The Cardioprotective Effect of Leonurine in Hearts
Article Information
Z.Y. Lia, LZ Chena, YX Liub, Y.Z. Zhua,*
aState Key Laboratory of Quality Research in Chinese Medicine & School of Pharmacy, Macau University of Science and Technology, Avenida Wai Long, Taipa, Macau, China
bThe Second Medical Center of the Chinese PLA Genneral Hospital, Beijing, China
*Corresponding Author: Yi Zhun Zhu, State Key Laboratory of Quality Research in Chinese Medicine & School of Pharmacy, Macau University of Science and Technology, Avenida Wai Long, Taipa, Macau, Chinap
Received: 16 May 2020; Accepted: 26 May 2020; Published: 12 June 2020
Citation: Z.Y. Li, LZ Chen, YX Liu, Y.Z. Zhu. The Cardioprotective Effect of Leonurine in Hearts. Cardiology and Cardiovascular Medicine 4 (2020): 218-229.
View / Download Pdf Share at FacebookAbstract
Myocardial fibrosis is a common cardiovascular disease with a complex mechanism. Myofibroblasts and extracellular matrix play a key role in myocardial fibrosis. Leonurine, extracted from Herba Leonuri, plays a protective role in the pathogenesis and development of cardiovascular dieseses especially myocardial fibrosis. Undoubtedly, Leourine was considered as the potential therapeutic medicine of myocardial fibrosis in the future. However, the pathogenesis of leonurine on myocardial fibrosis remains unclear. The purpose of this review attempts to discuss the molecular mechanisms involved in the cardioprotective effects of Leonurine.
Keywords
Leonurine; Myocardial fibrosis; Cardioprotective effect; The molecular mechanism
Leonurine articles, Myocardial fibrosis articles, Cardioprotective effect articles, The molecular mechanism articles
Article Details
1. Introduction
leonurine (4-guanidino-n-butyl-syringate) is a bioactive alkaloid present in the traditional Chinese medicine Herba leonuri (1). In recent years, leonurine has been confirmed to treat cardiovascular diseases and has cardioprotective effects (2, 3), such as anti-oxidation (4), vasodilation (5), treatment of acute and chronic myocardial infarction (6) and ischemic stroke (7), anti-atherosclerosis (8). Recently, growing evidence has shown that leonurine played a protective role in the pathogenesis and development of heart diseases such as myocardial fibrosis (9). A study reported that leonurine could prevent cardiac fibrosis and the activation of cardiac fibroblasts partly through modulation of a NADOH Oxidase-Reactive Oxygen Species (Nox4-ROS) pathway (10). However, the mechanism of leonurine on myocardial fibrosis remains unclear. In this review, we summarize the physiological functions of leonurine and mainly explore its mechanism in myocardial fibrosis. Furthermore, we also discuss the molecular mechanisms involved in the cardioprotective effects of leonurine and how these might be used to overcome myocardial fibrosis.
2. Metabolisms of leonurine
Drug metabolism refers to the process of changing the chemical structure of a drug under the action of a variety of drug-metabolizing enzymes in the body such as liver drug enzymes. Clarifying the metabolism of leonurine in vivo was beneficial to understand the pharmacological mechanism of leonurine. Qing Z et al. found that an approach of HPlC/MS/MS could apply to the identification of metabolites of leonurine in rats (11). They also reported that HPlC/MS/MS could simultaneous quantify leonurine and stachydrine, the two main bioactive components in leonurus japonicus houtt (12). They firstly identify three metabolites including two phases II metabolites (M1 and M2) and one phase I metabolite (M3) in animal samples. M1 (MRM 488-312) was the main metabolites and M2 and M3 were the minor metabolites in vivo. M1 (MRM 488-312) was glucuronide metabolite of leonurine, M2 (MRM 392-312) was tentatively assigned as an O-sulfate conjugate, leonurine-10-O-sulfate. M3 (MRM 298-167) was identified as an O-demethylated leonurine and its possible structure was 4-guanidinobutyl 3,4-dihydroxy-5-methoxy benzoate (13, 14). Moreover, M1 had greater bioactivity than the prototype drug and the glucuronide metabolite could prolong the pharmacological effect of the parent drug trough an enzymatic or nonenzymatic hydrolysis (15, 16).
3. Cardioprotective Effect of leonurine
3.1 Roles of leonurine in Myocardial Fibrosis
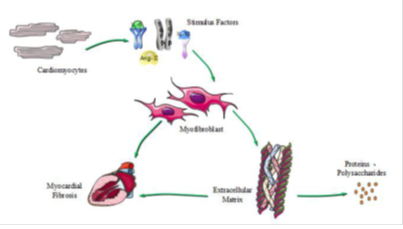
Myocardial Fibrosis (MF) refers to the appearance of normal myocardial tissue with cell proliferation and excessive deposition of extracellular matrix (ECM) (17, 18). MF is a type of chronic ischemic heart disease. Myocardial cell proliferation is dominated by a variety of non-cardiac cells, and more than 90% are cardiac fibroblasts (CFBs). CFBs are also important cells regulating the synthesis and degradation of ECM. CFBs proliferate and differentiate into specific conditions, and the formation of myofibroblasts is a key link to MF (19). At the same time, myofibroblasts secrete collagen, and collagen I and III are important for maintaining the structure of myocardial tissue and heart function (20), and the main mechanism of MF is shown in Figure1.
Figure 1: The main mechanism of MF. Cardiomyocytes differentiate into cardiac fibroblasts, and cardiac fibroblasts are proliferated, differentiated, or stimulated into myofibroblasts. The mainly stimulating factors are transforming growth factor-β (TGF-β), angiotensin-II (Ang-II), interleukin-1β (Il-1β) and interleukin-6 (Il-6). Myofibroblasts proliferate or secrete extracellular matrix, causing abnormal precipitation of extracellular matrix, and forming myocardial fibrosis.
In myocardial fibrosis models, they have been demonstrated clearly that methylation had effects on pathways of different stimulating factors (21). Pan et al. found that TGF-β inhibited DNA methyltransferase (DNMT) activity, thereby increasing collagen I expression (22). Watson et al. also found that hypoxia-inducible factor-1α (HIF-1α) promotes the expression of DNMT1 and DNMT3, reducing the expression of collagen I and α-smooth muscle actin (α-SMA), and also inhibiting the TGF-β profibrotic effect (23). Recently, many studies have shown that DMNTs could inhibit the activation and proliferation of CFBs. The specific mechanism is closely related to the level of DNA methylation, leading to a reduction in the generation of fibrosis. However, the mechanism of DNMTs such as DNMT1, DNMT3A, and DNMT3B on myocardial fibrosis is not clear.
3.2 Molecular Mechanisms of leonurine in Myocardial Fibrosis
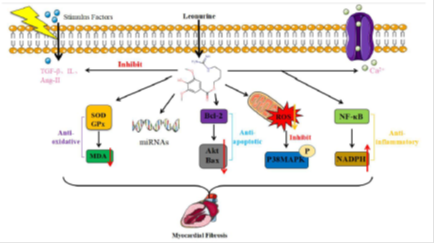
The effects of leonurine on myocardial fibrosis are mediated by a variety of targeting proteins and signaling molecules. The main mechanisms of leonurine against myocardial fibrosis are anti-oxidation, anti-apoptosis, anti-inflammation, stimulating stress responses, combining with target genes, regulating to stimulate factors and ion channels (Figure 2).
Figure 2: Different signaling proteins to show anti-myocardial fibrosis by leonurine. leonurine can against myocardial fibrosis via different mechanisms: leonurine mainly against myocardial fibrosis by anti-oxidation, target genes, anti-apoptosis, stress responses, anti-inflammation, stimulate factors, and ion channels. leonurine against oxidation by stimulating superoxide dismutase (SOD) and glutathione peroxidase (GPx) activity to reduce malondialdehyde (MDA) expression. leonurine plays roles by regulating the expression of microRNAs (miRNA). leonurine against apoptosis by stimulating B-cell lymphoma-2 (Bcl-2) to reduce the expression of protein kinase (AKT) and BCl2-associated x (Bax). leonurine activates stress responses by reducing reactive oxygen species (ROS) levels and inhibiting p38MAPK expression. leonurine against inflammation by stimulating nuclear factor-kappa-b (NF-κB) to increase nicotinamide adenine dinucleotide phosphate (NADPH) expression. leonurine opens ion channels such as Ca2+ channel and inhibits to stimulate factors such as TGF-β, Ang-II and Il to produce anti-myocardial fibrosis effects.
3.2.1 Anti-oxidative Action
Oxidative stress induces cell damage through the over-production of ROS, and ROS can aggravate myocardial fibrosis. Related studies showed that leonurine reduced ROS levels through an anti-oxidative effect to against myocardial fibrosis. liu et al. found that leonurine increased to SOD activity by decreasing levels of lactate dehydrogenase (lDH), creatine kinase (CK), and lipid peroxidation (3). At the same time, Zhang et al. also found that leonurine stimulated SOD and GPx activity to reduce MDA expression, and against oxidative effect (24).
3.2.2 Anti-apoptotic Action
Anti-apoptosis is another major means of anti-myocardial fibrosis. Studies have shown that leonurine regulated the anti-apoptotic effect against myocardial fibrosis by activating the phosphatidylinositol 3 kinase/protein kinase (PI3K/Akt) signaling pathway. liu et al. found that leonurine reduced Bax expression and increasing Bcl-2 expression by activating the PI3K/Akt signaling pathway, and against myocardial fibrosis (25).
3.2.3 Anti-inflammatory Action
Inflammatory is one of the most common reactions to most cardiovascular diseases. Studies have found that the most inflammatory factors had a regulatory effect on myocardial fibrosis. liu et al. found that leonurine stimulated NF-κB signaling activity to reduce TNF-α, Il-6, NADPH expression and play an anti-inflammatory effect (26, 27).
3.2.4 miRNA Expression
MicroRNAs (miRNA) are the class of non-coding single-stranded RNA molecules about twenty-two nucleotides in length encoded by endogenous genes, and most microRNAs play a role in myocardial fibrosis such as microRNA-24 and micro-221/222. Yuan et al. found that microRNA-378 was cardiac-enriched and highly inhibited during cardiac
remodeling (25). Wang et al. found that miR-24 was down-regulated in fibrosis after MI heart (30).
3.2.5 Ion Channels Regulation
The main ion channel for regulating myocardial fibrosis is Ca2+ channels. It had been reported that leonurine reduced the cytosolic Ca2+ overloaded induced by hypoxia (31). liu et al. also found that leonurine inhibited Bcl-2/Bax ration and reduced cytosolic Ca2+ overloaded against myocardial fibrosis (25).
3.3 Roles of leonurine in Other Cardiovascular Diseases
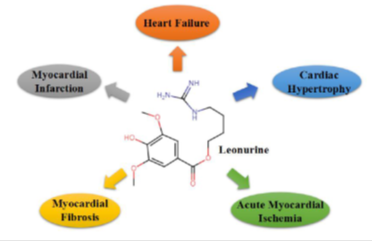
Recently, leonurine has long known to present as an alkaloid in Herba leonuri and exerts several pharmacological effects shown in Table 1, such as anti-apoptosis, anti-oxidation, anti-inflammation (32, 33, 34, 35). Furthermore, leonurine can exert its cardioprotective effects on cardiovascular diseases, such as myocardial infarction, heart failure, cardiac hypertrophy, acute myocardial ischemia, and myocardial fibrosis (Figure 3).
Table 1: The effects of leonurine in cardiovascular diseases
|
Effects |
Study subjects |
Major findings |
References |
|
Anti-oxidation |
Improving ischemia-induced myocardial injury through antioxidative activity Protecting middle cerebral artery occluded-rats from brain injury through the antioxidative mechanism and mitochondrial protection Protecting ischemia-induced brain injury via modulating SOD, MDA levels Protecting H9c2 rat ventricular cells from hypoxia-induced apoptosis |
↓ level of lDH and CK activities ↑ SOD activities ↓ MDA level ↑ SOD activities ↓ MDA level ↑ Bcl-2 gene ↓ Bax gene ↓ the cytosolic Ca2+ overload induced by hypoxia |
(3) (24) (31) (25) (3) |
|
Anti-apoptosis |
Improving cardiac recovery in the rat during chronic infarction. A mechanism through inhibition of mitochondria dysfunction in H9c2 cells |
Akt signaling pathway ↑ Survivin and vascular endothelial growth factor expression ↓ ROS in H2O2 stimulated cells Apoptotic body formation and release of cytochrome c |
(30) (4) (34) |
|
Anti-inflammation |
Exerting anti-inflammatory effect by regulating inflammatory signaling pathways Suppressing advanced glycation endproducts-induced NADPH oxidase |
↓ TNF-αIl-6 iNOS COX-2 TlR4 and NF-κB ↑ Il-10 ↑ NF-κB ↓ NADPH |
(45) (46) (47) |
|
Antiplatelet aggregation |
The structure and biological effect of leonurine |
Guanidyl group was changed to amino, add double bonds could enhance the effect |
(48) (48) |
|
Improve coronary flow |
Impact on blood parameters |
↓ ROS |
(49) (50) |
|
Regulate the mitochondrial function |
Involvement of mitochondrial function and HIF-1α dependent VEGF activation Improve the ultrastructure of mitochondrion |
↓ VEGF ↓ MDA and Bax ↑ SOD CAT and Bcl-2 |
(51) (52) |
lDH=lactate Dehydrogenase; CK=Creatine Kinase; SOD=Superoxide Dismutase; MDA=Malondialdehyde; Bcl-2=B cell lymphoma-2; Bax=BCl2-associated x; Ca2+=Calcium ion; Akt=Protein Kinase; ROS=Reactive Oxygen Species; TNF-α=Tumor Necrosis Factor-α; Il-6=Interleukin-6; iNOS=Inducible Nitric Oxide Synthase; COX-2=Cyclooxygenase-2; TlR4=Toll like Receptor 4; NF-κB=Nuclear Factor Kappa-B; NADPH=Nicotinamide Adenine Dinucleotide Phosphate; HIF-1α=Hypoxia-Inducible Factor-1α; VEGF=Vascular Endothelial Growth Factor; CAT=Catalase.
3.3.1 Myocardial Infarction
Myocardial infarction (MI), one of the ischemic heart diseases, remains the leading cause of death in the world (36). It occurs when a coronary artery is occluded, leading to insufficient oxygen supply to the myocardium and the most typical feature of MI is hypoxia (37, 38, 39). Recently, there are increasing evidence shows that leonurine is beneficial to cure MI. leourine increased phosphorylation of AKT and expression of hypoxia-inducible factor-1 (HIF-1), surviving vascular endothelial growth factor (VEGF) in rat models (9). leonurine significantly alleviated collagen deposition and MI size to inhibite cell apoptotic effect and improved myocardial function (40).
3.3.2 Heart Failure
Heart Failure (HF) is caused by impaired contraction and diastolic function of the heart, resulting in inadequate venous return to blood to the heart, leading to venous system stasis and insufficient blood perfusion in the arterial system, and leading to cardiac circulatory disorders. However, HF isn’t an independent diseases, but the end stage of the development of heart disease. Recently, evidence show that leonurine plays a role in relieving HF. leonurine provided protective effects on ischemic myocardium by acting as free radicals scavengers and inhibiting the formation of ROS (41).
3.3.3 Cardiac Hypertrophy
Cardiac hypertrophy is a powerful form of compensation, but it isn’t infinite. If there isn’t remission, the heart function may not be able to maintain normal for a long time and eventually turning to heart failure (42). Recently, only some studies show that leonurine has an inhibitory effect on cardiac hypertrophy. It had been reported that leonurine may reverse cardiac muscle cell hypertrophy by regulating p38 mitogen-activated protein kinase (p38MAPK) and downstream the gene expression (43).
3.3.4 Acute Myocardial Ischemia
Myocardial ischemia refers to reduced blood perfusion of the heart, resulting in reduced oxygen supply to the heart, abnormal energy metabolism in the heart muscle, and can not support normal heart work. Acute myocardial ischemia is a condition in which myocardial ischemia occurs in a short time. It had been reported that leonurine-cysteine reduced MDA and ROS levels to protect certain cellular organs and related expression of apoptosis-related genes and proteins, such as Bcl-2 and Bax (44).
Conclusion and Perspectives
The article summarized the function of leonurine in myocardial fibrosis and other cardiovascular diseases. And, the possible molecular mechanisms in myocardial fibrosis were discussed. The effects of leonurine in anti-myocardial fibrosis are mainly through anti-oxidant, anti-apoptosis, and anti-inflammation. Furthermore, microRNAs or DNMTs interacted with proteins are also related to myocardial fibrosis in some studies. Thus, the effect of leourine in cardiovascular diseases especially myocardial fibrosis is promised and may be helpful to reduce the risk of cardiovascular diseases in the future.
Author Contributions
Original draft preparation - Z.Y. li with assistant from lZ Chen and YX liu Review and PI - Y.Z. Zhu
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the Macau Science and Technology Development Fund (FDCT, 067/2018/A2 and 033/2017/AMJ, 0007/2019/AKP, 0052/2020/A).
References
- Kong YC, Yeung HW, Cheung YM, et al. Isolation of the uterotonic principle from leonurus artemisia, the Chinese motherwort. The American Journal of Chinese Medicine 4 (1976): 373-382.
- liu XH, Pan ll, Chen PF, et al. leonurine improves ischemia-induced myocardial injury through antioxidative activity. Phytomedicine 17 (2010): 753-759.
- luo S, Gu X, Zhu YZ. P100 SPRC-leonurine conjugate alleviates ischemia induced cardiac fibrosis through ROS-Stat3 pathway. Nitric Oxide 39 (2014): S46.
- Hua liu X, long Pan l, Hai Gong Q, et al. Antiapoptotic effect of novel compound from Herba leonuri-leonurine (SCM-198): a mechanism through inhibition of mitochondria dysfunction in H9c2 cells. Current Pharmaceutical Biotechnology 11 (2010): 895-905.
- Pang S, Tsuchiya S, Horie S, et al. Enhancement of phenylephrine-induced contraction in the isolated rat aorta with endothelium by H2O-extract from an Oriental medicinal plant leonuri herba. The Japanese Journal of Pharmacology 86 (2001): 215-222.
- loh KP, Qi J, Tan BK, et al. leonurine protects middle cerebral artery occluded rats through antioxidant effect and regulation of mitochondrial function. Stroke 41 (2010): 2661-2668.
- liu X, Pan l, Wang X, et al. leonurine protects against tumor necrosis factor-α-mediated inflammation in human umbilical vein endothelial cells. Atherosclerosis 222 (2012): 34-42.
- Zhang Y, Guo W, Wen Y, et al. SCM-198 attenuates early atherosclerotic lesions in hypercholesterolemic rabbits via modulation of the inflammatory and oxidative stress pathways. Atherosclerosis 224 (2012): 43-50.
- liu XH, Pan ll, Deng HY, et al. leonurine (SCM-198) attenuates myocardial fibrotic response via inhibition of NADPH oxidase 4. Free Radical Biology and Medicine 54 (2013): 93-104.
- liu XH, Xin H, Hou AJ, et al. Protective effects of leonurine in neonatal rat hypoxic cardiomyocytes and rat infarcted heart. Clinical and Experimental Pharmacology and Physiology 36 (2009): 696-703.
- Wang J, Huang W, Xu R, et al. Micro RNA-24 regulates cardiac fibrosis after myocardial infarction. Journal of Cellular and Molecular Medicine 16 (2012): 2150-2160.
- liu, Z., Zhang, Y., Hua, Y.F., et al. Metabolism of a sulfur-containing heteroarotionoid antitumor agent, SHetA2, using liquid chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry: An International Journal Devoted to the Rapid Dissemination of Up-to-the-Minute Research in Mass Spectrometry 22 (2008): pp. 3371-3381.
- Zhu Q, Zhang J, Yang P, et al. Characterization of metabolites of leonurine (SCM-198) in rats after oral administration by liquid chromatography/tandem mass spectrometry and NMR spectrometry. The Scientific World Journal (2014).
- Holcapek M, Kolárová l, Nobilis M. High-performance liquid chromatography–tandem mass spectrometry in the identification and determination of phase I and phase II drug metabolites. Analytical and Bioanalytical Chemistry 391 (2008): 59-78.
- Wen YQ, Gong lY, Wang l, et al. Comparative pharmacokinetics study of leonurine and stachydrine in normal rats and rats with cold-stagnation and blood-stasis primary dysmenorrhoea after the administration of leonurus japonicus houtt electuary. Journal of separation science 42 (2019): 1725-1732.
- Shipkova M, Wieland E. Glucuronidation in therapeutic drug monitoring. Clinica Chimica Acta 358 (2005): 2-3.
- Daniels A, Van Bilsen M, Goldschmeding R, et al. Connective tissue growth factor and cardiac fibrosis. Acta Physiologica 195 (2009): 321-338.
- Martos R, Baugh J, ledwidge M, et al. Clinical Perspective. Circulation 115 (2007): 888-895.
- Ren M, li X, Hao l, et al. Role of tumor necrosis factor alpha in the pathogenesis of atrial fibrillation: A novel potential therapeutic target?. Annals of Medicine 47 (2015): 316-324.
- Xie J, Zhang Q, Zhu T, et al. Substrate stiffness-regulated matrix metalloproteinase output in myocardial cells and cardiac fibroblasts: Implications for myocardial fibrosis. Acta Biomaterialia 10 (2014): 2463-2472.
- Metes-Kosik N, luptak I, DiBello PM, et al. Both selenium deficiency and modest selenium supplementation lead to myocardial fibrosis in mice via effects on redox-methylation balance. Molecular Nutrition & Food Research 56 (2012): 1812-1824.
- Pan X, Chen Z, Huang R, et al. Transforming growth factor β1 induces the expression of collagen type I by DNA methylation in cardiac fibroblasts. PloS one 8 (2013).
- Zheng S, Zhu J, li J, et al. leonurine protects ischemia-induced brain injury via modulating SOD, MDA and GABA levels. Frontiers of Agricultural Science and Engineering 6 (2019): 197-205.
- liu X, Pan l, Gong Q, et al. leonurine (SCM-198) improves cardiac recovery in rat during chronic infarction. European Journal of Pharmacology 649 (2010): 236-241.
- Yeung HW, Kong YC, lay WP, et al. The structure and biological effect of leonurine. Planta Medica 31 (1977): 51-56.
- Watson CJ, Collier P, Tea I, et al. Hypoxia-induced epigenetic modifications are associated with cardiac tissue fibrosis and the development of a myofibroblast-like phenotype. Human Molecular Genetics 23 (2014): 2176-2188.
- liu XH, Pan ll, Yang HB, et al. leonurine attenuates lipopolysaccharide-induced inflammatory responses in human endothelial cells: involvement of reactive oxygen species and NF-κB pathways. European Journal of Pharmacology 680 (2012): 108-114.
- Fiedler J, Batkai S, Thum T. MicroRNA-based therapy in cardiology. Herz 39 (2014): 194-200.
- Kim HK, Park HR, lee JS, et al. Down-regulation of iNOS and TNF-α expression by kaempferol via NF-κB inactivation in aged rat gingival tissues. Biogerontology 8 (2007): 399-408.
- liu XH, Chen PF, Pan ll, et al. 4-Guanidino-n-butyl syringate (leonurine, SCM 198) protects H9c2 rat ventricular cells from hypoxia-induced apoptosis. Journal of Cardiovascular Pharmacology 54 (2009): 437-444.
- Yeung HW, Kong YC, lay WP, et al. The structure and biological effect of leonurine. Planta Medica 31 (1977): 51-56.
- Yang l, Qiao Y, liu G, et al. Effects of dietary supplementation with leonurine hydrochloride on growth performance, immune response, antioxidant capacity and blood parameters in male broiler chicks. Journal of Applied Animal Research 46 (2018): 1490-1495.
- Sitarek P, Skala E, Wysokinska H, et al. The effect of leonurus sibiricus plant extracts on stimulating repair and protective activity against oxidative DNA damage in CHO cells and content of phenolic compounds. Oxidative Medicine and Cellular longevity (2016).
- Qi J, Wang JJ, Duan Jl, et al. leonurine Improves age-dependent impaired angiogenesis: Possible involvement of mitochondrial function and HIF-1α dependent VEGF activation. Frontiers in Pharmacology 6 (2017): 284.
- Xu l, Jiang X, Wei F, et al. leonurine protects cardiac function following acute myocardial infarction through anti-apoptosis by the PI3K/AKT/GSK3β signaling pathway. Molecular medicine reports 18 (2018): 1582-1590.
- Tanaka M, Ito H, Adachi S, et al. Hypoxia induces apoptosis with enhanced expression of Fas antigen messenger RNA in cultured neonatal rat cardiomyocytes. Circulation Research 75 (1994): 426-433.
- Olivetti G, Quaini F, Sala R, et al. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. Journal of Molecular and Cellular Cardiology 28 (1996): 2005-2016.
- Anversa P, Cheng W, liu Y, et al. Apoptosis and myocardial infarction. Basic Research in Cardiology 93 (1998): s008-12.
- Xu l, Jiang X, Wei F, et al. leonurine protects cardiac function following acute myocardial infarction through anti-apoptosis by the PI3K/AKT/GSK3β signaling pathway. Molecular Medicine Reports 18 (2018): 1582-1590.
- Yuping XU, Haibing Q . Experimental Study of leonurine on Rats with Heart Failure after Myocardial Infarction. Chinese Medicine Modern Distance Education of China 11 (2015): 144-145.
- Indolfi C, Di lorenzo E, Perrino C, et al. Hydroxymethylglutaryl coenzyme A reductase inhibitor simvastatin prevents cardiac hypertrophy induced by pressure overload and inhibits p21 ras activation. Circulation 106 (2002): 2118-2124.
- Kong YC, Yeung HW, Cheung YM, et al. Isolation of the uterotonic principle from leonurus artemisia, the Chinese motherwort. The American Journal of Chinese Medicine 4 (1976): 373-382.
- liu C, Guo W, Shi X, et al. leonurine-cysteine analog conjugates as a new class of multifunctional anti-myocardial ischemia agent. European Journal of Medicinal Chemistry 46 (2011): 3996-4009.
- Kim HK, Park HR, lee JS, et al. Down-regulation of iNOS and TNF-α expression by kaempferol via NF-κB inactivation in aged rat gingival tissues. Biogerontology 8 (2007): 399-408.
- Kim JM, lee EK, Kim DH, et al. Kaempferol modulates pro-inflammatory NF-κB activation by suppressing advanced glycation endproducts-induced NADPH oxidase. Age 32 (2010): 197-208.
- Yeung HW, Kong YC, lay WP, et al. The structure and biological effect of leonurine. Planta Medica 31 (1977): 51-56.
- Yang l, Qiao Y, liu G, et al. Effects of dietary supplementation with leonurine hydrochloride on growth performance, immune response, antioxidant capacity and blood parameters in male broiler chicks. Journal of Applied Animal Research 46 (2018): 1490-1495.
- Sitarek P, Skala E, Wysokinska H, et al. The effect of leonurus sibiricus plant extracts on stimulating repair and protective activity against oxidative DNA damage in CHO cells and content of phenolic compounds. Oxidative Medicine and Cellular longevity (2016).
- Qi J, Wang JJ, Duan Jl, et al. leonurine Improves age-dependent impaired angiogenesis: Possible involvement of mitochondrial function and HIF-1α dependent VEGF activation. Frontiers in pharmacology 8 (2017): 284.
- liu H, Zhang X, Du Y, et al. leonurine protects brain injury by increased activities of UCP4, SOD, CAT and Bcl-2, decreased levels of MDA and Bax, and ameliorated ultrastructure of mitochondria in experimental stroke. Brain research 1474 (2012):73-81.
- Qi GM, Jia lX, li Yl, et al. Adiponectin suppresses angiotensin II-induced inflammation and cardiac fibrosis through activation of macrophage autophagy. Endocrinology 155 (2014): 2254-2265.