Temporal Lobe Epilepsy in Multiple Sclerosis Resembling Autoimmune Limbic Encephalitis
Article Information
Fatme Seval Ismail1*, Christoph Andreas Lehrich2*, André Dik2, Christine Strippel2, Attila Rácz3, Sven Fuest4, Christoph Müller2, Andreas Johnen2, Stjepana Kovac2,Susanne Knake4,Rainer Surges3, Jörg Wellmer1, Christian E Elger2,3, Sven G Meuth2,5, Nico Melzer2,5
1Department of Neurology, University Hospital Knappschaftskrankenhaus, Ruhr University Bochum, Bochum, Germany
2Department of Neurology with Institute of Translational Neurology, University of Muenster, Muenster, Germany
3Department of Epileptology, University Hospital Bonn, Bonn, Germany
4Department of Neurology, University of Marburg, Marburg, Germany
5Department of Neurology, Medical Faculty, Heinrich-Heine University Düsseldorf, Düsseldorf, Germany
*Corresponding Author: Nico Melzer, Department of Neurology, Medical Faculty, Heinrich-Heine University of Düsseldorf, Moorenstraße 5, 40225 Düsseldorf, Germany.
Received: 10 October 2021; Accepted: 22 October 2021; Published: 14 December 2021
Citation: Ismail FS, Lehrich CA, Dik A, Strippel C, Racz A, Fuest S, Muller C, Johnen A, Kovac S, Knake S, Surges R, Welmer J, Elger CE, Meuth SG, Melzer N. Temporal Lobe Epilepsy in Multiple Sclerosis Resembling Autoimmune Limbic Encephalitis. Archives of Clinical and Medical Case Reports 5 (2021): 941-949.
View / Download Pdf Share at FacebookAbstract
Background: Temporal lobe seizures are typical symptoms in patients with Autoimmune Limbic Encephalitis (ALE), but also occur in the context of Multiple Sclerosis (MS). We here report a series of four patients with Temporal Lobe Epilepsy (TLE) and MS with features of ALE, focusing on clinical, MRI, EEG and serological findings.
Case series: Four patients diagnosed with relapsingremitting (RR) or primary progressive (PP) forms of MS according to the amended McDonald criteria from 2017 and TLE with additional features of ALE (according the Graus criteria from 2016) were retrospectively identified based on medical records from five German tertiary epilepsy centers. All patients (female) suffered from additional limbic symptoms including memory disturbances and depression. All patients had additional signs of bilateral temporomesial affection on MRI and bilateral temporal slowing and epileptiform activity on EEG. Three patients had CSFspecific oligoclonal bands. In two patients TLE with additional limbic symptoms preceded MS, in one patient MS preceded TLE, and in one patient TLE and MS occurred simultaneously. In all cases, serum and CSF samples were negative for well-characterized neuronal antibodies.
Conclusions: Our case series showed that TLE and MS symptoms may resemble the symptoms of ALE. Diagnosing of overlapping MS and ALE could not be made definitively because neither clinical symptoms nor temporomesial MRI findings could help clearly to distinguish between MS and antibody-negative ALE.
Keywords
Multiple sclerosis; Temporal lobe epilepsy; Autoimmune limbic encephalitis; MRI
Multiple sclerosis articles; Temporal lobe epilepsy articles; Autoimmune limbic encephalitis articles; MRI articles
SARS-CoV-2 articles SARS-CoV-2 Research articles SARS-CoV-2 review articles SARS-CoV-2 PubMed articles SARS-CoV-2 PubMed Central articles SARS-CoV-2 2023 articles SARS-CoV-2 2024 articles SARS-CoV-2 Scopus articles SARS-CoV-2 impact factor journals SARS-CoV-2 Scopus journals SARS-CoV-2 PubMed journals SARS-CoV-2 medical journals SARS-CoV-2 free journals SARS-CoV-2 best journals SARS-CoV-2 top journals SARS-CoV-2 free medical journals SARS-CoV-2 famous journals SARS-CoV-2 Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Multiple sclerosis articles Multiple sclerosis Research articles Multiple sclerosis review articles Multiple sclerosis PubMed articles Multiple sclerosis PubMed Central articles Multiple sclerosis 2023 articles Multiple sclerosis 2024 articles Multiple sclerosis Scopus articles Multiple sclerosis impact factor journals Multiple sclerosis Scopus journals Multiple sclerosis PubMed journals Multiple sclerosis medical journals Multiple sclerosis free journals Multiple sclerosis best journals Multiple sclerosis top journals Multiple sclerosis free medical journals Multiple sclerosis famous journals Multiple sclerosis Google Scholar indexed journals Temporal lobe epilepsy articles Temporal lobe epilepsy Research articles Temporal lobe epilepsy review articles Temporal lobe epilepsy PubMed articles Temporal lobe epilepsy PubMed Central articles Temporal lobe epilepsy 2023 articles Temporal lobe epilepsy 2024 articles Temporal lobe epilepsy Scopus articles Temporal lobe epilepsy impact factor journals Temporal lobe epilepsy Scopus journals Temporal lobe epilepsy PubMed journals Temporal lobe epilepsy medical journals Temporal lobe epilepsy free journals Temporal lobe epilepsy best journals Temporal lobe epilepsy top journals Temporal lobe epilepsy free medical journals Temporal lobe epilepsy famous journals Temporal lobe epilepsy Google Scholar indexed journals Autoimmune limbic encephalitis articles Autoimmune limbic encephalitis Research articles Autoimmune limbic encephalitis review articles Autoimmune limbic encephalitis PubMed articles Autoimmune limbic encephalitis PubMed Central articles Autoimmune limbic encephalitis 2023 articles Autoimmune limbic encephalitis 2024 articles Autoimmune limbic encephalitis Scopus articles Autoimmune limbic encephalitis impact factor journals Autoimmune limbic encephalitis Scopus journals Autoimmune limbic encephalitis PubMed journals Autoimmune limbic encephalitis medical journals Autoimmune limbic encephalitis free journals Autoimmune limbic encephalitis best journals Autoimmune limbic encephalitis top journals Autoimmune limbic encephalitis free medical journals Autoimmune limbic encephalitis famous journals Autoimmune limbic encephalitis Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals MRI articles MRI Research articles MRI review articles MRI PubMed articles MRI PubMed Central articles MRI 2023 articles MRI 2024 articles MRI Scopus articles MRI impact factor journals MRI Scopus journals MRI PubMed journals MRI medical journals MRI free journals MRI best journals MRI top journals MRI free medical journals MRI famous journals MRI Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals Torticollis articles Torticollis Research articles Torticollis review articles Torticollis PubMed articles Torticollis PubMed Central articles Torticollis 2023 articles Torticollis 2024 articles Torticollis Scopus articles Torticollis impact factor journals Torticollis Scopus journals Torticollis PubMed journals Torticollis medical journals Torticollis free journals Torticollis best journals Torticollis top journals Torticollis free medical journals Torticollis famous journals Torticollis Google Scholar indexed journals
Article Details
Abbreviations:
AHS: ammon´s horn sclerosis; ANA: anti-nuclear antibodies; ANCA: anti-neutrophil cytoplasmic antibodies; AMPAR: α-amino-hydroxy-methyl-isoxazolepropionic acid receptor; ACE: angiotensin converting enzyme; AQP4: aquaporin-4; CASPR2: contactin-associated protein-like 2; CMV: cytomegalovirus; ds-DNA: double stranded DNA antibodies; EBV: Epstein–Barr virus; ENA: extractable nuclear antigen antibodies; GABABR: gamma-amino butyric acid B receptor; GAD: glutamic acid decarboxylase; HHV-6: human herpesvirus 6; HSV1/2: herpes simplex virus ½; LGI1: leucine-rich, glioma inactivated 1; LE: limbic encephalitis; MOG: myelin oligodendrocyte glyco-protein; MS: multiple sclerosis; NMOSD: neuromyelitis optica spectrum disorders; NMDAR: N-methyl-D-aspartate receptor; OCB: oligoclonal bands; PPMS: primary progressive multiple sclerosis; RF: rheumatoid factor; RRMS: relapsing-remitting multiple sclerosis; VZV: varicella zoster virus
1. Background
Temporal lobe seizures are typical symptoms at disease onset in patients with autoimmune limbic encephalitis (ALE) due to inflammation of temporomesial structures [1], but patients especially with antibodies (abs) against intracellular neuronal antigens are prone to develop temporal lobe epilepsy (TLE) during the disease course [2-3]. Especially ALE with abs against intracellular glutamic acid decarboxylase 65 (GAD65) is associated with often pharmacoresistant (temporal lobe) epilepsy [2-4]. In a recent study, neuronal abs were found in 17% of patients with temporal lobe seizures and additional signs of limbic involvement [5]. The frequency of seizures/epilepsy in patients with other autoimmune CNS diseases such as multiple sclerosis (MS) ranges from 1.5% to 7.8% among the studies, but it is rare compared to the frequency in ALE patients [2-3, 6-7]. The causes for increased risk of epilepsy in MS patients are not completely understood. Previous study described a link between increasing disease duration, severity of MS and risk of epilepsy [7]. Further studies indicated that cortical inflammation and degenerative cortical grey matter lesions, especially in the temporal lobe, could enhance the susceptibility to seizures in MS [8-9]. One case of an association between seronegative possible ALE and MS has been published so far [10]. The association of autoimmune encephalitis (e.g., anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis) with demyelinating syndromes was previously described [11-14]. We here report a series of four patients with TLE and MS resembling ALE, focusing on clinical, MRI, EEG and serological findings.
2. Case series
Four patients diagnosed with MS according to the amended McDonald criteria from 2017 and TLE with additional features of ALE (according the Graus criteria from 2016) were retrospectively identified based on medical records from five German tertiary epilepsy centers [1, 15]. All patients were female, two of them had clinical and paraclinical manifestations of relapsing-remitting MS (RRMS) and two of primary progressive MS (PPMS). Detailed clinical and paraclinical features of the patients are summarized in Table 1.
All patients suffered from temporal lobe seizures with additional limbic symptoms including (figural and verbal) memory disturbances (either clinically or upon neuropsychological testing) and depression which occurred subacutely in temporal relation with each other. All patients had bilateral (anterior) temporal slowing and epileptiform activity on EEG. In two patients TLE with features of ALE preceded MS (case No. 1 and 4, Table 1) for 2 and 3 years respectively. The brain MRI showed left > right temporomesial T2-/FLAIR-signal and volume increase without contrast-enhancement in addition to MS-related changes in both cases (case no. 1, Figure 1 a; case no. 4, Figure 1 d). In one patient MS preceded TLE for 7 years. Bilateral Ammon´s horn sclerosis (AHS) was detected in this patient as signs of bilateral temporomesial affection on MRI in addition to MS signs (case no. 3, Figure 1 c). In another patient TLE and MS occurred simultaneously. The patient suffered from episodes of dysaesthesia of the right arm and optic neuritis in addition to limbic symptoms including figural and verbal memory disturbance, depression and epileptic seizures of temporal lobe origin at disease onset.
The brain MRI showed left temporomesial T2-/FLAIR-signal and volume increase with right AHS in addition to multiple periventricular, juxtacortical and infratentorial lesions consistent with MS (case no. 2, Figure 1 b). One patient had autoimmune thyreoditis as additional autoimmune comorbidity, but none had cancer. CSF investigations were performed in all patients and are shown in Table 1 at the time of diagnosis of TLE. None of the patients had CSF pleocytosis. Three patients had CSF-specific oligoclonal bands (OCBs).
In all cases, serum and CSF samples were negative for well-characterized abs against intracellular (Hu, Ri, Ma/Ta, CV2/CRMP5, amphiphysin, GAD65) and neural surface antigens (NMDAR, α-amino-hydroxy-methyl-isoxazolepropionic acid receptor (AMPAR), contactin-associated protein-like 2 (CASPR2), leucine-rich, glioma inactivated 1 (LGI1), gamma-amino butyric acid B receptor (GABABR), aquaporin-4 (AQP4), and myelin oligodendrocyte glyco-protein (MOG) typically associated with ALE and neuromyelitis optica spectrum disorders (NMOSD)). CSF samples were negative for viral (VZV, HSV1/2, CMV, EBV, HHV-6) and bacterial pathogens (borrelia burgdorferi, treponema pallidum, tropheryma whippeli), and an extensive panel for rheumatological-vasculitic disorders (ANA, ENA, ANCA, rheumatoid factor (RF), ds-DNA-antibodies, angiotensin converting enzyme (ACE), phospholipid-antibodies) was negative.
Two patients received specific immunotherapy for RRMS (case no. 2 and 3, Table 1) and the other 2 patients were treated with recurrent glucocorticoids for PPMS (case no. 1 and 4, Table 1). In addition, two patients received recurrent glucocorticoids for suspected ALE as a second disease entity besides MS (case no. 1 and 4, Table 1). All patients were also treated with anticonvulsive drugs and some also with antidepressant and antipsychotic drugs (Table 1).
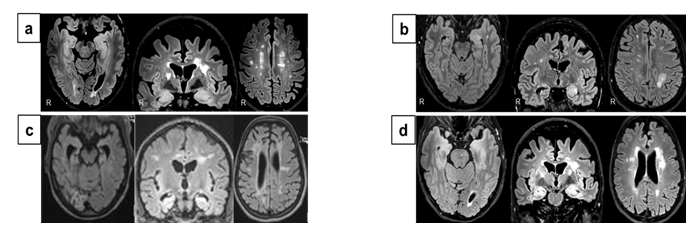
Figure 1: Bilateral temporomesial MRI changes in four patients with temporal lobe epilepsy and Multiple Sclerosis (MS) with features of autoimmune limbic encephalitis. (a,d) Transversal and coronal brain MRIs show left > right temporomesial FLAIR-hypersignal and volume increase (without contrast-enhancement, not shown) in addition to MS-related changes; (b) Left temporomesial FLAIR-hypersignal and volume increase with right Ammon´s horn sclerosis in addition to multiple periventricular, juxtacortical and infratentorial lesions consistent with MS on transversal and coronal brain MRIs; (c) Bilateral Ammon´s horn sclerosis and MS-related changes.
Table 1: Detailed clinical and paraclinical features of the patients with temporal lobe epilepsy and multiple sclerosis with features of autoimmune limbic encephalitis.
3. Discussion
Our case series shows that MS can cause limbic syndrome with temporal lobe seizures and features of autoimmune limbic encephalitis. Previous studies demonstrated occurrence of overlapping demyelinating syndromes and autoimmune encephalitis [10-14]. But diagnosing of these two entities as overlap is challenging in clinical practice. Differential diagnosis of limbic syndrome includes also a broad spectrum of non-autoimmune diseases e.g., CNS infections (herpes simplex virus encephalitis, HHV-6 encephalitis, Neurosyphilis, Whipple disease, HIV) or brain tumors (glioma) [1]. After extensive diagnostic work-up, alternative causes in our patients were excluded.
There are limited data on the frequency, clinical characteristics and outcome of patients with seronegative ALE. In a recent study, 7% of patients with ALE remained seronegative and older males were more affected [16]. None of these patients had additional demyelinating syndrome [16]. Patients with MS or ALE usually have different clinical features. In MS patients, according to location of lesions focal-neurological deficits represent the main symptoms. In ALE patients, temporal lobe seizures, memory deficits and psychiatric symptoms are the typical symptoms at disease onset due to inflammation of temporomesial structures [1]. In contrast, cognitive dysfunction in MS patients occur in more advanced stages of disease and correlates with the disease severity [17]. The frequency of seizures/epilepsy in patients with MS is rare compared to frequency in ALE patients [2-3, 6-7]. Etiopathogenesis of seizures in MS is not completely understood, but studies demonstrated MRI and histopathological atrophy with demyelination in the hippocampus of MS patients [18-20]. Otherwise, bilateral hippocampal T2/FLAIR abnormalities on brain MRI are related to ALE and one of the required criteria for diagnosis of definite ALE [1]. Of course, MRI cannot reliably distinguish between both possibilities in our cohort. But subacute limbic symptoms (especially seizures) in temporal relation with each other and in line with bilateral temporomesial MRI and EEG changes were part of the key features in our patients and could point to the diagnosis of ALE. In case no. 2, MS symptoms and TLE with additional limbic signs occurred simultaneously, but memory deficits and seizures are not typical at disease onset of MS. In case no. 3, MS preceded ALE symptoms for 7 years, hence bilateral AHS is maybe related to MS. However, bilateral temporomesial changes associated with temporal lobe seizures are more frequently caused by ALE than related to other causes [21]. In addition, it has been demonstrated that ALE usually starts as a subacute disease with uni- or bilateral swelling of temporomesial structures, which appear hyperintense on T2-/FLAIR-weighted sequences, but progressive temporomesial atrophy develops in most cases during the disease course [22]. Several MRI studies of MS patients have shown that hippocampal subregions have different susceptibility to damage and atrophy which was mainly associated with memory deficits (e.g., visuospatial/verbal memory and memory acquisition) and depressive symptoms, than with seizures [23-26].
According to diagnostic criteria of Graus et al., 2016 for definite Ab-negative ALE, reasonable exclusion of alternative causes is necessary, but in our patients diagnosed with MS, it is not possible to meet this criterion [1]. Following this, it is difficult to distinguish clearly between MS and Ab-negative ALE and diagnosing of overlap syndrome cannot be made definitively. All of our patients received immunotherapy, but unfortunately, lack of detailed follow-up data hampered analysis of the treatment-outcome. Nevertheless, occurrence of TLE with additional limbic symptoms in MS patients should prompt detailed future follow-up of the clinical and paraclinical outcome because of possible overlap syndrome. Supplementary antibody diagnostic can be helpful for exclusion or confirmation of ALE.
4. Conclusions
In conclusion, all our patients were female which is in line with MS populations. Temporal lobe seizures with memory disturbances and depressive symptoms occur as key features more frequently at disease onset in ALE, but can be also related to MS during the disease course. Bilateral temporomesial T2/FLAIR changes on brain MRI are one of the required diagnostic criteria for definite ab-negative ALE which was fulfilled in all of our patients. On the other hand, temporal lobe damage including hippocampal structures was reported as possible cause for seizures in MS patients. Furthermore, there is no doubt about the diagnosis of MS in our patients. So, it is more likely that MS symptoms in our case series may resemble the symptoms of ALE.
References
- Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 15 (2016): 391-404.
- Spatola M, Dalmau J. Seizures and risk of epilepsy in autoimmune and other inflammatory encephalitis. Curr Opin Neurol 30 (2017): 345-353.
- Steriade C, Britton J, Dale RC, et al. Acute symptomatic seizures secondary to autoimmune encephalitis and autoimmune-associated epilepsy: Conceptual definitions. Epilepsia 61 (2020): 1341-1351.
- Malter MP, Helmstaedter C, Urbach H, et al. Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol 67 (2010): 470-478.
- Ismail FS, Spatola M, Woermann FG, et al. Diagnostic challenges in patients with temporal lobe seizures and features of autoimmune limbic encephalitis. Eur J Neurol 00 (2021): 1-8.
- Kavcic A, Hofmann WE. Unprovoked seizures in multiple sclerosis: Why are they rare?. Brain Behav 7 (2017): e00726.
- Burman J, Zelano J. Epilepsy in multiple sclerosis: A nationwide population-based register study. Neurology 89 (2017): 2462-2468.
- Calabrese M, De Stefano N, Atzori M, et al. Extensive cortical inflammation is associated with epilepsy in multiple sclerosis. J Neurol 255 (2008): 581-586.
- Nicholas R, Magliozzi R, Campbell G, et al. Temporal lobe cortical pathology and inhibitory GABA interneuron cell loss are associated with seizures in multiple sclerosis. Mult Scler 22 (2016): 25-35.
- Karaaslan Z, Mercan Ö, Tüzün E, et al. A Case of Seronegative Limbic Encephalitis with Multiple Sclerosis: A Possible Overlapping Syndrome. Am J Case Rep 18 (2017): 64-66.
- Titulaer MJ, Höftberger R, Iizuka T, et al. Overlapping demyelinating syndromes and anti–N-methyl-D-aspartate receptor encephalitis. Ann Neurol 75 (2014): 411-428.
- Kruer MC, Koch TK, Bourdette DN, et al. NMDA receptor encephalitis mimicking seronegative neuromyelitis optica. Neurology 74 (2010): 1473-1475.
- Lekoubou A, Viaccoz A, Didelot A, et al. Anti-N-methyl-D-aspartate receptor encephalitis with acute disseminated encephalomyelitis-like MRI features. Eur J Neurol 19 (2012): e16-e17.
- Pennington C, Livingstone S, Santosh C, et al. N-methyl D-aspartate receptor antibody encephalitis associated with myelitis. J Neurol Sci 317 (2012): 151-153.
- Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17 (2018): 162-173.
- Graus F, Escudero D, Oleaga L, et al. Syndrome and outcome of antibody-negative limbic encephalitis. Eur J Neurol 25 (2018): 1011-1016.
- Rocca MA, Amato MP, De Stefano N, et al. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol 14 (2015): 302-317.
- Pagani E, Rocca MA, Gallo A, et al. Regional brain atrophy evolves differently in patients with multiple sclerosis according to clinical phenotype. AJNR Am J Neuroradiol 26 (2005): 341-346.
- Geurts JJ, Bö L, Roosendaal SD, et al. Extensive hippocampal demyelination in multiple sclerosis. J Neuropathol Exp Neurol 66 (2007): 819-827.
- Calabrese M, Castellaro M, Bertoldo A, et al. Epilepsy in multiple sclerosis: The role of temporal lobe damage. Mult Scler 23 (2017): 473-482.
- Bien CG, Urbach H, Schramm J, et al. Limbic encephalitis as a precipitating event in adult-onset temporal lobe epilepsy. Neurology 69 (2007): 1236-1244.
- Urbach H, Soeder BM, Jeub M, et al. Serial MRI of limbic encephalitis. Neuroradiology 48 (2006): 380-386.
- Rocca MA, Barkhof F, De Luca J, et al. MAGNIMS Study Group (2018) The hippocampus in multiple sclerosis. Lancet Neurol 17 (2018): 918-926.
- Longoni G, Rocca MA, Pagani E, et al. Deficits in memory and visuospatial learning correlate with regional hippocampal atrophy in MS. Brain Struct Funct 220 (2015): 435-444.
- Planche V, Koubiyr I, Romero JE, et al. Regional hippocampal vulnerability in early multiple sclerosis: dynamic pathological spreading from dentate gyrus to CA1. Hum Brain Mapp 39 (2018): 1814-1824.
- Kiy G, Lehmann P, Hahn HK, et al. Decreased hippocampal volume, indirectly measured, is associated with depressive symptoms and consolidation deficits in multiple sclerosis. Mult Scler 17 (2011): 1088-1097.
