campaign database. Crit Care Med 43 (2015): 567-573.
- Thomas?Rueddel DO, Poidinger B, Weiss M, et al. Hyperlactatemia is an independent predictor of mortality and denotes distinct subtypes of severe sepsis and septic shock. J Crit Care 30 (2015): 431-436.
- Filho RR, Rocha LL, Correa TD, et al. Blood lactate levels cutoff and mortality prediction in sepsis-time for a reappraisal? A Retrospective Cohort Study. Shock 46 (2016): 480-485.
- Bolvardi E, Malmir J, Reihani H, et al. The role of lactate clearance as a predictor of organ dysfunction and mortality in patients with severe sepsis. Mater Sociomed 28 (2016): 57-60.
- Newcombe VF, Williams GB, Outtrim JG, et al. Microstructural basis of contusion expansion in traumatic brain injury: insights from diffusion tensor imaging. J Cereb Blood Flow Metab 33 (2013): 855-862.
- Patel AB, Lai JC, Chowdhury GM, et al. Direct evidence for activity-dependent glucose phosphorylation in neurons with implications for the astrocyte-to-neuron lactate shuttle. Proc Natl Acad Sci USA 111 (2014): 5385-5390.
- TeSlaa T, Teitell MA. Techniques to monitor glycolysis. Methods Enzymol 542 (2014): 91-114.
- Keri LH Carpenter, Ibrahim Jalloh, Peter J Hutchinson. Glycolysis and the significance of lactate in traumatic brain injury. Front Neurosci 9 (2015): 112.
- Bouzat P, Sala N, Suys T, et al. Cerebral metabolic effects of exogenous lactate supplementation on the injured human brain. Intensive Care Med 40 (2014): 412-421.
