The Effect of Conservative Periodontal Therapy at Patients with Systemic Diseases
Article Information
Ilma Robo*, Saimir Heta, Fjona Hamzai, Vera Ostreni
Department of Dentistry, Albanian University, Tirana, Albania
*Corresponding author: Ilma Robo, Department of Dentistry, Albanian University, Tirana, Albania
Received: 11 September 2019; Accepted: 01 October 2019; Published: 21 October 2019
Citation:
Ilma Robo, Saimir Heta, Fjona Hamzai, Vera Ostreni. The Effect of Conservative Periodontal Therapy in Patients with Systemic Diseases. Archives of Internal Medicine Research 2 (2019): 040-049.
View / Download Pdf Share at FacebookAbstract
Introduction: The purpose of this study is to evaluate the clinical outcomes of non-surgical periodontal therapy specifically for psychiatric, diabetic, nephropathic and gastrointestinal patients. The clinical presentation of the diseases of these patients is followed by the selection of some of the patient typologies according to the above classification, followed by the documented evaluation of the periodontal prior status and 1 week of non-surgical periodontal non-surgical posttraumatic treatment.
Materials and methods: The study was conducted in a total of 311 patients, out of which 206 were ill patients included in the assessment, of which 80 were cardiac patients, 76 diabetic patients, 43 nephropathic patients, 7 gastrointestinal patients included in the study ours, after meeting the inclusion criteria. Patients included in the study are divided by age, sex and socio-health status.
Results: Outcome results in delayed treatment in diabetic patients with oral cavity injuries. The minimum amount of bacterial plaque in patients with gastrointestinal diseases is the most typical element of this category. The appearance of gingival hypertrophy in patients with hypertension treated with calcium-blockers and the faintest gingivitis during treatment of nephropathy patients are the typical features found in the relevant patient category.
Patient's vulnerability to combinations of diseases that follow and encourage each other was higher in the cardiac and diabetic relationship than in nephropathy and diabetes patients. Cardiac patients, if we talk about the healing process, in the probability values, reacted more slowly than diabetic patients. This element should be further followed by further analysis of the risk factors of these categories versus the occurr
Keywords
Diabetes, Cardiovascular, Gastrointestinal, Nephropathy, Periodontal therapy
Diabetes articles Diabetes Research articles Diabetes review articles Diabetes PubMed articles Diabetes PubMed Central articles Diabetes 2023 articles Diabetes 2024 articles Diabetes Scopus articles Diabetes impact factor journals Diabetes Scopus journals Diabetes PubMed journals Diabetes medical journals Diabetes free journals Diabetes best journals Diabetes top journals Diabetes free medical journals Diabetes famous journals Diabetes Google Scholar indexed journals Cardiovascular articles Cardiovascular Research articles Cardiovascular review articles Cardiovascular PubMed articles Cardiovascular PubMed Central articles Cardiovascular 2023 articles Cardiovascular 2024 articles Cardiovascular Scopus articles Cardiovascular impact factor journals Cardiovascular Scopus journals Cardiovascular PubMed journals Cardiovascular medical journals Cardiovascular free journals Cardiovascular best journals Cardiovascular top journals Cardiovascular free medical journals Cardiovascular famous journals Cardiovascular Google Scholar indexed journals Gastrointestinal articles Gastrointestinal Research articles Gastrointestinal review articles Gastrointestinal PubMed articles Gastrointestinal PubMed Central articles Gastrointestinal 2023 articles Gastrointestinal 2024 articles Gastrointestinal Scopus articles Gastrointestinal impact factor journals Gastrointestinal Scopus journals Gastrointestinal PubMed journals Gastrointestinal medical journals Gastrointestinal free journals Gastrointestinal best journals Gastrointestinal top journals Gastrointestinal free medical journals Gastrointestinal famous journals Gastrointestinal Google Scholar indexed journals Nephropathy articles Nephropathy Research articles Nephropathy review articles Nephropathy PubMed articles Nephropathy PubMed Central articles Nephropathy 2023 articles Nephropathy 2024 articles Nephropathy Scopus articles Nephropathy impact factor journals Nephropathy Scopus journals Nephropathy PubMed journals Nephropathy medical journals Nephropathy free journals Nephropathy best journals Nephropathy top journals Nephropathy free medical journals Nephropathy famous journals Nephropathy Google Scholar indexed journals Periodontal therapy articles Periodontal therapy Research articles Periodontal therapy review articles Periodontal therapy PubMed articles Periodontal therapy PubMed Central articles Periodontal therapy 2023 articles Periodontal therapy 2024 articles Periodontal therapy Scopus articles Periodontal therapy impact factor journals Periodontal therapy Scopus journals Periodontal therapy PubMed journals Periodontal therapy medical journals Periodontal therapy free journals Periodontal therapy best journals Periodontal therapy top journals Periodontal therapy free medical journals Periodontal therapy famous journals Periodontal therapy Google Scholar indexed journals Dentistry articles Dentistry Research articles Dentistry review articles Dentistry PubMed articles Dentistry PubMed Central articles Dentistry 2023 articles Dentistry 2024 articles Dentistry Scopus articles Dentistry impact factor journals Dentistry Scopus journals Dentistry PubMed journals Dentistry medical journals Dentistry free journals Dentistry best journals Dentistry top journals Dentistry free medical journals Dentistry famous journals Dentistry Google Scholar indexed journals non-surgical periodontal articles non-surgical periodontal Research articles non-surgical periodontal review articles non-surgical periodontal PubMed articles non-surgical periodontal PubMed Central articles non-surgical periodontal 2023 articles non-surgical periodontal 2024 articles non-surgical periodontal Scopus articles non-surgical periodontal impact factor journals non-surgical periodontal Scopus journals non-surgical periodontal PubMed journals non-surgical periodontal medical journals non-surgical periodontal free journals non-surgical periodontal best journals non-surgical periodontal top journals non-surgical periodontal free medical journals non-surgical periodontal famous journals non-surgical periodontal Google Scholar indexed journals posttraumatic articles posttraumatic Research articles posttraumatic review articles posttraumatic PubMed articles posttraumatic PubMed Central articles posttraumatic 2023 articles posttraumatic 2024 articles posttraumatic Scopus articles posttraumatic impact factor journals posttraumatic Scopus journals posttraumatic PubMed journals posttraumatic medical journals posttraumatic free journals posttraumatic best journals posttraumatic top journals posttraumatic free medical journals posttraumatic famous journals posttraumatic Google Scholar indexed journals hypertension articles hypertension Research articles hypertension review articles hypertension PubMed articles hypertension PubMed Central articles hypertension 2023 articles hypertension 2024 articles hypertension Scopus articles hypertension impact factor journals hypertension Scopus journals hypertension PubMed journals hypertension medical journals hypertension free journals hypertension best journals hypertension top journals hypertension free medical journals hypertension famous journals hypertension Google Scholar indexed journals diabetic patients articles diabetic patients Research articles diabetic patients review articles diabetic patients PubMed articles diabetic patients PubMed Central articles diabetic patients 2023 articles diabetic patients 2024 articles diabetic patients Scopus articles diabetic patients impact factor journals diabetic patients Scopus journals diabetic patients PubMed journals diabetic patients medical journals diabetic patients free journals diabetic patients best journals diabetic patients top journals diabetic patients free medical journals diabetic patients famous journals diabetic patients Google Scholar indexed journals
Article Details
1. Introduction
Periodontal therapy aims to maintain the balance of destructive factors and formative and remodeling factors in periodontal structures. Therapy aims to maintain the level of periodontal structures up to the stage where the destruction has resulted, as the consequence of the bacterial flora and the systemic effect of the disease by the suffering organism [1, 2]. Conservative periodontal therapy, regardless of the protocols defined in the work protocol, is expressed in clinical outcomes ranging from systemic diseases, gravity, and patient control to them. These results are measurable and clinically controllable, even as evaluators of various stages of agitation or disease improvement [3-7]. Periodontal disease diagnosis is usually based on clinical data and radiographic estimates, while gingiva assessment in the acute phase can be done by clinical examination, including gingival sulcus depth recording and gingival bleeding [8-12].
The purpose of this study is to evaluate the clinical outcomes of non-surgical periodontal therapy specifically for psychiatric, diabetic, nephropathic and gastrointestinal patients. The clinical presentation of the diseases that these patients experience is included in the first part of the study, followed by the selection of some of the patient typologies of the above classification, followed by a documented parental periodontal evaluation and 1 week of non-surgical periodontal posttraumatic patient. In the group of systemic diseases selected cardiovascular diseases, diabetes, hypertonic and gastrointestinal diseases, always stating in the fact that these diseases have a typical clinical appearance of t oral cavity, expressed depending on the duration of the disease, so for as long as the systemic effects of of the disease have appeared in the oral cavity [1]. These selected diseases, specifically have the clinical appearance of the oral cavity and despite the oral protocol of oral therapy affect the healing of the wounds in the mouth, and the duration of full periodontal healing. Periodontal therapy for these patients, specifically according to certain diseases, has certain protocols and stages of application for achieving the ultimate goal of protecting the level of periodontal tissue destruction [13-15].
2. Materials and Methods
After recording the history of each of the patients and it was found that the patients were in their psychiatric, diabetic, nephropathic and gastrointestinal disorder under treatment for specific diseases, in the attendance of the parodontologist, it was proceeded with a clinical examination of the oral cavity. Oral examination was performed on soft tissue and soft tissues of the oral mucous membrane, always respecting the air drying procedure and then checking free eye differences in oral mucosal relief. Telling the patient, the purpose of the study and the ongoing procedure, his consensus was required. The study was conducted in a total of 311 patients, out of which 206 were ill patients involved in the assessment, out of which 80 were cardiac patients, 76 diabetic patients, 43 nephropathic patients, 7 gastrointestinal patients included in our study after completing inclusion criteria. Patients included in the study are divided by age, sex and socio-health status. Each patient involved in the study is well-informed, and then agreed with full consensus to become part of the study, and then proceed with the established protocol.
3. Results
After collecting the data in the baseline table of excellence, they were processed for purpose of displaying the results of the study according to the tables below.
|
Patients |
Healthy |
% of healthy |
Ill patients |
% of ill |
Total |
|
Female |
65 |
62% |
102 |
49.5% |
167 |
|
Male |
40 |
38% |
104 |
50.5% |
144 |
|
Total |
105 |
100% |
206 |
100% |
311 |
Table 1: Number and percentage of sick and healthy patients are presented in the table.
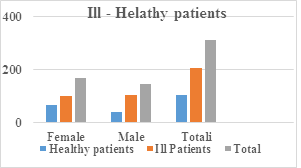
Figure 1: The graph shows numerically and in percentage the healthier and healthier patients.
|
Patients |
Cardiac |
Diabetic |
Nephropathy |
Gastrointestinal |
|
Female |
33 |
40 |
35 |
6 |
|
Male |
47 |
36 |
8 |
1 |
|
Total |
80 |
76 |
43 |
7 |
Table 2: Distribution of patients in male ratio: female, according to cardiac diseases, diabetes, nephropathy and gastrointestinal diseases.
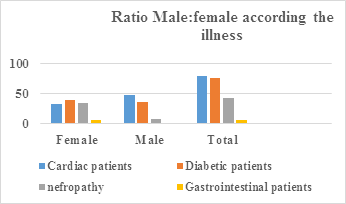
Figure 2: Shows the distribution of patients in male ratio: female, according to cardiac, diabetes, nephropathic and gastrointestinal disorders.
|
Patients |
Patients cardiac-diabetes |
% |
|
Female |
38 |
19% |
|
Male |
22 |
11% |
|
Total |
60 |
30% |
Table 3: Intercourse between cardiac diseases and diabetes.
|
Patients |
Nephropathy and Diabetes |
% |
|
Female |
16 |
17% |
|
Male |
7 |
7% |
|
Total |
23 |
24% |
Table 4: Intercourse between Nephropathy and Diabetes.
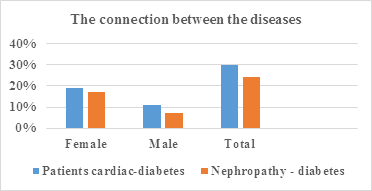
Figure 3: Intercourse between diseases: cardiac-diabetes, nephropathy-diabetes.
|
Probing values |
Before |
% |
After treatment |
% |
|
0-3mm |
22 |
28% |
36 |
45% |
|
4-6mm |
43 |
54% |
36 |
45% |
|
7mm |
15 |
19% |
8 |
10% |
|
Total |
80 |
100% |
80 |
100% |
Table 5: Probability values for cardiac patients.
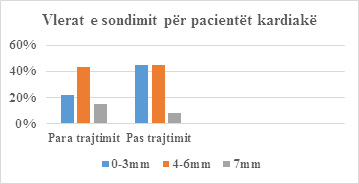
Figure 4: Patient Probe Values.
|
Probing values |
Before |
% |
After |
% |
|
0-3mm |
23 |
30% |
56 |
74% |
|
4-6mm |
47 |
62% |
20 |
26% |
|
7mm |
6 |
8% |
0 |
0% |
|
Totali |
76 |
100% |
76 |
100% |
Table 6: Probing values for diabetic patients.
|
Probing values |
Before |
% |
After |
% |
|
0-3mm |
7 |
16% |
10 |
23% |
|
4-6mm |
28 |
65% |
28 |
65% |
|
7mm |
8 |
19% |
5 |
12% |
|
Totali |
43 |
100% |
43 |
100% |
Table 7: Probing values for nephropathic patients.
|
Probing values |
Before treatment |
% |
After treatment |
% |
|
0-3mm |
5 |
71% |
7 |
100% |
|
4-6mm |
2 |
29% |
0 |
0% |
|
7mm |
0 |
0% |
0 |
0% |
|
Totali |
7 |
100% |
7 |
100% |
Table 8: Probing values for gastrointestinal patients.
|
Probing values |
Before treatment |
After treatment |
|
Cardiac |
42% |
39.5% |
|
Diabetes |
45% |
13% |
|
Nefropathy |
37% |
35.5% |
|
Gastrointestinal |
32% |
5% |
Table 9: Values of hemorrhage index for cardiac, diabetic, nephropathic, gastrointestinal patients.
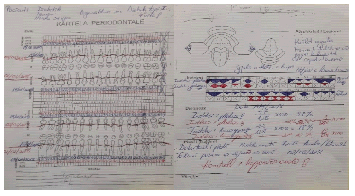
Figure 5: Female diabetic patient, age 54, diagnosed with Type I diabetes for 7 years. This patient has recorded periodontal diagrams before and 1 week after the last period of periodontal treatment.
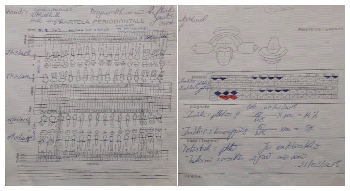
Figure 6: Patient male of 40 years with gastrointestinal reflux problems, diagnosed for 3 years. The photo is the first sheet of periodontal card - the probe diagram.
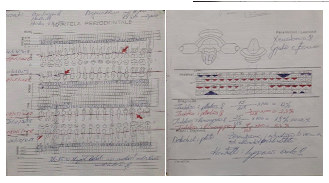
Figure 7: Patient suffering from hypertension, diagnosed with this disease for 15 years. The picture shows the probe diagram before and after treatment.
Periodontal disease and arteriosclerosis tend to increase their appearance over time. Disease caused by vascular changes could increase the patient's sensitivity to the appearance of periodontal diseases. On the other hand, periodontal diseases also have a significant effect on the appearance of atherosclerosis through the mechanisms of activation of macrophages. Hypertension is another disease that has an effect on the appearance of periodontal diseases, depending on the medications to which these patients are treated [16-19]. Calcium channel blocker medications give inflammatory gingivitis hypertrophy that is stimulating to appear further and agitating [20-25]. From recent literature studies, it is logical that the level of cholesterol in the blood is higher than that in patients suffering from atherosclerosis [25-32]. The level of cholesterol in the blood has a direct correlation with increased depth of pocket in patients with atherosclerosis [33-35]. Again, blood cholesterol levels are associated with increased depth of the pockets even in patients in the control group.
If we are talking about diabetics, high-grade glucose in saliva and gingival fluid is present in the disease pack. This high glucose level stems from the presence of increased glucose levels in the blood, or by non-control of this level due to systemic disease. Having a high amount of glucose in the saliva, the possibility of creating bacterial plaque in the marginal gingival is added. Diabetic patients have oral cavity pH in the direction of more acid than patients who do not suffer from these systemic diseases. Diabetic patients always notice delays in wound healing. This delay is explained by the slow motion of neutrophils to the inflammatory areas, as it is known that the first stage of healing is the cleansing phase of the neutrophils area that perform the phagocytosis of any unwanted element in the wound.
Recent studies point out that patients who are suffering from Type I diabetes mellitus are more susceptible to periodontal and lumbar disease, and that such problems increase with age and increase in age [37].
Patients with kidney problems are responsible for debiting the processes performed by these organs. Knowing that they filter the unnecessary elements of the blood, are understood in kidney dysfunction, these elements will tend to be deposited in the host tissues.Patients with gastrointestinal problems are related to the treatment of periodontal diseases at the point of having caution when prescribing certain medications, as their absorption is affected by medicines taken for the treatment of gastrointestinal diseases, under the control of the acidic pH of the stomach. Gastrointestinal reflux affects the emptying of gastric contents through the oral cavity. This content, being in acid form, stomach acid, sharpens the enamel surface significantly reflecting the dentin underneath it. This phenomenon is well-known with the emblematic description. This phenomenon was also evident in the patients involved in the study. Gastric content affects not only strong structures in the oral cavity, but also soft structures, mucosal tissues, which again suffer the acidification of the gastric contents.
4. Conclusions
Regardless of the choice of patients, it was found that the vulnerability of individuals, in general, to cardiac diseases, diabetes, kidney disease, gender segregation, was the same. Patient's vulnerability to combinations of diseases that follow and encourage each other was higher in the cardiac and diabetic relationship than in nephropathy and diabetes patients. Cardiac patients, if we talk about the healing process, in the probability values, reacted more slowly than diabetic patients. This element should be further followed by further analysis of the risk factors of these categories versus the occurrence of periodontal diseases. The hemorrhage index expressed significant reductions during the healing process, in descending order, the highest changes in diabetic patients, followed in gastrointestinal, nephropathic patients and the last were cardiac patients. Systemic diseases are directly related to the periodontal status of patients affected by them. This connection is secondary because, as the systemic diseases cause periodontal disease, the latter path is also worthwhile, since periodontal diseases also cause systemic diseases, which even endanger the patient's life.
References
- Michael G Newman, Henry H Takey, Ms Fermin A Carranza, et al. Odont; Clinical Periodontology; Ninth edition. Mosby (2004).
- Teeuw WJ, Slot DE, Susanto H, Gerdes VE, Abbas F, D'Aiuto F, et al. Treatment of periodontitis improves the atherosclerotic profile: a systematic review and meta-analysis. Journal of Clinical Periodontology 41 (2014): 70-79.
- Jirina Bartova, Pavla Sommerova, Yelena Lyuya-Mi, Jaroslav Mysak, Jarmila Prochazkova, Jana Duskova, et al. Periodontitis as a Risk Factor of Atherosclerosis. Journal of Immunology Research (2014): 9.
- Goodson JM. Antimicrobial strategies for treatment of periodontal diseases. Periodontol 5 (1994):142-168.
- Slots J, Rams TE. Antibiotics in Periodontal therapy: Advantages and disadvantages. J Clin Periodontol 17 (1990): 479-493.
- Sakkelari D, Goodson JM, Kolokotronis A. Concentration of 3 tetracyclines in plasma, GCF and Saliva. J Clin Periodontol 27 (2000): 53-60.
- Vidal F, Figueredo CM, Cordovil I, Fischer RG. Periodontal therapy reduces plasma levels of interleukin-6, C-reactive protein, and fibrinogen in patients with severe periodontitis and refractory arterial hypertension. J Periodontol 80 (2009): 786-91.
- Hussain M, Stover CM, Dupont A. P. gingivalis in Periodontal Disease and Atherosclerosis-Scenes of Action for Antimicrobial Peptides and Complement. Front Immunol 6 (2015): 45.
- Mackie IJ, Kitchen S, Machin SJ, Lowe GD. Haemostais and Thrombosis Task Force of the British Committee for standards in Haematology. Guidelines for fibrinogen assays. Br J Haemotol 121 (2003): 396-304.
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 32 (2009): S13–S61.
- Steveen L. Bricker, Robert P. Langlais, Craig S. Miller. Oral Diagnosis, Part One. Chapter “Endocrine Disorders" (2002): 470-481.
- Oral Medicine chapter "Diabetes Mellitus" 651-665.
- Idf Diabetes Atlas, seventh edition, executive summary (2015).
- wikipedia.
- Ahmed N. Advanced glycation endproducts-role in pathology of diabetic complications. Diabetes Res Clin Pract 67 (2005): 3-21.
- Craig RG. Ref: Interactions between chronic renal disease and periodontal disease. oral dis 14 (2008): 1-7.
- Tom D Daley, Jerrold E Armstrong. Oral manifestations of gastrointestinal diseases. Can J Gastroenterol 21 (2007): 241-244.
- Moazzez R, Bartlett D, Anggiansah A. Dental erosion, gastrooesophageal reflux disease and saliva: How are they related? J Dent 32 (2004): 489-94.
- Barron RP, Carmichael RP, Marcon MA, Sandor GK. Dental erosion in gastroesophageal reflux disease. J Can Dent Assoc 69 (2003): 84-89.
- Little JW. Eating disorders: Dental implications. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 93 (2002): 138-43.
- Kumar V, Fausto N, Abbas A. Robbins and Cotran’s Pathologic Basis of Disease. 7th edn. Philadelphia: Elsevier Saunders (2005).
- Neville BW, Damm DD, Allen CM, Bouquot JE. Oral & Maxillofacial Pathology. 2nd edn. Philadelphia: WB Saunders (2002).
- Bohmer T, Mowe M. The association between atrophic glossitis and protein-calorie malnutrition in old age. Age Ageing 29 (2000): 47-50.
- Drinka PJ, Langer E, Scott L, Morrow F. Laboratory measurements of nutritional status as correlates of atrophic glossitis. J Gen Intern Med 6 (1991): 137-40.
- Field EA, Speechley JA, Rugman FR, Varga E, Tyldesley WR. Oral signs and symptoms in patients with undiagnosed B12 deficiency. J Oral Pathol Med 24 (1995): 468-470.
- Chaudhry SI, Philpot NS, Odell EW, Challacombe SJ, Shirlaw PJ. Pyostomatitis vegetans associated with asymptomatic ulcerative colitis: A case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 87 (1999): 327-330.
- Ruiz-Roca JA, Berini-Aytes L, Gay-Escoda C. Pyostomatitis vegetans. Report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 99 (2005): 447-454.
- Katz J, Shenkman A, Stavropoulos F, Melzer E. Oral signs and symptoms in relation to disease activity and site of involvement in patients with inflammatory bowel disease. Oral Dis 9 (2003): 34-40.
- Ranjitkar S, Kaidonis JA, Smales RJ. Gastroesophageal reflux disease and tooth erosion. Int J Dent (2012).
- Silva MA, Damante JH, Stipp AC. Gastroesophageal reflux disease: New oral findings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 91 (2001): 301-310.
- Vakil N van Zanten Sv, Kahrilas P, Dent J, Jones R, Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 101 (2006): 1900-1920.
- Pace F, Pallotta S, Tonini M, Vakil N, Bianchi Porro G. Systematic review: gastro-oesophageal reflux disease and dental lesions. Aliment Pharmacol Ther 27 (2008): 1179-86.
- Vinesh E, Masthan K, Kumar MS, Jeyapriya SM, Babu A, Thinakaran M. A Clinicopathologic Study of Oral Changes in Gastroesophageal Reflux Disease, Gastritis, and Ulcerative Colitis. J Contemp Dent Pract 17 (2016): 943-947.
- Corrêa MC, Lerco MM, Henry MA. Study in oral cavity alterations in patients with gastroesophageal reflux disease. Arq Gastroenterol 45 (2008): 132-136.
- Talley N. Self-reported Halitosis and Gastro-esophageal Reflux Disease in the General Population. J Gen Intern Med 23 (2008): 260–266.
- Rosy Valensia, Sri Lelyati C. Masulili, Robert Lessang, Basuni Radi. Association between blood cholesterol level with periodontal status of coronary heart disease patients. Published Online (2017).
- Rokhsareh Sadeghi, Ferial Taleghani, Samira Mohammadi, Zahra Zohri. The Effect of Diabetes Mellitus Type I on Periodontal and Dental Status. J Clin Diagn Res 11 (2017): ZC14-ZC17.
