Systemic Inflammation: A Risk Factor for Osteoporosis in Pre- and Post-menopausal Women
Article Information
Yasovanthi Jeedigunta1*, Shehnaz Sultana2, Rajender Rao Kalashikam1, Raghunath Manchala1
1National Institute of Nutrition, Jamai Osmania, Telangana, India
2Institute of Genetics and Hospital for Genetic Diseases, Osmania University, Telangana, India
*Corresponding author: Dr. J Yasovanthi, Department of Endocrinology and Metabolism, National Institute of Nutrition, Jamai Osmania, Hyderabad – 500 007, Telangana, India
Received: 28 September 2020; Accepted: 06 October 2020; Published: 19 October 2020
Citation:
Yasovanthi Jeedigunta, Shehnaz Sultana, Rajender Rao Kalashikam, Raghunath Manchala. Systemic Inflammation: A Risk Factor for Osteoporosis in Pre- and Post-menopausal Women. Journal of Women’s Health and Development 3 (2020): 423-431.
View / Download Pdf Share at FacebookAbstract
Background: Inflammatory markers like Interleukin 6 (IL-6), tumor necrosis factor α (TNF α) and high sensitivity C-reactive protein (hs CRP), play an important role in the pathogenesis of osteoporosis. The present study aims to investigate the relationship between markers of inflammation, BMD and to correlate these markers with estradiol levels in pre- and post-menopausal women.
Methods: A total of 169 subjects with low BMD along with 170 age matched controls were studied. Serum concentrations of hs CRP, IL-6, TNF α and estradiol levels were measured by ELISA method.
Results: Bone specific ALP, acid phosphatase, hsCRP, IL-6 and TNF α, levels were significantly high in osteoporotic subjects (p<0.05) when compared with controls. hsCRP levels were positively correlated with IL-6 (r = 0.247, p <0.001) and TNF α levels (r =0.397, p< 0.001) in osteoporotic women.
Conclusion: Inflammation is associated with low bone mass and therefore an independent risk factor for osteoporosis.
Keywords
Osteoporosis, CRP, Interleukin-6, Tumor necrosis factor α, Estradiol
Osteoporosis articles Osteoporosis Research articles Osteoporosis review articles Osteoporosis PubMed articles Osteoporosis PubMed Central articles Osteoporosis 2023 articles Osteoporosis 2024 articles Osteoporosis Scopus articles Osteoporosis impact factor journals Osteoporosis Scopus journals Osteoporosis PubMed journals Osteoporosis medical journals Osteoporosis free journals Osteoporosis best journals Osteoporosis top journals Osteoporosis free medical journals Osteoporosis famous journals Osteoporosis Google Scholar indexed journals CRP articles CRP Research articles CRP review articles CRP PubMed articles CRP PubMed Central articles CRP 2023 articles CRP 2024 articles CRP Scopus articles CRP impact factor journals CRP Scopus journals CRP PubMed journals CRP medical journals CRP free journals CRP best journals CRP top journals CRP free medical journals CRP famous journals CRP Google Scholar indexed journals Interleukin-6 articles Interleukin-6 Research articles Interleukin-6 review articles Interleukin-6 PubMed articles Interleukin-6 PubMed Central articles Interleukin-6 2023 articles Interleukin-6 2024 articles Interleukin-6 Scopus articles Interleukin-6 impact factor journals Interleukin-6 Scopus journals Interleukin-6 PubMed journals Interleukin-6 medical journals Interleukin-6 free journals Interleukin-6 best journals Interleukin-6 top journals Interleukin-6 free medical journals Interleukin-6 famous journals Interleukin-6 Google Scholar indexed journals Tumor necrosis factor ? articles Tumor necrosis factor ? Research articles Tumor necrosis factor ? review articles Tumor necrosis factor ? PubMed articles Tumor necrosis factor ? PubMed Central articles Tumor necrosis factor ? 2023 articles Tumor necrosis factor ? 2024 articles Tumor necrosis factor ? Scopus articles Tumor necrosis factor ? impact factor journals Tumor necrosis factor ? Scopus journals Tumor necrosis factor ? PubMed journals Tumor necrosis factor ? medical journals Tumor necrosis factor ? free journals Tumor necrosis factor ? best journals Tumor necrosis factor ? top journals Tumor necrosis factor ? free medical journals Tumor necrosis factor ? famous journals Tumor necrosis factor ? Google Scholar indexed journals Estradiol articles Estradiol Research articles Estradiol review articles Estradiol PubMed articles Estradiol PubMed Central articles Estradiol 2023 articles Estradiol 2024 articles Estradiol Scopus articles Estradiol impact factor journals Estradiol Scopus journals Estradiol PubMed journals Estradiol medical journals Estradiol free journals Estradiol best journals Estradiol top journals Estradiol free medical journals Estradiol famous journals Estradiol Google Scholar indexed journals pre-menopausal articles pre-menopausal Research articles pre-menopausal review articles pre-menopausal PubMed articles pre-menopausal PubMed Central articles pre-menopausal 2023 articles pre-menopausal 2024 articles pre-menopausal Scopus articles pre-menopausal impact factor journals pre-menopausal Scopus journals pre-menopausal PubMed journals pre-menopausal medical journals pre-menopausal free journals pre-menopausal best journals pre-menopausal top journals pre-menopausal free medical journals pre-menopausal famous journals pre-menopausal Google Scholar indexed journals smokers articles smokers Research articles smokers review articles smokers PubMed articles smokers PubMed Central articles smokers 2023 articles smokers 2024 articles smokers Scopus articles smokers impact factor journals smokers Scopus journals smokers PubMed journals smokers medical journals smokers free journals smokers best journals smokers top journals smokers free medical journals smokers famous journals smokers Google Scholar indexed journals X-ray articles X-ray Research articles X-ray review articles X-ray PubMed articles X-ray PubMed Central articles X-ray 2023 articles X-ray 2024 articles X-ray Scopus articles X-ray impact factor journals X-ray Scopus journals X-ray PubMed journals X-ray medical journals X-ray free journals X-ray best journals X-ray top journals X-ray free medical journals X-ray famous journals X-ray Google Scholar indexed journals osteopenia articles osteopenia Research articles osteopenia review articles osteopenia PubMed articles osteopenia PubMed Central articles osteopenia 2023 articles osteopenia 2024 articles osteopenia Scopus articles osteopenia impact factor journals osteopenia Scopus journals osteopenia PubMed journals osteopenia medical journals osteopenia free journals osteopenia best journals osteopenia top journals osteopenia free medical journals osteopenia famous journals osteopenia Google Scholar indexed journals lumbar spine articles lumbar spine Research articles lumbar spine review articles lumbar spine PubMed articles lumbar spine PubMed Central articles lumbar spine 2023 articles lumbar spine 2024 articles lumbar spine Scopus articles lumbar spine impact factor journals lumbar spine Scopus journals lumbar spine PubMed journals lumbar spine medical journals lumbar spine free journals lumbar spine best journals lumbar spine top journals lumbar spine free medical journals lumbar spine famous journals lumbar spine Google Scholar indexed journals
Article Details
1.Introduction
Osteoporosis is characterized by the loss of bone mass and deterioration of bone microarchitecture with a resulting increase in bone fragility and, therefore, susceptibility to fractures [1]. Currently Osteoporosis is attributed to various endocrine, metabolic and environmental factors. Emerging clinical and molecular evidence suggests that inflammation also exerts significant influence on bone turnover, inducing osteoporosis. Numerous pro-inflammatory cytokines have been implicated in the regulation of osteoblast and osteoclasts and an activated immune profile has been hypothesized as an important risk factor in the occurrence of the disorder [2].
During the past decade, considerable evidence has accumulated that estrogen deficiency dramatically alters the dependency of bone cells on several cytokines, including interleukin 6 (IL-6), interleukin -1(IL-1), and tumor necrosis factor α (TNFα) for the role of cytokines in bone loss due to estrogen deficiency [3-5]. Although these data provide strong evidence for the involvement of cytokines due to estrogen deficiency most of the results have been derived from in vitro and animal studies. However, evidence that these inflammatory cytokines play a similar role in human studies is limited. Preliminary studies on cytokines and bone mineral density [6-9] or bone loss [10, 11] in post-menopausal women have shown inconsistent and contradictory results.
Further the associations between circulating inflammatory markers, estradiol levels and BMD in pre-menopausal women have not been reported. Therefore, the aim of this study was to estimate the serum bone specific alkaline phosphate (ALP, bone formation marker), bone specific tartrate resistant acid phosphatase (TRAP, bone resorption marker) and to determine the association between serum hsCRP ,IL-6 ,TNF α (markers of inflammation) and bone mineral density as well as estradiol levels in pre and post-menopausal women.
2. Materials and Methods
2.1 Study subjects
A total of 169 osteoporotic Indian women from a high socio- economic group attending the bone health program of Vijaya Diagnostic Centre, Himayat Nagar, Hyderabad were included in the study. Subjects affected by disorders of mineral metabolism or by conditions known to cause secondary osteoporosis, such as hyperthyroidism, cortisol excess, malabsorption syndromes and malignancies were excluded. Likewise, individuals on drugs known to affect calcium metabolism, including corticosteroids, Thyroid hormones, anticonvulsants, diuretics as well as bisphosphonates, Fluorides were excluded from the study.
70 premenopausal women (mean age 40.7 ± 5.1 years; range 30 -45 years) having regular cycles and T score ≤ -2.5 at spine were considered as osteoporotic. The women were non pregnant, non-lactating at the time of sample collection. Postmenopausal women with cessation of menses for at least one year (mean age 56.6 ± 7.8; range 50-70 years) and T score ≤ -2.5 at spine were taken as osteoporotic individuals for the study. Individuals were excluded if they had undergone hysterectomy prior to natural menopause. None of the women were on estrogen replacement therapy. None of the subjects were alcoholic nor smokers. All the subjects were interviewed in accordance with a structured questionnaire in order to collect clinical data, including a history of fracture which was verified by medical records. Osteoporotic fractures included clinically recognized fractures of the hip, spine, or distal fore arm which resulted from minimal or moderate trauma. Among the 169 osteoporotic individuals none of them had fractures at the time of sample collection. Written informed consent was obtained from all the individuals and the study was approved by the ethical committee of the Institute. Standing height and weight were recorded. Physical built was expressed as body mass index, calculated as the weight (in kilograms) divided by the square of the height (in meters).
2.2 BMD measurements
Bone mineral density was obtained from anterior –posterior lumbar spine (L2-L4) scanning with Dual energy X-ray absorptiometry (Lunar GE medical systems, Prodigy 6.80, DF+10243). Subjects were categorized according to the WHO definition, osteopenia was diagnosed at –2.5< T -score <–1.0 SD, and osteoporosis was diagnosed at T-score ≤ –2.5 SD at any site. T-score ≤ 1 SD is considered normal. All the individuals were analyzed by the same DEXA machine to avoid minor variations if any.
2.3 Biochemical measurements
Serum total alkaline phosphatase and bone specific alkaline phosphatase, total tartrate resistant acid phosphatase and bone acid phosphate, were measured by spectroscopy. [12-14] Serum calcium and phosphorus levels were determined by atomic absorption spectrophotometry (AAS). Enzyme linked immunosorbent assay (ELISA) test was carried out to determine the serum levels of IL-6, TNF α, hs CRP and estradiol using kits obtained from Pharmingen opt EIATM BD Biosciences, Diagnostics Biochem, Canada Inc.and Equipar Diagnostici Italy respectively.
2.4 Statistical analysis
The results are expressed as mean ± SD. Differences in the parameters between the Pre- and post-menopausal women were compared using Students t-test. Pearson’s correlation co-efficient was used to find the strength of the association between serum IL-6, TNF α and hs CRP. Relationship between estradiol levels and IL-6, TNF α and hs CRP were performed by Pearson correlation. All statistical analysis was performed with SPSS statistical software (version 15), with p value less than 0.05 considered statistically significant.
3. Results
The clinical characteristics of the study subjects are shown in Table 1. The mean age of the pre-menopausal women was 40.7 ± 5.1years in osteoporotic group and 39.2 ± 7.4 years in the control group, in post-menopausal women it was 58.6 ± 7.8 years in osteoporotic group and 56.7 ± 6.8 years in the controls. Weight and BMI were significantly higher (p<0.05) in pre-and post-menopausal osteoporotic subjects when compared to the controls.
|
Variables |
Premenopausal women (n=140) |
Post-menopausal women (n=199) |
||||
|
Controls |
Osteoporotics |
P- value |
Controls |
Osteoporotics |
P-value |
|
|
N=70 |
N=70 |
N=99 |
N=100 |
|||
|
Age (years) |
39.2 ± 7.4 |
40.7 ± 5.1 |
0.16 |
56.7 ± 6.8 |
58.6 ± 7.8 |
0.06 |
|
BMI(kg/m2) |
23.1 ± 5.0 |
24.1 ± 4.8 |
0.04 |
25.4 ± 5.1 |
26.0 ± 3.6 |
0.004 |
|
Duration of menopause (years) |
NA |
NA |
NA |
8.4 ± 6.2 |
11.0 ± 6.1 |
0.003 |
|
Age at menopause (years) |
NA |
NA |
NA |
49.5 ± 5.3 |
45.8 ± 5.3 |
0.001 |
|
T-score |
-1.0 ± 0.7 |
-2.6 ± 0.3 |
<0.001 |
-1.2 ± 0.4 |
-2.98 ± 0.6 |
0.007 |
|
Z-score |
-1.5 ± 0.9 |
-2.1 ± 0.6 |
0.009 |
-1.5 ± 0.5 |
-3.6 ± 1.1 |
<0.001 |
Table 1: Clinical characteristics of the study subjects.
We could not find any statistical significance (p > 0.05) in serum calcium and phosphorus levels between osteoporotics and controls (Table 2). Total ALP and bone ALP were significantly high in osteoporotics when compared to controls (p<0.05). Tartrate resistance acid phosphatase was also significantly high in patients when compared to controls (Table 2). There was no significant difference (p>0.05) in serum estradiol levels of premenopausal osteoporotic women in comparison with their controls whereas statistically significant values were obtained in postmenopausal osteoporotic women when compared to controls. Markers of inflammation, IL-6, TNF α and hs CRP levels were significantly high (p<0.001) in all osteoporotic subjects in comparison with controls. (Table 2).
|
Variables |
Premenopausal women (n=140) |
Post-menopausal women (n=199) |
||||
|
Controls |
Osteoporotics |
P- value |
Controls |
Osteoporo tics |
P-value |
|
|
N=70 |
N=70 |
N=99 |
N=100 |
|||
|
Calcium(mg/dl) |
8.74 ± 0.43 |
9.09 ± 0.40 |
NS |
9.26 ± 0.31 |
9.38 ± 0.35 |
NS |
|
Phosphorus(mg/dl) |
3.48 ± 0.43 |
4.0 ± 0.52 |
NS |
4. 19 ± 0.62 |
4.52 ± 0.75 |
NS |
|
Total ALP(IU/L) |
69.40 ± 4.60 |
80.0 ± 7.54 |
<0.001 |
101.0 ± 6.70 |
103.0 ± 5.05 |
0.005 |
|
Bone ALP(IU/L) |
24.50 ± 5.48 |
38. 0 ± 4.20 |
0.02 |
40.20 ± 4.10 |
47.0 ± 6.53 |
0.01 |
|
Total TRAP (IU/L) |
7. 0 ± 3.60 |
10.5 ± 2.63 |
0.002 |
8.1 ± 1.26 |
11.5 ± 1.78 |
0.007 |
|
Bone TRAP (IU/L) |
3.40 ± 0.78 |
4.92 ± 1.36 |
0.001 |
3.65 ± 1.20 |
5.3 ± 1.85 |
0.002 |
|
hsCRP (mg/l) |
0.7 ± 0.21 |
1.90 ± 0.20 |
0.001 |
1.3 ± 0.20 |
2.2 ± 0.30 |
0.001 |
|
IL-6 (pg/ml) |
2.40 ± 0.41 |
3.0 ± 0.40 |
0.001 |
2.8 ± 0.41 |
4. 0 ± 0.92 |
0.001 |
|
TNF α (pg/ml) |
2.73 ± 0.40 |
3.5 ± 0.71 |
0.002 |
2.8 ± 0.40 |
5.2 ± 1.10 |
0.001 |
|
17ß Estradiol (pg/m) |
89.65 ± 14.50 |
75.87 ± 11.82 |
NS |
36.0 ± 3.0 |
21.0 ± 4.5 |
0.007 |
Table 2: Biochemical and inflammatory markers of the study subjects.
When we compared pre-menopausal osteoporotic women with post-menopausal osteoporotic women all the above said inflammatory markers were significantly high in post-menopausal osteoporotic women with p value <0.05 (Table 3).
|
Variables |
Premenopausal Osteoporotic women |
Postmenopausal Osteoporotic women |
P value |
|
Estradiol pg/ml |
89.65 ± 14.50 |
21.0 ± 3.4 |
<0.0001 |
|
hs CRP mg/l |
1.9 ± 0.2 |
2.2 ± 0.3 |
<0.004 |
|
IL-6 Pg/ml |
3.0 ± 0.4 |
4.0 ± 0.9 |
<0.001 |
|
TNF α (pg/ml) |
3.5 ± 0.7 |
5.2 ± 1.1 |
<0.001 |
The results are given as mean ± SD, p value <0.05 is considered significant
Table 3: Comparison of inflammatory markers according to menopausal status.
According to WHO classification when the subjects were categorized into normal, osteopenia and osteoporotic, we found that the serum hs CRP (F=29.90, p=0.000) IL-6 (F=16.30,p=0.000) and, TNF α (F=3.754, p=0.05) levels were significantly high in osteoporotic subjects compared to controls. The significant decrease in bone mineral density and increase in the levels of inflammatory markers in post- menopausal osteoporotic women indicate a rapid bone loss than pre- menopausal osteoporotic women (Table 2). Osteopenic subjects had also higher concentrations of inflammatory markers hs CRP (F= 5.073, p=0.026) and IL-6 (F=6.604, p=0.016) when compared to controls but TNF α levels did not reach statistical significance (F=0.390, p=0.533) in these subjects. Serum hs CRP (a), IL-6 (b) and TNF α (c) concentrations among controls, osteopenics and osteoporotic subjects are shown in figure 1. Data reported as estimated mean ± S.E values in all the subjects.
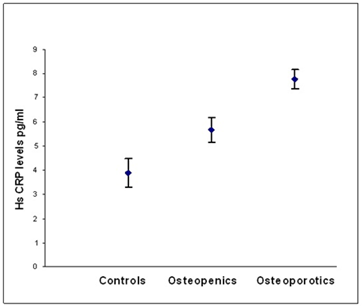
Figure 1a: Relationship between hs CRP levels and BMD status.
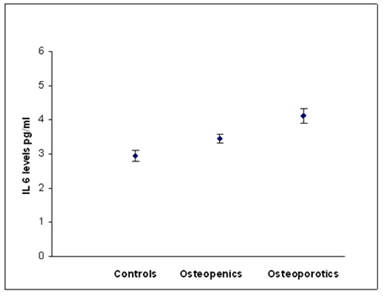
Figure 1b: Relationship between IL-6 and BMD status.
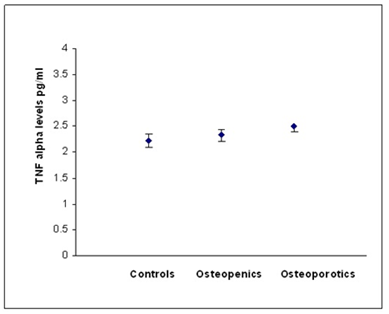
Figure 1c: Relationship between TNF alpha and BMD status.
4. Discussion
Emerging clinical and molecular evidence suggests that factors involved in inflammation are linked with those critical for bone physiology and remodeling, supporting the theory that inflammation significantly contributes to the etiopathogenesis of osteoporosis [15, 16]. Decline in ovarian function with menopause is associated with spontaneous increase in pro inflammatory cytokines. Experimental and clinical studies strongly support a link between the increased state of pro inflammatory cytokine activity and postmenopausal bone loss. Cytokines in the microenvironment of the bone, such as interleukins 1 and 6 (IL-1 and IL-6), tumor necrosis factor (TNF), interferon (IFN), affect the bone-remodeling process by regulating the differentiation as well as the activity of osteoblasts and osteoclasts [17-20]. Over the past decades, numerous reports have been published demonstrating that natural or surgical menopause increases blood, bone marrow, and monocytic levels of IL-1, IL-6, TNF, and the related factors IL-1R [4, 21] In spite of these observations, controversy persists concerning the specific contribution of each of these factors to bone loss. Recent data from a number of laboratories have indicated a potential critical role for certain pro inflammatory cytokines, both in the normal bone remodeling process and in the pathogenesis of peri menopausal and late-life osteoporosis [21, 22].
However, there is no consensus about serum IL-6 levels in menopause. Previous studies have failed to demonstrate a regulatory effect of ovarian steroids on IL-6 levels in human bone cells and animals [23-25]. Furthermore, McKane et al also suggested that there was no difference in serum IL-6 levels between pre- and post-menopausal women [26]. However, Rachon et al reported that estrogen deprivation results in IL-6 production by the peripheral blood mononuclear cells in postmenopausal women [27]. They reported that the mean serum levels of IL-6 in postmenopausal women were significantly higher than in young women. Abrahamsen et al reported that serum IL-6 was associated with an increase in BMD at lumbar spine in pre-menopausal women [10]. In contrast Salamone et al reported that the production of IL-1, IL-6 and/or TNF-α by peripheral blood monocytes positively correlated with bone resorption or spinal bone loss in healthy pre- [28] and postmenopausal women [29].
In the present study all the three markers of inflammation (hs CRP, IL-6 and TNF α) were found to be significantly high in osteoporotic subjects when compared to controls. When we compared the above said markers in pre-menopausal osteoporotic and post-menopausal osteoporotic women we found significantly higher levels of hs crp , IL-6 and TNF α in the latter group. Our study corroborates earlier findings that estrogen deficiency dramatically alters the dependency of bone cells on several cytokines, including interleukins 6 (IL-6) IL-1 and tumor necrosis factor (TNF) [3-5]. Tartre resistant bone acid phosphatase a marker of bone resorption is significantly high (p<0.001) in osteoporotic patients when compared to controls and also the elevated levels of IL-6, hs CRP and TNF α in pre- and post-menopausal osteoporotic women suggest that inflammation is associated with low bone mass. Further we also found that the serum hs CRP levels correlated positively with both IL-6 and TNF α concentrations in pre and postmenopausal osteoporotic women.
Results of the present study reveal that inflammation is involved in bone metabolism. However, the mechanisms linking bone metabolism are not clear. Accelerated bone loss due to menopause –induced estrogen deficiency is the major cause of postmenopausal osteoporosis [30] where inflammatory processes are known to up-regulate many cytokines, such as IL-1, IL-6 and TNF-α, which strongly stimulate CRP production from the liver as well as induce bone resorption [31, 32]. Increased bone resorption may result in increased bone turnover and decreased BMD [33-36]. But the mechanism involved in pre-menopausal women is not clear. The strong family history of osteoporosis, especially in the premenopausal women, may provide further support for current theories of a genetic predisposition to osteoporosis.
5. Conclusion
Our observations clearly indicate that elevated levels of serum hs CRP, IL-6 and TNF α concentrations in both pre and postmenopausal women are associated with low BMD. Thus, inflammation alone could be an important risk factor in the occurrence of the disease and targeted anti-inflammatory therapy may play a potential role in the prevention of rapid bone loss.
Conflict of Interest
All the authors declare no conflict of interest.
Acknowledgements
The authors are thankful to Indian Council of Medical Research (ICMR), New Delhi for financial support.
References
- Sambrook P, Cooper C. Lancet 367 (2006): 2010-2018
- Lia Ginaldi, Maria critina, DI Benedetto, Massimo De Martinis. Osteoporosis inflammation and ageing. Immunity and ageing 2 (2005): 1742-4933-2-14.
- Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med 332 (1995): 305-311.
- Pacifici R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res 11 (1996): 1043-1051.
- Jilka RL. Cytokines, bone remodeling, and estrogen deficiency: a 1998 update. Bone 23 (1998): 75-81.
- Khosla S, Peterson JM, Egan K, Jones JD, Riggs BL. Circulating cytokine levels in osteoporotic and normal women. J Clin Endocrinol Metab 79 (1994): 707-711.
- Kania DM, Binkley N, Checovich M, Havighurst T, Schilling M, Ershler WB. Elevated plasma levels of interleukin-6 in postmenopausal women do not correlate with bone density. J Am Geriatr Soc 43 (1995): 236-239
- Papadopoulos NG, Georganas K, Skoutellas V, Konstantellos E, Lyritis GP. Correlation of interleukin-6 serum levels with bone density in postmenopausal women. Clin Rheumatol 16 (1997): 162-165
- McKaneWR, Khosla S, Peterson JM, Egan K, RiggsBL. Circulating levels of cytokines that modulate bone resorption: effects of age and menopause in women. J Bone Miner Res 9 (1994): 1313-1318.
- Abrahamsen B, Bonnevie-Nielsen V, Ebbesen EN, Gram J, Beck-Nielsen H. Cytokines and bone loss in a 5-year longitudinal study–hormone replacement therapy suppresses serum soluble interleukin-6 receptor and increases interleukin-1-receptor antagonist: the Danish Osteoporosis Prevention Study. J Bone Miner Res 15 (2000): 1545-1554
- Scheidt-Nave C, Bismar H, Leidig-Bruckner G, Woitge H, Seibel MJ, Ziegler R, et al. Serum interleukin 6 is a major predictor of bone loss in women specific to the first decade past menopause. J Clin Endocrinol Metab 86 (2001): 2032-2042.
- Schiele F, Artur Y, Floc'h AY, Siest G. Total, tartrate-resistant, and tartrate-inhibited acid phosphatases in serum: biological variations and reference limits. Clin Chem 34 (1988): 685-690
- M J Cadeau, A Malkin. Relative heat stability for the identification of serum alkaline phosphatase isoenzymes. Clin Chim Acta 45 (1973): 235-242.
- Methods of Enzymatic Analysis - Bergmeyer HU. Academic Press 2 (1974): 856-860.
- Arron JR, Choi Y. Bone versus immune system. Nature 408 (2000): 535-536.
- Lorenzo J. Interactions between immune and bone cells: new insights with many remaining questions. J Clin Invest 106 (2000): 749-752.
- Wallach S, Avioli LV, Feinbllatt JD. Cytokines and bone metabolism. Calcif Tissue Int 53 (1993): 293-296.
- Ishimi Y, Miyamura C, Jin CH, Akatsu T, Abe E, Nakamura Y, et al. IL-6 is produced by osteoblasts and induces bone resorption. J Rheumatol 145 (1990): 3297-3303.
- Littlewood J, Russell J, Harvey OR, Hughes D E, Russell R G, Gowen M, et al. The modulation of the expression of IL-6 and its receptor in human osteoblasts in vitro. Endocrinology 129 (1991): 1513-1520.
- Natale VM, Filho WJ, Duarte AJS. Does the secretion of cytokines in the periphery reflect their role in bone metabolic diseases? Mechanisms of Ageing and Development 94 (1997): 17-23.
- Manolagas SCV, Jilka RL. Bone marrow, cytokines and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl. J Med 332 (1995): 305-311.
- Horowitz MC. Cytokines and estrogen in bone: Anti-osteoporotic effects. Science 260 (1993): 626-627.
- Chaudhary LR, Spelsbert TC, Rggs BL. Production of various cytokines by normal human osteoblast-like cells in response to interleukin-1 beta and tumor necrosis factor alpha: lack of regulation by 17-beta estradiol. Endocrinology 130 (1992): 2528-2534.
- Rickard D, Russel G, Gowen M. Oestradiol inhibits the release of tumor necrosis factor but not interleukin-6 from adult human osteoblasts in vitro. Osteoporos Int 2 (1992): 94-102.
- Rifas L, Kenney JS, Marcelli M, Pacifici R, Cheng SL, Dawson LL, et al. Production of interleukin-6 in human osteoblasts and human bone marrow stromal cells: evidence that induction by interleukin-1 and tumor necrosis factor alpha is not regulated by ovarian steroids. Endocrinology 136 (1995): 4056-4067.
- McKane WR, Khosla S, Peterson JM, Egan K, Riggs BL. Circulating levels of cytokines that modulate bone resorption: effects of age and menopause in women. J Bone Miner Res 9 (1994): 1313-1318.
- Rachon D, Mysliwska J, Suchecka-Rachon K, Wieckiewicz J, Mysliwski A. Effect of oestrogen deprivation on interleukin-6 production by peripheral blood mononuclear cells of postmenopausal women. J Endocrinol 172 (2002): 387-395.
- Salamone LM, Whiteside T, Friberg D, Epstein RS, Kuller LH, Cauley JA. Cytokine production and bone mineral density at the lumbar spine and femoral neck in premenopausal women. Calcif Tissue Int 63 (1998): 466-470.
- Cohen-Solal ME, Graulet AM, Denne MA, Gueris J, Baylink D, de Vernejoul MC. Peripheral monocyte culture supernatants of menopausal women can induce bone resorption: involvement of cytokines. J Clin Endocrinol Metab 77 (1993): 1648-1653.
- Riggs BL, Melton III LJ. Involutional osteoporosis. N Engl J Med 314 (1986): 1676 -1686.
- Weinhold B, Ruther U. Interleukin-6-dependent and independent regulation of the human C-reactive protein gene. Biochem J 327 (1997): 425-429.
- Yoshida N, Ikemoto S, Narita K, Sugimura K, Wada S, Yasumoto R, et al. Interleukin-6, tumour necrosis factor alpha and interleukin-1beta in patients with renal cell carcinoma. Br J Cancer 86 (2002): 1396-1400.
- Muller B. Cytokine imbalance in non-immunological chronic disease. Cytokine 18 (2002): 334-339.
- Jilka RL, Hangoc G, Girasole G, Passeri G, Williams DC, Abrams JS, et al. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science 257 (1992): 88-91.
- Gowen M, Mundy GR. Actions of recombinant interleukin1, interleukin 2, and interferon-gamma on bone resorption in vitro. J Immunol 136 (1986): 2478-2482
- Bertolini DR, Nedwin GE, Bringman TS, Smith DD, Mundy GR. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature 319 (1986): 516-518.
