The Effect of Combined Clove and Cinnamon extracts on Growth and Survival of Escherichia fergusonii and Salmonella typhimurium in milk pre and post fermentation
Article Information
Betty A Ogwaro1,2*, Elizabeth A O’Gara2,3, Dave J Hill1,2 and Hazel Gibson1,2*
1Faculty of Science and Engineering, Wolverhampton School of Sciences, University of Wolverhampton, Wulfruna Street, WVI ILY, UK
2Faculty of Science and Engineering, Research Institute for Healthcare Science, University of Wolverhampton, Wulfruna Street, Wolverhampton, WVI ILY, UK
3Faculty of Science and Engineering, School of Medicine and Clinical Practice, University of Wolverhampton, Wulfruna Street, Wolverhampton, WV1 1LY, UK.
*Corresponding Author: Betty A. Ogwaro, Faculty of Science and Engineering, Wolverhampton School of Sciences, University of Wolverhampton, Wulfruna Street, WVI ILY, UK
Hazel Gibson, Faculty of Science and Engineering, Wolverhampton School of Sciences, University of Wolverhampton, Wulfruna Street, WVI ILY, UK
Received: 04 January 2022; Accepted: 10 February 2022; Published: 23 March 2022
Citation: Betty A. Ogwaro, Elizabeth A. O’Gara, Dave J. Hill and Hazel Gibson. The Effect of Combined Clove and Cinnamon extracts on Growth and Survival of Escherichia fergusonii and Salmonella typhimurium in milk pre and post milk fermentation. Journal of Food Science and Nutrition Research 5 (2022): 430-451.
View / Download Pdf Share at FacebookAbstract
The antimicrobial activities of extracts of clove buds (CL) and cinnamon bark (CE) were investigated individually and in combination in fermenting and fermented full cream milk against Escherichia fergusonii and Salmonella typhimurium. Clove and cinnamon were extracted for their essential oils (EOs) and eugenol and cinnamaldehyde were the major components representing 60-80% of the total oils. Preheated milk was inoculated with 1% (v/v) of a mixed culture of Lactobacillus delbrueckii subspecies bulgaricus (NCIMB 11778) and Streptococcus thermophilus (NCIMB 10387) and incubated at 25 or 37oC for 24 h. E. fergusonii or S. typhimurium (3 x 105 CFU/mL) were introduced into the milk pre - or post-fermentation. CL and CE were added at the same time as the pathogens based on their Minimum Inhibitory Concentrations of 0.25% for both pathogens as follows: CL and CE at 0.25% each alone; 0.0625% CL/0.1875% CE; 0.125% CL/ 0.125% CE; and 0.1875% CL/0.0625% CE. When added at the start of fermentation at 25oC, the CL and CE combinations inhibited the growth of E. fergusonii, and S. typhimurium, whereas at 37oC and associated with more rapid and higher acidification, the CL and CE combinations showed a marked antimicrobial activity against both pathogens. In post fermentation inoculated milk, survival of E. fergusonii and S. typhimurium was reduced by all CL and/ or CE treatments and were undetectable in samples containing 0.0625% CE with 0.1875% CL and 0.1875% CE with 0.0625% CL within 48 to 72 h of storage. The extent of the effect was most marked in the samples fermented at 37oC compared to 25oC and related to the pH after fermentation of 4.2 ±0.2 and 5.0 ±0.2 respectively. The use of these extracts both during fermentation and storage have the potential to enhance the microbiological safety of these products.
Keywords
Natural antimicrobial, Clove extract, Cinnamon extract, Escherichia fergusonii, Salmonella typhimurium, Traditional fermented milk, Pre and post fermentation contamination
Natural antimicrobial articles; Clove extract articles; Cinnamon extract articles; Escherichia fergusonii articles; Salmonella typhimurium articles; Traditional fermented milk articles; Pre and post fermentation contamination articles
Natural antimicrobial articles Natural antimicrobial Research articles Natural antimicrobial review articles Natural antimicrobial PubMed articles Natural antimicrobial PubMed Central articles Natural antimicrobial 2023 articles Natural antimicrobial 2024 articles Natural antimicrobial Scopus articles Natural antimicrobial impact factor journals Natural antimicrobial Scopus journals Natural antimicrobial PubMed journals Natural antimicrobial medical journals Natural antimicrobial free journals Natural antimicrobial best journals Natural antimicrobial top journals Natural antimicrobial free medical journals Natural antimicrobial famous journals Natural antimicrobial Google Scholar indexed journals Clove extract articles Clove extract Research articles Clove extract review articles Clove extract PubMed articles Clove extract PubMed Central articles Clove extract 2023 articles Clove extract 2024 articles Clove extract Scopus articles Clove extract impact factor journals Clove extract Scopus journals Clove extract PubMed journals Clove extract medical journals Clove extract free journals Clove extract best journals Clove extract top journals Clove extract free medical journals Clove extract famous journals Clove extract Google Scholar indexed journals Cinnamon extract articles Cinnamon extract Research articles Cinnamon extract review articles Cinnamon extract PubMed articles Cinnamon extract PubMed Central articles Cinnamon extract 2023 articles Cinnamon extract 2024 articles Cinnamon extract Scopus articles Cinnamon extract impact factor journals Cinnamon extract Scopus journals Cinnamon extract PubMed journals Cinnamon extract medical journals Cinnamon extract free journals Cinnamon extract best journals Cinnamon extract top journals Cinnamon extract free medical journals Cinnamon extract famous journals Cinnamon extract Google Scholar indexed journals Escherichia fergusonii articles Escherichia fergusonii Research articles Escherichia fergusonii review articles Escherichia fergusonii PubMed articles Escherichia fergusonii PubMed Central articles Escherichia fergusonii 2023 articles Escherichia fergusonii 2024 articles Escherichia fergusonii Scopus articles Escherichia fergusonii impact factor journals Escherichia fergusonii Scopus journals Escherichia fergusonii PubMed journals Escherichia fergusonii medical journals Escherichia fergusonii free journals Escherichia fergusonii best journals Escherichia fergusonii top journals Escherichia fergusonii free medical journals Escherichia fergusonii famous journals Escherichia fergusonii Google Scholar indexed journals Salmonella typhimurium articles Salmonella typhimurium Research articles Salmonella typhimurium review articles Salmonella typhimurium PubMed articles Salmonella typhimurium PubMed Central articles Salmonella typhimurium 2023 articles Salmonella typhimurium 2024 articles Salmonella typhimurium Scopus articles Salmonella typhimurium impact factor journals Salmonella typhimurium Scopus journals Salmonella typhimurium PubMed journals Salmonella typhimurium medical journals Salmonella typhimurium free journals Salmonella typhimurium best journals Salmonella typhimurium top journals Salmonella typhimurium free medical journals Salmonella typhimurium famous journals Salmonella typhimurium Google Scholar indexed journals Traditional fermented milk articles Traditional fermented milk Research articles Traditional fermented milk review articles Traditional fermented milk PubMed articles Traditional fermented milk PubMed Central articles Traditional fermented milk 2023 articles Traditional fermented milk 2024 articles Traditional fermented milk Scopus articles Traditional fermented milk impact factor journals Traditional fermented milk Scopus journals Traditional fermented milk PubMed journals Traditional fermented milk medical journals Traditional fermented milk free journals Traditional fermented milk best journals Traditional fermented milk top journals Traditional fermented milk free medical journals Traditional fermented milk famous journals Traditional fermented milk Google Scholar indexed journals Pre and post fermentation contamination articles Pre and post fermentation contamination Research articles Pre and post fermentation contamination review articles Pre and post fermentation contamination PubMed articles Pre and post fermentation contamination PubMed Central articles Pre and post fermentation contamination 2023 articles Pre and post fermentation contamination 2024 articles Pre and post fermentation contamination Scopus articles Pre and post fermentation contamination impact factor journals Pre and post fermentation contamination Scopus journals Pre and post fermentation contamination PubMed journals Pre and post fermentation contamination medical journals Pre and post fermentation contamination free journals Pre and post fermentation contamination best journals Pre and post fermentation contamination top journals Pre and post fermentation contamination free medical journals Pre and post fermentation contamination famous journals Pre and post fermentation contamination Google Scholar indexed journals milk production articles milk production Research articles milk production review articles milk production PubMed articles milk production PubMed Central articles milk production 2023 articles milk production 2024 articles milk production Scopus articles milk production impact factor journals milk production Scopus journals milk production PubMed journals milk production medical journals milk production free journals milk production best journals milk production top journals milk production free medical journals milk production famous journals milk production Google Scholar indexed journals fermented milk articles fermented milk Research articles fermented milk review articles fermented milk PubMed articles fermented milk PubMed Central articles fermented milk 2023 articles fermented milk 2024 articles fermented milk Scopus articles fermented milk impact factor journals fermented milk Scopus journals fermented milk PubMed journals fermented milk medical journals fermented milk free journals fermented milk best journals fermented milk top journals fermented milk free medical journals fermented milk famous journals fermented milk Google Scholar indexed journals
Article Details
1. Introduction
Pastoralists are increasingly producing milk for the market as well as for subsistence, contributing to the growth of a production subsector with notable resilience to climate variability and change. Milk presents livelihood opportunities to most of the rural populations in Africa ranging from farmers, processors, shopkeepers, and other stakeholders in the dairy chain [1, 2]. Milk and milk products have excellent nutrients as milk consists of fats, proteins, minerals, vitamins, carbohydrates, and water [3]. Milk, however, is extremely susceptible to spoilage by microorganisms. The quality control of milk is an essential aspect of the dairy chain [4] to ensure milk product safety and public health. With lack of good quality water, unreliable electricity, and poor keeping quality of the milk, contamination of the milk is unavoidable even if the milk was pasteurised or under very hygienic conditions of milk production [4]. Contamination of milk can occur at any stage during handling from production side, storage to marketing between animal and consumers [5]. This number may increase considerably according to the type of bacteria, their virulence, and surrounding conditions especially temperature. Consumption of unpasteurised milk has led to many outbreaks and pasteurised milk has also been responsible for causing illness through post process contamination. Many Africans prefer sour, or acidified milk made from raw milk where there is no denaturing of milk proteins by heat treatment process, or destruction of natural flavours in the milk [6,7]. Rural African farmers incorporate some spices into fermenting/fermented milk either as whole (unbroken) spices or as coarse powders to improve the flavour and aroma of the product [8]. Cava et al. [9] reported the effect of essential oils of some spices on pathogens in milk. Furthermore, Ogwaro et al. [10] and Cava et al. [11] reported on the effect of fractional combinations of spices against various food pathogens. There are, however, no reports on the effects of combined essential oils of cloves with cinnamon on Escherichia fergusonii and Salmonella typhimurium in fermented milk. Escherichia fergusonii, is a Gram-negative, rod-shaped bacterium formerly known as, Enteric Group 10 due to its biochemically distinct nature compared to other species and bio-groups of Enterobacteriaceae. It is one of the five members of Escherichia which can be found in the intestines of human beings [12]. E. fergusonii was isolated from raw milk and some dairy products in Egypt [13]. According to Sherwood and Clegg [14], E. fergusonii grows optimally at 37-40oC under aerobic conditions but temperature range of growth extends from 21- 45oC so the bacterium is well suited to growth in tropical African temperatures. However, its prevalence and survival in milk and milk products is not yet well documented. Salmonellae are Gram negative short rods bacteria of the family Enterobacteriaceae. They have caused outbreaks of illness around the world and continue to be a major concern for the dairy industry. They are widespread in the environment and appear in a wide variety of foods and food ingredients [15]. Salmonellae have been isolated in raw milk [16]. In principle, using raw milk increases the likelihood of Salmonellae being present in the product. Salmonella grow optimally between 35 to 37oC, but they can grow at much lower temperatures too. The pH range for growth of Salmonellae is between 6.5 and 7.5 but it can also grow readily in an acidic environment [17]. Under laboratory condition, Salmonellae grew at pHs as low as 4.05, 4.10 and 4.40 in the presence of hydrochloric, acetic, and lactic acids respectively [18]. Yoghurt has been reported to be effective in controlling growth of Salmonella [19]. However, Adams and Hall [20] and Alvarez-Ordonez, [21] reported that inhibition of Salmonellae in the presence of lactic acid bacteria depended on the type of lactic acid bacteria, incubation temperature, and size of inoculum of lactic cultures. Clove, (Syzygium aromaticum L. Myrtaceae) also known as ‘champion’ spice is an aromatic plant widely cultivated in tropical and subtropical countries. It is one of the most intensely flavoured spices and extensively used in foods and hot drinks such as tea and many brands of tomato ketchup [22]. Clove oil is a mixture of different compounds with three main active ingredients and is rich in volatile compounds and antioxidants such as eugenol, β-caryophyllene, and α-humulene. Clove oil has biological activity relevant to human health including antimicrobial, antioxidant, and insecticidal, antifungal, and antiviral activity [23]. Antimicrobial activity of cloves has been reported by many researchers [11, 24]. Xu et al. [25] reported that clove could destroy the cell walls and membranes of microorganisms, and permeate the cytoplasmic membranes or enter the cells, then inhibit the normal synthesis of DNA and proteins. Eugenol accounts for at least 60-80% of the composition and thus is the main active component. The remaining 20-40% consists of eugenol acetate and β -caryophyllene and alpha-humulene [26]. Xu et al. [25] also noted that eugenol could inhibit the production of amylase and proteases in Bacillus cereus and Burt [27] reported that clove has the ability to deteriorate cell wall causing cell lysis. Cinnamon (Cinnamomum zeylanicum) on the other hand is from the Lauraceae family and is used widely in the preparation of tea and foods for its flavour and aroma. The three main components are cinnamaldehyde, cinnamyl acetate and cinnamyl alcohol. Cinnamon is reported to have antimicrobial compounds [28, 29] and it is active as an antibacterial, anti-allergic, anti-ulcerogenic, antipyretic and antioxidant [25]. Research has shown that cinnamon extract was more effective than cinnamon essential oil [11, 30, 31]. The aim of this study was to assess the antimicrobial activity of a mixture of clove and cinnamon extracts on the growth and survival of E. fergusonii and S. typhimurium in pasteurized full milk during and post fermentation
2. Materials and Methods
2.1 Media for growth
Tryptone Soy Broth (TSB, LAB004) Tryptone Soy Agar (TSA, LAB011); de Man-Rogosa-Sharpe Agar (MRSA LAB098); de Man Rogosa-Sharpe Broth (MRSB LAB093) and M17 Agar (LAB092) were all purchased from Lab M Limited (Bury, UK) and M17 Broth (CM0817) was purchased from Thermo Fisher Scientific (Loughborough, UK). All the media were prepared according to the manufacturers’ instructions. M17 Agar and M17 broth were used for S. salivirus subspecies thermophilus [32]. 50 mL of 10% sterilized lactose was added to 1 litre of M17A or M17B before use [33]. The sample was tested by streaking on the agar or inoculating in the broth using a typical S. thermophilus colony and incubated at 37°C for 24h. MRSA and MRSB were used for the enumeration of L. delbrueckii subspecies bulgaricus [34], Violet Red Bile Agar (VRBA, CM0107B, Thermo Fischer Scientific, Loughborough, UK) for the enumeration of E. fergusonii and Xylose Lysine Deoxycholate (XLD) (CM0469, Thermo- Fisher Scientific, Loughborough, UK) for Salmonella typhimurium. Sterile quarter-strength Ringers Solution (BR 0052, Thermo Fischer Scientific, Loughborough, UK) was used as an isotonic diluent for the bacterial cells. All media were prepared with deionized water.
2.2 Microorganisms and Culture Conditions
Escherichia fergusonii (UCC 585) was isolated and identified from a traditional African yoghurt. S. typhimurium (NCIMB 10248) L. delbrueckii subspecies bulgaricus (NCIMB 11778) and Streptococcus thermophilus (NCIMB 10387) were obtained from the National Collection of Industrial Food and Marine Bacteria (UK). Before each experiment, a culture from a freeze-dried vial, maintained at -20°C was activated in TSB for S. typhimurium; and in M17 Broth for S. thermophilus incubated for 24 h at 37°C ±0.1°C and streaked onto TSA, and M17 Agar respectively; and L. delbrueckii subspecies bulgaricus was activated in MRSB, pH 5.5 ±0.2 and incubated under anaerobic condition at 37 ±0.1°C for 48-72 h. The pH of MRS agar and broth were adjusted with 1N HCL to pH 5.5 ±0.2.
The cultures were then streaked on MRSA and incubated in anaerobic gas jar at 37°C for 48-72 h. Resuscitated microorganisms were sub-cultured twice before use in the experiments. All activated cultures were maintained on slants at 4ºC and were sub-cultured monthly. For the growth of S. thermophilus,M17 Agar or M17 Broth were used. 50 ml of 10% sterilized lactose was added to the M17 A or M17 B before use. The sample was tested streaking on the agar or inoculating in the broth using typical S. thermophilus culture incubated at 37oC for 24h. To prepare the inoculum, one pure isolated colony from a plate agar was transferred to TSB for S. typhimurium and E. fergusonii, M17B for S. thermophilus and MRSB (pH 5.5) for L. delbrueckii subspecies bulgaricus. The latter was incubated in anaerobic gas jar. All the cultures were incubated at 37°C for 24 h with shaking (150 rpm). L. delbrueckii subspecies bulgaricus grew better at pH 5.0-5.5 while S. thermophilus grew well at pH 6.0. The lactic acid bacteria were inoculated at approx. 106 CFU/mL.
To obtain the viable counts for E. fergusonii or S. typhimurium during the antimicrobial challenge test, serial dilutions were made in ¼ strength Ringer’s solution and 100 µL was plated out on VBRA and brilliant green agar respectively incubated for 24 h at 37°C. Viable counts were recorded after the incubation period.
2.3 Spices for this study
Spices in this study were clove buds and cinnamon barks (Table 1), purchased from the local market in Juba, South Sudan
2.4 Extraction of essential oils
The spices were first washed with sterile distilled water then dried in a drying cabinet at 50°C for 72 h. The dried spices were then crushed individually using a sterilized mortar and then ground with an electric grinder to coarse smaller particles before extraction. Clove and cinnamon essential oils (EOs) were extracted using Soxhlet extractor according to the methods of Azwanida [35]. Briefly, 100 g of each ground powder was weighed in a 50 mL extraction thimble. This was placed inside the main chamber of the Soxhlet extractor. 500 mL of HPLC grade methanol (Sigma Aldrich, UK) was put in a 1000 mL round bottom flask and was placed on the electric heater of the extractor. The heater was turned on to 50°C (methanol’s boiling point). Continuous extraction took place by re-fluxing the solvent until the solvent was clearly colourless. Using a rotary evaporator, the methanol was evaporated at fixed temperature of 50°C until all the methanol was completely evaporated off, leaving a thick essential oil. The samples were either used immediately or kept at -20°C until use (used within one month). The compositions of EOs from the spices were determined by Gas Chromatography-Mass Spectrometry (GC-MS).
|
Common name |
Scientific name |
Part of the plant |
Extraction method |
|
Cinnamon |
Cinnamomum zeylanicum |
cinnamon bark |
Steam distillation |
|
Clove |
Eugenia caryophyllata |
clove buds |
Steam distillation |
Table 1: Spices selected in this study and the oil extraction methods
2.5 Milk sample preparation
Whole, full cream pasteurized milk (pH 6.7 ±0.1) was purchased from a supermarket one day before the experiment was performed and at least 6 to 7 days before its use-by date. Sterile test tubes containing 10 mL of milk were heated by steam for 30 min at 85°C and transferred immediately to cool in a water bath set at 40°C. The sterility of the samples was confirmed by streaking on to petri dishes of TSA and incubating at 37°C for 4 h.
2.6 Pre-screening for antimicrobial activity
Antimicrobial activity was tested by agar-well diffusion method. 1000 µL of undiluted overnight inoculum of either E. fergusonii or S. typhimurium was inoculated in cooled (45oC) but still molten 20 ± 2 mL TSA. After mixing, the agar was poured into the Petri dish in duplicates. Another 20 ml of molten agar (without the bacteria), in duplicate was poured in sterile petri dishes as control. This was allowed to set for 1-2 hours in the laminar flow (LAF) cabinet then four, equal distance holes were punched in each plate of the agar. To each well, 150 µL of the individual or combined spice extract was added and left to stand for about 1 hour to allow the extract to diffuse. The plates were then incubated for 24 hours at 37°C. The antimicrobial activity was evaluated by measuring the Zone of inhibition (ZOI) using a ruler and expressed in millimetres (mm).
2.7 Determination of Minimum Inhibitory Concentration (MIC)
MIC was determined by broth dilution methods. 1% (v/v) stock solution of each EO of spice extract was prepared in methanol (solvent). To determine the minimum inhibitory concentration of the EOs, 12 test tubes, each containing 5.0 mL of pasteurised milk were set up. From the 1.0% of spice extract prepared before, a 2-fold serial dilution was carried out in tubes 1-10 to dilute to 0.156%. The 11th and 12th tubes contained pasteurised milk only. 100 µL of the suspended E. fergusonii or S. typhimurium was then added to test tubes 1-11 giving a final concentration of approx. 105 CFU/mL in each tube. Counts were initially performed to ensure that the size of the inoculum was in the range desired (x 105 CFU/mL). The control sample (Tube 11) contained E. fergusonii or S. typhimuriumithout the spice extracts. Tube 12 contained pasteurised milk only (sterility control). All the test tubes were then incubated at 37°C for 24h. Following the incubation, the samples were serially diluted (1:10) in quarter Ringer’s solutions and appropriate dilutions were plated on TSA plates.
The plates were incubated at 37°C for 24h. The lowest concentration of the EO treatment that inhibited visible growth of the pathogen after incubation was taken as the MIC of the treatment. The minimum bactericidal concentration (MBC) was carried out by plating 100 µL from each tube was plated on TSA plates and the MBC was defined as the lowest concentration at which no growth was observed.
2.8 Effect of combined essential oils of cloves (CL) and cinnamon (CE) extracts on E. fergusonii and S. typhimurium during fermentation (pre-fermentation contamination)
The CL and CE were used alone or in combination with each other at ¼, ½ and ¾ MIC (Table 2) to determine the antimicrobial effectiveness againstE. fergusoniior S. typhimurium when contamination occurs pre- fermentation or post fermentation. For the pre-fermentation contamination, milk samples were inoculated with 1% (v/v, about 106 CFU/ mL) of a mixed culture ofL. delbrueckiisubspeciesbulgaricus andS.thermophilus. To assess the effect of the combined CL with CE on growth of the pathogens during fermentation, 100 µL of a diluted overnight culture ofeither E. fergusoniior S. typhimurium was added to all the 6 tubes to give a final concentration of approx. 105 CFU/mL was added to each sample along with the treatments of CL and CE either alone or in combinations as shown in table 2. Triple sets of the test tubes were then incubated for 24 h at 25°C or 37°C. Tube No. 1 was inoculated with the test bacterium but without the spice extracts. This was included in each experiment and served as control. Viable counts during fermentation were enumerated at 0, 2, 4, 6, 8, 10, 12 and 24 h. The cell counts were converted into Log10 CFU/mL and plotted against time.
|
Tube No. |
CL conc. (%) |
CE conc (%) |
MIC of CL |
MIC of CE |
|
1 |
0 |
0 |
0 |
0 |
|
2 |
0.25 |
0 |
1 |
0 |
|
3 |
0.0625 |
0.1875 |
1/4 |
3/4 |
|
4 |
0.125 |
0.125 |
1/2 |
1/2 |
|
5 |
0.1875 |
0.0625 |
3/4 |
1/4 |
|
6 |
0 |
0.25 |
0 |
1 |
Table 2: Concentrations of CL and CE evaluated for antimicrobial effects against E. fergusonii and S. typhimurium.
2.9 Effect of combined essential oils of cloves extract (CL) and cinnamon extract (CE) on survival of E. fergusonii and S. typhimurium during storage (post-fermentation contamination)
For the study of post contamination effect of the extracts on the survival of the bacteria, initially a set of pasteurized full cream milk samples were inoculated with 1% (v/v) of mixed culture of lactic acid bacteria (L. delbrueckii subspecies bulgaricus and S. thermophilus) only and were incubated at 25oC or 37oC for 24 h. After the fermentation, approx. 105 CFU/mL stationary phase cultures of E. fergusonii or S. typhimurium was added to each sample along with the combined treatments of CL and CE (Table 1). Each treatment was stored at 25oC for 120 h. Viable counts for E. fergusonii and S. typhimurium were determined by plating 100 µL of each sample on VRBA or XLD respectively at 0 [start of storage], 24, 48, 72,120, and 144 h during storage.
2.10 pH measurement
The pH of the samples was measured with a Mettler Toledo Delta 320 pH meter, at room temperature. Readings were taken before inoculation (negative control), immediately after inoculation (T=0) and then at every sampling point.
2.11 Measurement of titratable acidity
20 g of a well shaken yoghurt or un-fermented milk was weighed accurately into a 250-mL Elenmeyer flask, 40 mL of boiled and cooled distilled water was added to it. With a sterile pipette, 2-3 drops of phenolphthalein was added in the milk as an indicator of end point. The content of the flask was titrated against 0.1N sodium hydroxide (NaOH) until the sample changed colour to persistent light pink. The initial and final readings on the meniscus burette were recorded, prior to starting the titration and at the end point, respectively.
The amount (mL) of 0.1N NaOH titrated was calculated by subtracting the initial volume from the final volume to give the amount of NaOH used to reach the endpoint. This was performed at least three times per sample. The amount percent lactic acid was then calculated using the equation:

2.12 Statistical Analysis
All experiments were performed in triplicate. The data were expressed as the mean ± SD. The differences between means (P<0.05) were compared by one-way analysis of variance (ANOVA) using Prism graph pad (USA), version 9.0.
3.0. Results
3.1 Chemical composition of methanol clove buds and cinnamon bark extracts
The chemical composition of methanol clove buds and cinnamon barks were identified by Gas Chromatography- Mass Spectrometer (GC-MS). Quantitative calculations were based on the relative areas of the corresponding GC signals. Seven components were identified in clove bud’s extract. Eugenol was the major component comprising of 68.76% followed by Caryophyllene then Phenol, 2-methoxy-4-(2-propenyl) acetate (Table 3). Seven compounds were identified in cinnamon barks extracts (Table 4). The major component was cinnamaldehyde comprising of 83.54% followed by alfa-Copaene and cis-Calamenene, alpha-Muurolene.
|
No. |
Compound |
Retention Time (min) |
Relative content (%) |
|
1 |
Methylamine, N,N-dimethyl- |
1.51 |
8.28 ±0.3 |
|
2 |
Eugenol |
11.44 |
68.77 ±0.3 |
|
3 |
Phenol, 2-methoxy-3-(2-propenyl)- |
11.51 |
1.16 ±0.3 |
|
4 |
Caryophyllene |
12.33 |
11.04 ±0.3 |
|
5 |
Humulene |
12.76 |
1.44 ±0.3 |
|
6 |
Phenol, 2-methoxy-4-(2-propenyl)-, acetate |
13.54 |
7.01 ±0.3 |
|
7 |
(4-Acetylphenyl)phenylmethane |
15.52 |
2.30 ±0.3 |
Relative content values are mean ± standard deviation (n=3)
Table 3: The analysis of chemical composition of clove buds extracts as analysed by GC-MS.
|
No. |
Compound |
Retention Time (min) |
Relative content (%) |
|
1 |
Cinnamaldehyde, (E)- |
10.23 |
83.46 ±0.25 |
|
2 |
alfa-Copaene |
11.72 |
3.02 ±0.45 |
|
3 |
Coumarin |
12.55 |
1.81 ±0.5 |
|
4 |
gamma-Muurolene |
13.02 |
0.86 ±0.5 |
|
5 |
alpha-Muurolene |
13.29 |
2.24 ±0.35 |
|
6 |
cis-Calamenene |
13.58 |
2.84 ±0.35 |
|
7 |
2-Propenal, 3-(2-methoxyphenyl)- |
13.63 |
5.76 ±0.35 |
Relative content values are mean ± standard deviation (n=3) ± SD
Table 4: The analysis of chemical composition of cinnamon extracts as analysed by GC-MS
3.2 Preliminary assessment of the antimicrobial activity of CL and CE by agar well diffusion
The initial screening of the antimicrobial activity of CL or CE against E. fergusonii and S. typhimurium was carried out using agar well diffusion. CL and CE extracts were assayed in TSA using six concentrations (1%; 0.5%; 0.25%; 0.125%; 0.0625% and 0.0312%). The zones of inhibition of the cloves or cinnamon extracts against E. fergusonii and S. typhimurium are presented on Table 5. At 1% (w/v) concentration of CL or CE, the zones of inhibition ranged from 16-18 mm and 14-15 ±0.5 mm for E. fergusonii or S. typhimurium respectively. At 0.5 and 0.25% (w/v), similar zones were observed, 8.9 ± 0.5 mm and 7-8 ±0.5 mm for both pathogens but at 0.125% (w/v), cloves extract showed no ZOI against E. fergusonii. At 0.0625%, no zone was detected for E. fergusonii nor S. typhimurium. In view of the observations in the agar well diffusion method, the MIC of the extracts was assessed with the broth dilution assay with concentration from 1 to 0.0156%. The MIC was found to be 0.25% and MBC was 1% for both clove and cinnamon extract for both E. fergusonii and S. typhimurium.
3.3 Effect of combined concentrations of CL and CE on the growth of E. fergusonii and S. typhimurium and change in pH of milk during fermentation at 25 or 37oC
3.3.1 Growth of E. fergusonii in milk during fermentation at 25 or 37oC: Figure 1A shows growth curves of E. fergusoniiin the LAB fermenting milk with various concentrations of CL and CE at 25oC. E. fergusonii grew to a similar level in all the samples in the initial 4 h then, in the control and the sample with 0.25% CE alone, it increased from the initial 105CFU/mL to 109 CFU/mL (doubling times 0.68 h). Growth of the bacterium in the samples treated with the combined concentrations of the EOs resulted in lower counts than the counts in the control samples. The most potent combination at the fermentation temperature of 25oC was 0.1875% CL (three-quarters MIC) combined with 0.0625% CE (one-quarter MIC). Growth in this sample was slower (doubling time 1.5 h) than in the control sample (doubling time 0.68 h). At this concentration the cell counts were approx. 106 CFU/mL, 1 log unit lower than the initial contamination level and approximately 2.4 log units lower than the counts in the control samples.
The next most effective combination was the 0.125% CL (one half MIC) with 0.125% CE (one-half MIC). In the other treated samples, the bacterium grew to approx. 108CFU/mL and 108.5 CFU/mL respectively. At this temperature, the pH of the milk declined from initial pH 6.8 ±0.1 to 6.5 (±0.2) in all the treated samples after 4 h then to pH 5.3 (±0.2). The pH with 0.25% CL only was higher than in all the samples even in the control. This was in line with titratable acidity being lowest in that sample too. Statistical analysis showed that there was no significant difference (p>0.05) between the control and treated samples in the initial 8 hours at 25oC. After this time, there was a significantly less growth in the treated samples except CE alone. Figure 1B shows the growth pattern when the milk was fermented at 37oC in the absence or presence of combinations of CL and CE. In the absence of extracts and in the sample with 0.25% CE alone, the bacterium grew to approx. 109 CFU/mL in the first 12 h of incubation (doubling time 0.56 h and 0.41 h respectively) and subsequently in the control (samples without treatment), it increased by approx. 1 log unit more than in the sample incorporated with combined CL 0.25% alone.
However, in the sample with 0.25% CE alone the increase was less as seen in figure 1B. Although CE alone was not statistically significantly different to the control, all other CE/CL treatments significantly reduced the E.fergusonii population (p<0.05) and 0.1875% CL/ 0.0625% CE to undetectable levels in 6 h.
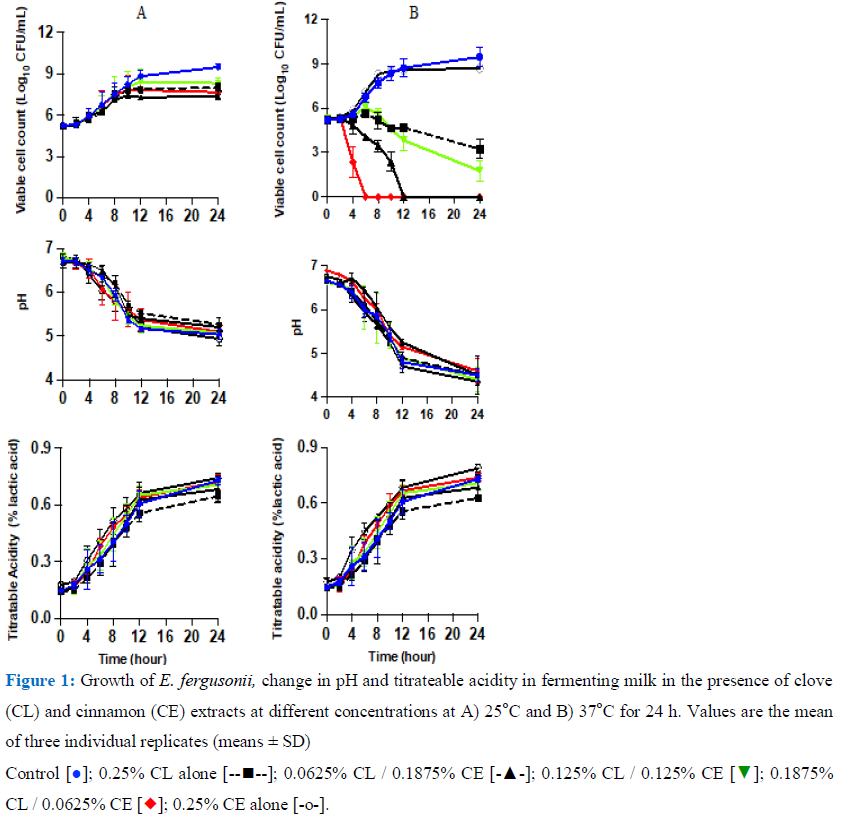
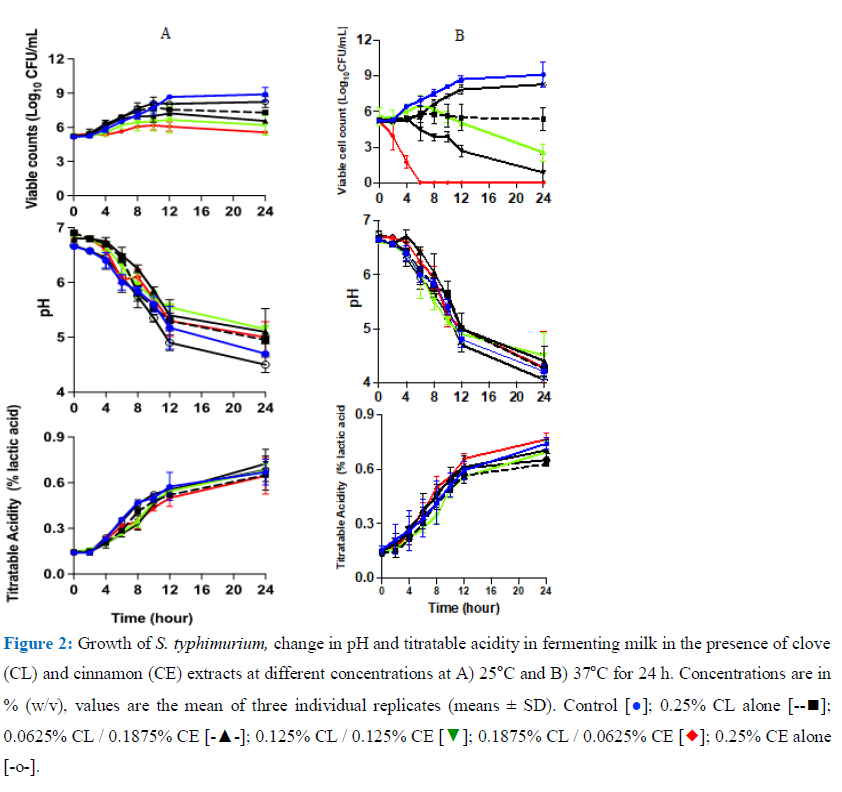
3.3.2 Growth of S. typhimurium in milk during fermentation at 25 or 37oC: The growth curves of S. typhimurium milk fermenting at 25oC are shown in Figure 2A. S. typhimurium showed a similar growth pattern in response to all treatments in the first 4 h. In the control sample, the bacterium then increased from 105CFU/mL to 108 CFU/mL (doubling time 0.86 min) after 10 h then to 109 CFU/mL after 24 h fermentation. Whereas the samples treated with combined extracts: 0.0625% CL / 0.1875% CE; 0.1875% CL / 0.0625% CE and the 0.125% CL / 0.125% CE, at 24 h the viable cell counts were 106 CFU/mL; 106.5 CFU/mL and 107 CFU/mL respectively with doubling times of 1.5 h. The pH declined in all the samples and was lowest in samples containing 0.25% CE only. It declined from pH 6.8 (± 0.1) to pH p.5 (± 0.1) after 12 h of fermentation. The highest pH was in the sample containing combined 0.125% CL with 0.125% CE with pH 5.1(± 0.1) after 24 h. The TA of the samples was in the range of 0.61-0.7% lactic acid in all the samples (Figure 2A). Growth curves of S. typhimurium at milk fermented at 37oC are presented in figure 2B. The results showed that growth was inhibited in all the treated samples except for the sample with 0.2% CE% alone which was approx. 1 log unit lower that the control sample. In samples treated with 0.1875% CL with 0.0625% CE, the S.typhimurium population was reduced as soon as the fermentation commenced and was not detected after 6 h of fermentation. In samples treated with 0.0625% CL / 0.1875% CL (one quarter MIC of CL with three quarter MIC of CE) and containing 0.125% CL /125%CE (one half with one half), cell numbers declined to below 103 CFU/mL and 103 CFU/mL respectively. The acidity of the samples increased with the pH around 4.5 and TA ranged between 0.7% - 0.8% lactic in sample with 0.25% CL. The pH in all the samples declined similarly up to 12 hours and this was reflected by an increase in titratable acidity over this period. This shows that lactic fermentation had progressed in the presence of CE/CL but the addition of the extracts contributed to the reduction in the pathogen levels. With continued fermentation, the pH declined in all the samples to pH 4.1–4.6 (±0.2) after 24 h.
3.4 Effect of combined concentrations of CL and CE on the survival of E. fergusonii and S. typhimurium and change in pH post fermentation
3.4.1 Survival of E. fergusonii during storage in milk fermented at 25°C or 37oC and stored at 25oC: Milk was fermented at 25oC for 24 h thereafter, various concentrations of CL mixed with CE were added to it as well as 105CFU mL-1 E. fergusonii was co-inoculated in it (post fermentation contamination) then stored for 120 h at 25oC (Figure 3A). After 48, 72, and 96 h of storage, the viable cell counts of the bacterium declined to undetectable level in milk inoculated with concentrations of 0.0625% CL / 0.1875% CE, 0.1875% CL / 0.0625% CE and 0.125% CL/ 0.125% CE respectively. With continued storage, a reduction in the bacterial numbers in the rest of the samples was observed and after 96 and 120 h there was a reduction of approximately 2.5 - 2.3 log units in samples with 0.125% Cl and 0.125% CE respectively. Th pH of the sample continued to decline although slowly with the pH in the range of 4.8 - 5.2 (±0.2) and declined to 4.4 -5.0 (±0.2). Another set of milk was fermented at 37°C for 24 h and then various concentrations of CL mixed with CE were added concurrently with E. fergusonii and stored for 5 days at 25oC (Figure 3B). The pH of the sample declined over the period of storage to a pH range of 3.5-2.8. At this temperature, the organism was undetectable in the control samples after 120 h as a consequence of this pH, but a more rapid decline was observed in all treated samples except CE alone.
3.4.2. Survival of S. typhimurium during storage in milk fermented at 25°C or 37oC then stored at 25oC: For these samples, milk was fermented at 25oC or 37oC for 24h and then different concentrations of CL and CE singly or in combination were added. These samples were also spiked with approx. 105 CFU/mL S. typhimurium and then stored at 25oC for 120 h (Figure 4A and B). In milk fermented at 25oC and then stored, S. typhimurium declined from the onset to undetectable levels after just after 48 in milk containing 0.0625% CL /0.1875% CE and within 72 h of storage in samples with 0.1875% CL / 0.0625% CE. In milk fermented at 37oC, the decline of the pathogen was even more marked with undetectable levels within 48 h in all treated samples.
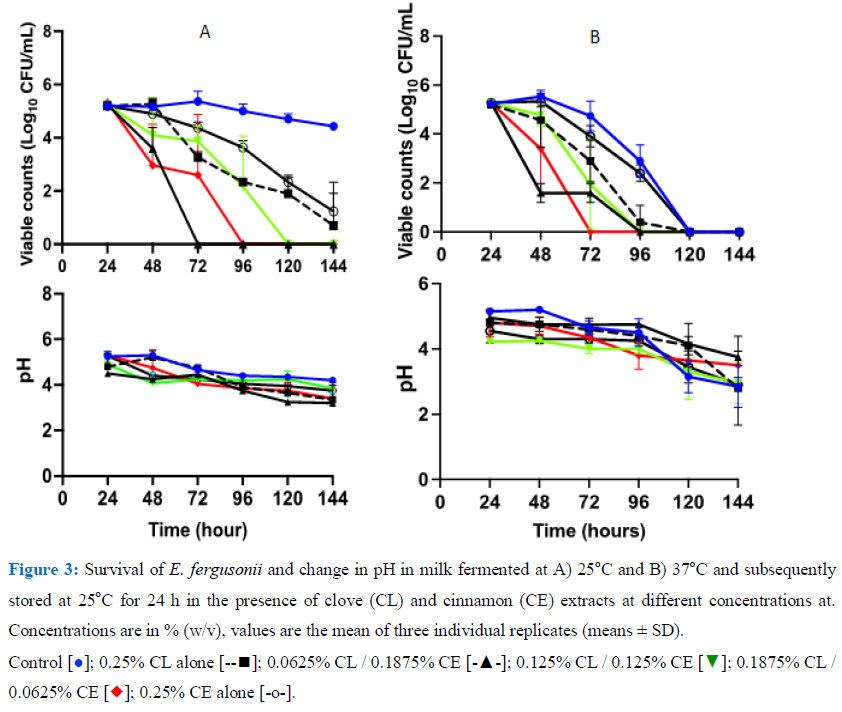
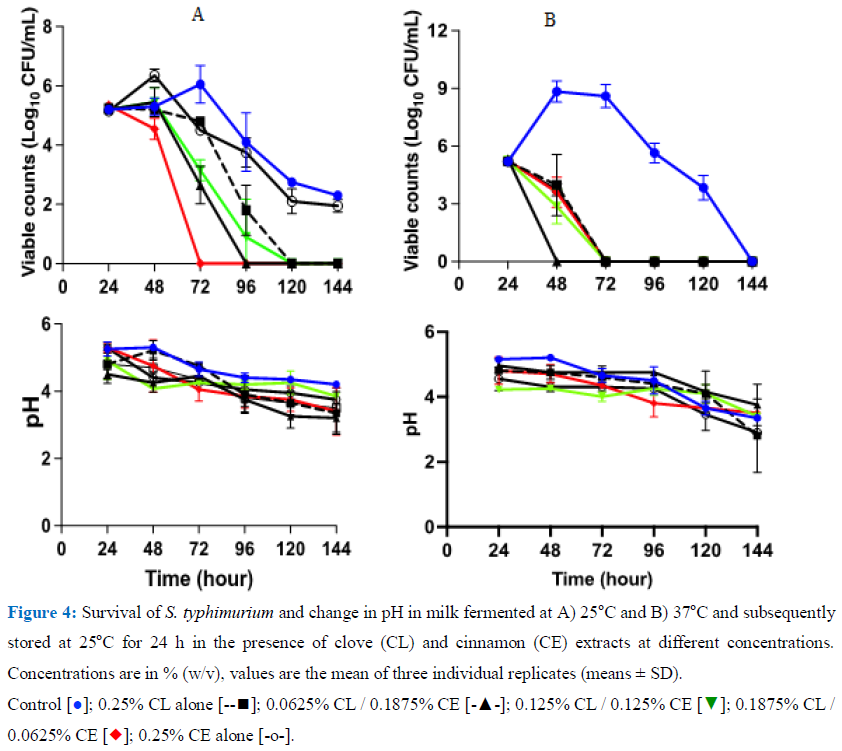
4. Discussion
4.1. Chemical composition of methanol clove buds and cinnamon bark extracts
Spices have been used all over the world in cuisine and beverages to impart extra flavour. EOs and volatile products of plant secondary metabolism have been used in food industry and medical research. The major constituents in cloves extract were identified as being eugenol (68%), caryophyllene (12.3%); and eugenol acetate (8%) (Table 2) and is agreement with the literature [36-40]. These reports focus on the constituents in cloves from the different places around the world but this the first report on analysis of clove buds sold in the markets in South Sudan. The antimicrobial activity displayed by CL and CE can be assigned to their high content in eugenol or cinnamaldehyde as reported by some researchers [41,42] but also could be influenced by the minor components. Ceylan and Fung [43] reported that phenolic, aldehyde and ketones were the principal compounds of cinnamon EO. However, in 2009, Goni et al. [44] reported that, the major components of cinnamon EO are trans-cinnamaldehyde or cinnamaldehyde. These are consistent with the results in this study, cinnamaldehyde was the major component with 83.46%. Li et al. [45] and Marongiu et al., [48] identified a range from 66.26-81.97% and 66.28-77.21% in cassia bark respectively. Shan et al., and Chairunnisa et al. [49, 50] reported a lower value of trans-cinnamaldehyde (56.10%) in cassia bark whilst Zhang [46] reported the highest content of 92.40%. According to Alma et al. [38] eugenol was 89.6% of clove bud and similar values were reported by Santinet al. [37] with 89.6% of the clove oil was eugenol. The report of Lv et al. [47] reported a slightly lower level of eugenol of 77.45%. In this study eugenol accounted for 68.77% which is consistent with the range reported by Uddin et al., [51] of 60-90% eugenol in cloves from different areas. Hazzit et al., [52]) however, concluded that spices do differ in components even if they were from the same species. Purseglove et al., [53]) reported that post-harvest processing, pre-treatment before extractions as well as the methods of extractions and treatment after the extract all affect the constituents of spices. According to Belcher [54] eugenol content of the oil is dependent on the time taken to distil the product and could vary within 0.41% - 3.11%. Not only the extraction method and Cinnamomum species affect the yield, but research have found that also the age and segment (top, center and lower) part of the tree do influence the yield. Besides, there are variations in the methods of extractions of spices or even processing method by different researchers which could have resulted into the variation of the quality and quantity and the constituent compositions of the spice extracts [55, 56].
4.2 Antimicrobial activity of CL and CE by agar well diffusion
The methanol extracts of clove and cinnamon were effective against E. fergusonii and S. typhimurium with zones of inhibition ranging from14 to 18 mm. The ZOIs of cloves against E. fergusonii or S. typhimurium were not significantly different at all the concentrations. There are reports that E. fergusonii is an emerging drug resistant pathogen [12,57] but a similar sensitivity to clove extract was found here to S. typhimurium. Ampicillin (10 µg) was included for comparison and the ZOI were 14.2±0.5 and 16 ± 0.3 mm for E. fergusonii and S. typhimurium respectively. According to CLSI [58], resistance is shown when the ZOI to ampicillin (10 µg) are < 13 mm; intermediate if ZOI are 14-16 mm and susceptible if >17 mm. This means both organisms show intermediate susceptibility to ampicillin. In comparison with clove and cinnamon extracts using this scale, at the highest concentration, E. fergusonii was susceptible to clove and showed intermediate susceptibility to cinnamon extract. Whereas S. typhimurium was susceptible to clove extract and showed intermediate sensitivity to cinnamon extract. In contrast, Galivance [59] reported that with agar well diffusion, cinnamon oil showed a stronger antimicrobial than clove oil against E. coli which is a close relative to E. fergusonii.
4.3 Effect of combined CL and CE on growth of E. fergusonii and S. typhimurium and change in pH of milk fermented at 25 or 37°C
The effect of CL and CE on growth and survival of E. fergusonii and S. typhimurium were investigated during fermentation by the LAB (pre-fermentation contamination). At 25oC, E. fergusonii and S. typhimurium grew in all the samples during the 24 h of fermentation, although growth was slightly reduced in the samples treated with the spice extracts. The results show that contamination of the milk before fermentation allows the pathogens to grow early in the fermentation when the acidity is low. Acidification is an important measure to control the growth and survival of pathogens and spoilage microorganisms. However, yoghurt and other acidic foods have been implicated in the foodborne outbreaks caused by pathogens such as E. coli O157:H7 [60]. Acid adaptation and increased resistance of pathogens such as Salmonella spp. have been reported [61,62]. Similar growth and survival of Salmonella typhimurium during fermentation of kefir a traditional fermented milk of Ethiopia was found [63]. E. fergusonii is reported as a drug resistant pathogen however, there no report on its resistance to fermentation acids. The temperature of the milk fermentation exerted a marked effect on the bacteria with 37°C resulting to faster reduction in viable numbers of the pathogens than 25°C which was associated with a lower pH due to the acid production at the higher temperature (Figures 1A and B). Reduction of pH during fermentation with the inclusion of the spice extracts also showed that the activities of lactic acid bacteria was not affected. At 37oC, the pathogens were completely inactivated after twenty-four hours of incubation with 0.1875% CL/0.0625% CE and 0.0625% CL/ 0.1875% CE. Inhibition of both E. fergusonii and S. typhimurium was enhanced due to the more rapid acidification of the milk at the higher temperature by the lactic acid bacteria. The observation in the present study suggests that the use of a higher temperature than the traditional fermentation temperature can restrict pathogen growth and would improve and enhance product quality of the fermented milk. The results suggest that both CL and CE are effective EOs capable of improving the preservation effects of milk fermentation against E. fergusonii or S. typhimurium. Clove extract showed a stronger antimicrobial effect than cinnamon extract when applied individually. The fractional combination of clove extract to cinnamon extract showed an additive effect against both E. fergusonii and S. typhimurium. The extent of the effect depended on the concentration of the EOs. Ogwaro et al. [64] reported similar results when black pepper extract was combined with clove extract. This work has shown that cloves combined with cinnamon enhances the inhibition of the bacteria. According to Moon et al. [65], at low pH, the antimicrobial molecules bind better to the hydrophobic zone of the membrane, where they are diluted in the lipid phase, improving their activities on bacteria and fungi. The increased inhibition at 37oC, the higher temperature, could be due to increased fluidity of the cytoplasmic membrane that occurs at warmer temperatures [65]. This effect is temperature dependent and explain the enhanced effect at the higher temperature. In addition, to temperature of fermentation, the effect of a combination of the spices showed a higher antimicrobial activity compared to a single application of both EOs. These findings may be useful for food applications, but their effect on sensory quality of various foods need to be studied. Moreover, where the amount of CL was higher than CE, the inhibition was increased and indicates that CL may act as a facilitator for enhancing the activity of CE. In an earlier report, Sinenky and Horváth et al. [66, 67] reported the ability of cinnamaldehyde to inhibit amino acid decarboxylases in Enterobacter aerogenes and suggested that this could be due to carbonyl group binding to proteins, thus preventing the action of amino acid decarboxylases. Trans-cinnamaldehyde is capable of inhibiting E. coli and Salmonella by gaining access to the periplasm and inhibiting the activity of transmembrane ATPase as well as without gaining access to the periplasm as well as the deeper parts of the bacterial cell [68]. The effect of the EOs depended on the composition of the mixture between the two oil extracts as well as the temperature of incubation.
4.4 Effect of combined concentrations of CL and CE on the survival of E. fergusonii and S. typhimurium and change in pH post fermentation
When the antimicrobial was applied post-fermentation then stored, the EOs were more effective than when applied pre-fermentation. This suggest that for optimum pathogen inactivation, the EOs need to be added once the pH has been reduced by the LAB fermentation. It was also observed that applying the EOs post milk fermentation promoted faster inhibitory effects (Figures 3 and 4). The pH decreased further during storage indicating that the lactic acid bacteria continued to be active degrading any lactose which was still remained in the yoghurt. The gradual decrease in pH during storage supports the report of Singh et al., [69] who also reported that acidity of fermented milk increased gradually during storage period of fermented milk. The growth limiting pH could be dependent on several factors, most importantly the acid molecule. Besides, the effect of fermentation temperature, relative oxygen supply as yoghurt becomes anaerobic after the curds are formed and contributes to inhibition of growth. As previously, during storage it was also evident that the antimicrobial activities of clove extract were stronger than that of cinnamon extract. The results also suggest that incorporation of EO into yoghurt (post fermentation) is more effective in inhibiting survival ofE. fergusonii and S. typhimurium, whereas application pre-fermentation of milk resulted to lower inhibitory effect. One main hurdle in using essential oils in fermented milk is that the quantity applied by the farmers are not sufficient as single components besides the negative organoleptic and colour change effects when added in amount sufficient to cause antimicrobial effect. Exploiting synergies between several compounds is suggested as a solution to this problem. Furthermore, during storage over a long period of time, essential bioactive components are lost quickly due to volatilzation, chemical degradation, and certain other physical chemical reactions. The stability, bioavailability, and bioactivity of the bioactive components could be resolved through encapsulation. Encapsulation is already used to preserve unstable food bioactive components, flavoring substances, and essential oil components by preventing their contact with moisture, light, and oxygen. In conclusion, this study has demonstrated that CL in combination with CE inhibited the growth of the pathogens E.fergusonii and S. typhimurium during fermentation and were more effective at 37 than 25oC. Addition of CL in combination with CE post fermentation where the pH is lower significantly reduced the survival of these pathogens. The use of these extracts both during fermentation and storage have the potential to enhance the microbiological safety of these products.
Author Contributions
B.O. performing methodology and writing original draft. H.G. and B.O. were the main persons responsible for planning the experiments and interpretation of the data; H.G., B.O., L.O.G, and D.H. were involved in the editing, reviewing and preparation of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding, but we would like to thank the Government of South Sudan for facilitating Betty Ogwaro through paying her monthly salaries through the study period and accepting her to take time off her duties to carry on the research.
Data availability statement
The data presented in this study are openly available.
Acknowledgments
We would like to acknowledge the contributions of the technical staff in the Microbiology and Chemistry laboratories for their technical support.
Conflicts of interest
The authors declare no conflicts of Interest.
References
- Musinga MD, Kimenye, Kivolonz P. The camel milk industry in Kenya. Results of a study commissioned by SNV to explore the potential of camel milk from Isiolo District to access sustainable formal markets. SNV world, The Hague, Netherlands: Nairobi, Kenya. (2008).
- Marshall E, Mejia D. Traditional Fermented Food and Beverages for Improved Livelihoods. Diversity Booklet 21, FAO, Rome (2011).
- Zhiqian Liu, Cheng Li, Jennie Pryce,et al.Comprehensive Characterization of Bovine Milk Lipids: Phospholipids, Sphingolipids, Glycolipids, and Ceramides.Journal of Agricultural and Food Chemistry 68 (2020): 6726-6738.
- Ahmedsham M, Amza N, Tamiru M. Review on milk and milk product safety, quality assurance and control. International Journal of Livestock Production 9 (2018): 67-78.
- Brock TD, Madigan MT.Biology of microorganisms,6th New Jersey: Prentice Hall (1991): 345
- Karenzi E, Mashaka A, Nshimiyimana AM, et al. Kivuguto traditional fermented milk and the dairy industry in Rwanda. A review. Biotechnology, Agronomy and Society and Environment 17 (2013): 383-391
- Misihairabgwi J, Cheikyoussef A. Traditional fermented foods and beverages of N Journal of Ethnic Foods 4 (2017): 145-153.
- Vallerdu-Queralt A, Regueiro J, Martinez-Huelamo M, et al. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: rosemary, thyme, oregano, cinnamon, cumin, and bay. Food Chemistry (2014): 299-307.
- Cava-Roda M, Taboada-Rodríguez A, Valverde-Franco MT, et al. Antimicrobial Activity of Vanillin and Mixtures with Cinnamon and Clove Essential Oils in ControllingListeriamonocytogenesandEscherichia coliO157:H7 in Milk.Food Bioprocess Technology5 (2012): 2120-2131
- Ogwaro BA, O’Gara EA, Hill DJ, et al. A Study of the Antimicrobial Activity of Combined Black Pepper and Cinnamon Essential Oils against Escherichia fergusonii in Traditional African Yoghurt. Foods 10 (2021): 28-47.
- Cava-Roda R, Taboada-Rodríguez A, López-Gómez A, et al. Synergistic antimicrobial activities of combinations of vanillin and essential oils of cinnamon bark, cinnamon leaves, and cloves. Foods 10 (2021): 1406.
- Savini V, Catavitello C, Talia M, et al. Multidrug-resistant Escherichia fergusonii: a case of acute cystitis.Journal of Clinical Microbiology 46 (2008): 1551-1552.
- Saad NM, Sabreen MS, Amin WF, et al. Prevalence ofEscherichia albertiiand otherEscherichiaspecies in raw milk and some dairy products in Assiut City, EgyptJournal of American Science 8 (2012): 333-341.
- Sherwood HP, Clegg LFL. Further studies of incubation at 44 °C: As a test for ‘faecal coli’Epidemiology Infections42 (1942): 45-54.
- Foster EM. The problem of salmonella in foods. Food Technology 23 (1969): 1178.
- Currie RW. Raw milk and human gastrointestinal disease in humans. Clinical Infectious Disease 43 (2006): 610-615.
- El-Gazzar FE, Marth EH. Dairy Foods: Salmonellae, Salmonellosis and Dairy Foods: A review. Journal of Dairy Science 75 (1992): 2327-2337.
- Chang, Shang-Tzen, Pin-Fun Chen, et al. Antibacterial activity of leaf essential oils and their constituents from Cinnamomum osmophloeum.Journal of ethnopharmacology77 (2001): 123-127.
- Goel MC,KulshresthaDC, MarthEH,et al. Fate of coliforms in yogurt, buttermilk, sour cream, and cottage cheese during refrigerated storage. Journal of milk and Food Technology 34(1971): 54.
- Adams MR, Hall CJ. Growth inhibition of foodborne pathogens by lactic acid and acetic acids and their mixtures. International Journal of Food Science and Technology 23 (1988): 287-292.
- Alvarez-Ordonez A, Prieto M, Bernard A, et al. The acid Tolerance response of Salmonella spp. An adaptive strategy to survive in stressful environments prevailing in foods and host. Food Research International 45 (2011): 482-492.
- Parle M, Deepa K. Clove: a champion spice: International Journal of Research Ayurveda Pharmacology. 2 (2011): 47-54.
- Chaieb K, Hajlaoui H, Zmantar T, et al. The chemical composition and biological activity of clove essential oil,Eugenia caryophyllata(Syzigium aromaticumL. Myrtaceae): A short review.Phytotherapy Research 21 (2007): 501-506.
- Kuang X, Li B, Kuang R, et al. Granularity and antibacterial activities of ultra-fine cinnamon and clove powders.Journal of Food Safety 31 (2011): 291-296.
- Xu JG, Liu T, Hu QP, et al. Chemical composition, antibacterial properties, and mechanism of action of essential oil from clove buds againstStaphylococcus aureus.Molecules 21 (2016): 1194
- Haro-González JN, Castillo-Herrera GA, Martínez-Velázquez M, et al. Clove Essential Oil (Syzygium aromaticumL. Myrtaceae): Extraction, Chemical Composition, Food Applications, and Essential Bioactivity for Human Health. Molecules 26 (2021): 6387.
- Burt S. Essential oils: Their antibacterial properties and potential applications in foods-A review.International Journal of Food Microbiology 94 (2004): 223-253.
- Ranasinghe P, Jayawardana R, Galappaththy P, et al. Efficacy and safety of “true” cinnamon (Cinnamomum zeylanicum) as a pharmaceutical agent in diabetes: A systematic review and meta-analysis.Diabetic. Medicine29 (2012): 1480-1492.
- Lomarat P, Phanthong P, Wongsariya K, et al. Bioautography-guided isolation of antibacterial compounds of essential oils from Thai spices against histamine-producing bacteria.Pakistan Journal of Pharmaceutical Sciences 26 (2013): 473-477
- Lai PK, Roy J. Antimicrobial and chemo-preventive properties of herbs and spices.Current Medical Chemistry11 (2004): 1451-1460.
- Gupta C, Garg AP, Uniyal RC, et al. Comparative analysis of the antimicrobial activity of cinnamon oil and cinnamon extract on some food-borne microbes.African Journal Microbiology Research 2 (2008): 247-251.
- Terzaghi BE, Sandine WE. Improved medium for lactic Streptococci and their bacteriophages.Applied Microbiology29 (1975): 807-813.
- Shankar PA, Davies FL. Recent developments in yoghurt starters: A note on the suppression ofLactobacillus bulgaricusin media containing beta-glycerophosphate and application of such media to selective isolation ofStreptococcus thermophilusfrom yoghurt.Society of Dairy Technology30 (1977): 28-30.
- De Man JC, Rogosa M, Sharpe EM. A medium for the cultivation of Lactobacilli.Journal Applied Bacteriology 23 (1960): 130-135.
- Azwanida NN. A review on the extraction methods use in medicinal plants, principle, strength and limitation.Med. Aromatic Plants4 (2015): 167-0412.
- Fei L, Yi-cheng D, Xian-qian YE, et al. Antibacterial Effect of Cinnamon oil Combined with Thyme or Clove oil. Agricultural Science 10 (2011): 1482-1487.
- Santin JR, Lemos M, Klein-Junior LC, et al. Gastroprotective activity of essential oil of Syzygium aromaticum and its major component eugenol in different animal models. Naunyn-Schmied Arch Pharmacol. 383 (2010): 149-158.
- AlmaMK, Ertas M, Nitz S, et al. Chemical composition and content of essential oil from the bud of cultivated Turkish clove (Syzygium aromaticun) Bio Resource 2 (2007): 265-269.
- Guan W, Li S, Yan R, et al. Comparison of essential oils of clove buds extracted with supercritical carbon dioxide and other three traditional extraction methods.Food Chemistry101 (2007): 1558-1564.
- Jirovetz L, Buchbauer G, Stoilova I, et al. Chemical composition and antioxidant properties of clove leaf essential oil.Journal of Agriculture and Food Chemistry 54 (2006): 6303-6307.
- López-Malo A, Palou E, Alzamora SM. Naturally occurring compounds-plant sources. InAntimicrobials in Food, 3rd edtn, Davidson, P.M., Sofos, J.N., Branen, A.L., Eds.; CRC Press: New York, NY, USA (2005): 429-451.
- Siddiqua S, Anusha BA, Ashwini LS, et al. Antibacterial activity of cinnamaldehyde and clove oil: Effect on selected foodborne pathogens in model food systems and watermelon juice. Journal of Food Science and Technology 52 (2014): 5834-5841.
- Ceylan E, Fung D, Sabah JR. Antimicrobial activity and synergistic effect of cinnamon with sodium benzoate or potassium sorbate in controllingEscherichia coliO157: H7 in apple juice.Journal of Food Science 69 (2004): M102-M106.
- Goñi P, López P, Sánchez C, et al. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chemistry 116 (2009): 982-989.
- Li, Yan-qun, De-xin Kong, et al. Analysis and evaluation of essential oil components of cinnamon barks using GC–MS and FTIR spectroscopy.Industrial Crops and Products 41 (2013): 269-278.
- Marongiu B, Alessandra P, Silvia Po, et al. Supercritical CO2 extract of Cinnamomum zeylanicum: chemical characterization and antityrosinase activity.Journal of agricultural and Food Chemistry 55 (2007): 10022-10027.
- Shan B, Cai YZ, Brooks JD, et al. Antibacterial properties and major bioactive components of cinnamon stick (Cinnamomum burmannii): Activity against foodborne pathogenic bacteria. Journal of Agricultural and Food Chemistry 55 (2007): 5484-5490.
- Chairunnisa, Tamhid HA, Nugraha AT. Gas chromatography – Mass spectrometry analysis and antibacterial activity of Cinnamomum burmanii essential oil to Staphylococcus aureus and Escherichia coli by gaseous contact. Proceedings from the 14th International Symposium on Therapeutic Ultrasound 1823 (2017): 020073.
- Zhang Y, Liu X, Wang Y, et al. Antibacterial activity and mechanism of cinnamon essential oil againstEscherichia coliandStaphylococcus aureus. Food Control 59 (2016): 282-289.
- Lv, Fei, Hao L, et al. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms.Food Research International 44 (2011): 3057-3064.
- Uddin MA, Shahinuzzaman M, Rana MS et al. Study of chemical composition and medicinal properties of volatile oil from clove buds (Eugenia caryophyllus). International Journal of Pharmaceutical Science Research 8 (2017): 895-99.
- Hazzit M., Baaliouamer A, Verissimo A, et al. Chemical composition, and biological activities of Algerian Thymus oils 116 (2009): 714-721.
- Purseglove JW, Brown EG, Green CL, et al. Spices: Longman Group Limited, London. Volumes 1 and 2 (1981).
- Belcher EFM. The Distillation of Clove Oils. In Christian, Karl (ed.). Perfume and Essential Oil Review. (1965): 148-151.
- Hardman R. Spices and Herbs: their families, secretary tissues and pharmaceutical aspects. Tropical Product Institute. Conference Proceedings. TPI, London (1973): 23-33.
- Kramer RE. Antioxidants in clove. J. Amer Oil Chem Soc 62 (1985): 111-113.
- Adesina T, Nwinyi O, De N, et al. First Detection of Carbapenem-ResistantEscherichia fergusoniiStrains Harbouring Beta-Lactamase Genes from Clinical Samples. Pathogens 8 (2019): 164.
- Clinical and Laboratory Standards Institute, Performance Standards for antimicrobial susceptibility Testing. Seventeenth Informational Supplement, Wayne, Pa. USA, 2010 M100 S17. CLSI.
- Galivanche AT. Antimicrobial Activity of Clove Oil and Cinnamon Oil against Escherichia coli. California State Science Project (2013): J1604.
- Griffin PM, Tauxe RV. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic coli and the associated haemolytic uremic syndrome. Epidemiology Reviews 13 (1991): 60-98.
- Goverd KA, Beech FW, Hobbs R, et al. The occurrence and survival of coliforms and salmonellas in apple juice and cider. Journal of Applied Bacteriology 46 (1979): 521-530.
- Foster JW, Hall HK. Adaptive acidification tolerance response of Salmonella typhimurium. Journal of Bacteriology 172 (1990): 771-778.
- Karagözlü LN, Karagözlü C, Ergönül B. Survival Characteristics of coli O157:H7, S. typhimurium and S. aureus during Kefir Fermentation. Czech J of Food Sci. 25 (2007): 202-207.
- Ogwaro BA, O’Gara EA, Hill DJ, et al. A Study of the Antimicrobial Activity of Combined Black Pepper and Cinnamon Essential Oils against Escherichia fergusonii in Traditional African Yoghurt. Foods 10 (2021): 2847.
- Moon DD, Delaquis P, Toivonen P, et al. Effect of vanillin on the fate ofListeria monocytogenesandEscherichia coliO157: H7 in a model apple juice medium and in apple juice.Food Microbiology 23 (2006): 169-174.
- Sinenky M. Homeoviscous adaptation- A homeostatic process that regulates viscosity of membrane lipids inEscherichia coli. Natl. Acad. Sci. USA 71 (1974): 522-525.
- Horváth I, Glatz A, Varvasovszki V, et al. Membrane physical state controls the signalling mechanism of the heat shock response in Synechocystis PCC 6803: Identification of hsp17 as a “fluidity gene” Natl. Acad. Sci. USA.95 (1998): 3513-3518.
- Hyldgaard M, Mygind T, Meyer RL. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front Microbiology 3 (2012): 1-24.
- Singh, Bijender, Gotthard Kunze, et al. Developments in biochemical aspects and biotechnological applications of microbial phytases.Biotechnology Molecular Biology Review6 (2011): 69-87.
