Synergism between manuka honey and penicillin antibiotics against Escherichia coli
Article Information
DeCarolis Mara1, Tessler Madison1, DeCarolis Emidio1, Rampakakis Emmanouil2*
1Evidence-Based Practices Strategies, Montreal, Canada
2Medical Affairs, Clinfo Health Research, Montreal, Canada
*Corresponding Author: Rampakakis Emmanouil, 9400 Henri-Bourassa Blvd. West, H4S1N8, Saint-Laurent, QC, Canada
Received: 03 September 2019; Accepted: 17 September 2019; Published: 23 September 2019
Citation: DeCarolis M, Tessler M, DeCarolis E, Rampakakis E. Synergism between manuka honey and penicillin antibiotics against Escherichia coli. Journal of Food Science and Nutrition Research 2 (2019): 270-273.
View / Download Pdf Share at FacebookAbstract
Previous studies have suggested synergistic effects between manuka honey and different antibiotics against Staphylococcus aureus. In the present study we have tested in vitro the antibacterial efficacy against Escherichia coli of manuka honey with or without ampicillin and penicillin using the disc diffusion method. Similar synergistic effects of manuka honey with ampicillin and penicillin were observed, particularly in early phases of treatment. Overall, these results support the potential use of natural antimicrobials such as manuka honey either alone or in combination with synthetic agents to lower their therapeutic dose and prevent antibiotic resistance.
Keywords
Antibiotics, Antibiotic resistance, Natural, Synthetic, Manuka honey
Antibiotics articles, Antibiotic resistance articles, Natural articles, Synthetic articles, Manuka honey articles
Antibiotics articles Antibiotics Research articles Antibiotics review articles Antibiotics PubMed articles Antibiotics PubMed Central articles Antibiotics 2023 articles Antibiotics 2024 articles Antibiotics Scopus articles Antibiotics impact factor journals Antibiotics Scopus journals Antibiotics PubMed journals Antibiotics medical journals Antibiotics free journals Antibiotics best journals Antibiotics top journals Antibiotics free medical journals Antibiotics famous journals Antibiotics Google Scholar indexed journals Antibiotic resistance articles Antibiotic resistance Research articles Antibiotic resistance review articles Antibiotic resistance PubMed articles Antibiotic resistance PubMed Central articles Antibiotic resistance 2023 articles Antibiotic resistance 2024 articles Antibiotic resistance Scopus articles Antibiotic resistance impact factor journals Antibiotic resistance Scopus journals Antibiotic resistance PubMed journals Antibiotic resistance medical journals Antibiotic resistance free journals Antibiotic resistance best journals Antibiotic resistance top journals Antibiotic resistance free medical journals Antibiotic resistance famous journals Antibiotic resistance Google Scholar indexed journals Natural articles Natural Research articles Natural review articles Natural PubMed articles Natural PubMed Central articles Natural 2023 articles Natural 2024 articles Natural Scopus articles Natural impact factor journals Natural Scopus journals Natural PubMed journals Natural medical journals Natural free journals Natural best journals Natural top journals Natural free medical journals Natural famous journals Natural Google Scholar indexed journals Synthetic articles Synthetic Research articles Synthetic review articles Synthetic PubMed articles Synthetic PubMed Central articles Synthetic 2023 articles Synthetic 2024 articles Synthetic Scopus articles Synthetic impact factor journals Synthetic Scopus journals Synthetic PubMed journals Synthetic medical journals Synthetic free journals Synthetic best journals Synthetic top journals Synthetic free medical journals Synthetic famous journals Synthetic Google Scholar indexed journals Manuka honey articles Manuka honey Research articles Manuka honey review articles Manuka honey PubMed articles Manuka honey PubMed Central articles Manuka honey 2023 articles Manuka honey 2024 articles Manuka honey Scopus articles Manuka honey impact factor journals Manuka honey Scopus journals Manuka honey PubMed journals Manuka honey medical journals Manuka honey free journals Manuka honey best journals Manuka honey top journals Manuka honey free medical journals Manuka honey famous journals Manuka honey Google Scholar indexed journals therapeutic dose articles therapeutic dose Research articles therapeutic dose review articles therapeutic dose PubMed articles therapeutic dose PubMed Central articles therapeutic dose 2023 articles therapeutic dose 2024 articles therapeutic dose Scopus articles therapeutic dose impact factor journals therapeutic dose Scopus journals therapeutic dose PubMed journals therapeutic dose medical journals therapeutic dose free journals therapeutic dose best journals therapeutic dose top journals therapeutic dose free medical journals therapeutic dose famous journals therapeutic dose Google Scholar indexed journals carbohydrates articles carbohydrates Research articles carbohydrates review articles carbohydrates PubMed articles carbohydrates PubMed Central articles carbohydrates 2023 articles carbohydrates 2024 articles carbohydrates Scopus articles carbohydrates impact factor journals carbohydrates Scopus journals carbohydrates PubMed journals carbohydrates medical journals carbohydrates free journals carbohydrates best journals carbohydrates top journals carbohydrates free medical journals carbohydrates famous journals carbohydrates Google Scholar indexed journals antibiotic activity articles antibiotic activity Research articles antibiotic activity review articles antibiotic activity PubMed articles antibiotic activity PubMed Central articles antibiotic activity 2023 articles antibiotic activity 2024 articles antibiotic activity Scopus articles antibiotic activity impact factor journals antibiotic activity Scopus journals antibiotic activity PubMed journals antibiotic activity medical journals antibiotic activity free journals antibiotic activity best journals antibiotic activity top journals antibiotic activity free medical journals antibiotic activity famous journals antibiotic activity Google Scholar indexed journals European Union articles European Union Research articles European Union review articles European Union PubMed articles European Union PubMed Central articles European Union 2023 articles European Union 2024 articles European Union Scopus articles European Union impact factor journals European Union Scopus journals European Union PubMed journals European Union medical journals European Union free journals European Union best journals European Union top journals European Union free medical journals European Union famous journals European Union Google Scholar indexed journals
Article Details
1. Introduction
Although the therapeutic properties of honey have been known for centuries, its antiseptic and antimicrobial properties have been discovered recently. Consisting mainly of carbohydrates, honey also contains several enzymes, proteins, aminoacids, vitamins, lipids, minerals, and phytochemicals which vary considerably by type of honey and are believed to exert health benefits [1, 2]. The antibacterial activity of honey has been attributed to different factors, including hydrogen peroxide, low pH, high osmolarity, and methylglyoxal (MGO) [3-6]. Among the different types of honey, manuka honey has attracted attention for its antimicrobial effects in numerous bacterial pathogens [7] receiving regulatory approvals for clinical use as a wound care agent in the European Union, the USA, New Zealand and others. Of particular interest is that, despite attempts to induce bacterial resistance to manuka honey in the laboratory, there has been no such report which may be due to the complex composition of honey and the multiple active components [8]. Furthermore, multi-resistant bacterial strains have been shown to remain sensitive to manuka honey suggesting a broad spectrum of action and highlighting its potential clinical usefulness [9]. With increasing concerns over antimicrobial resistance worldwide [10], there has been much focus on the potential use of natural antimicrobials [11, 12]. The objective of this study was to compare the antibiotic activity of manuka honey with commonly used synthetic antibiotics, specifically ampicillin and penicillin, as well as to explore any synergistic effects.
2. Materials and Methods
2.1 Inoculum and plate preparation
Commercially available agar plates were spread-plated with approximately 200 µl suspension from an overnight liquid culture of Escherichia coli (E. coli) incubated at 37°C for 48 hours.
2.2 Antimicrobial susceptibility test
Antimicrobial susceptibility test was done using the disc diffusion method as previously described [13]. In each plate six discs were placed, one each containing ampicillin (10 μg), penicillin (10 units), and manuka honey (1 drop), two containing manuka honey with ampicillin or penicillin, respectively, and an empty disc (negative control). The plates were then incubated immediately at 37°C and the diameters of the inhibition zones were measured every 24 hours.
2.3 Data analysis
Data-driven simulation was used to generate 100 observations per treatment group at each timepoint using the SAS procedure PROC SIMNORMAL and the parameters of the input data set. Subsequently, mixed models with repeated measures taking into consideration the correlation of iterations over time were used to compare the antimicrobial activity of ampicillin, penicillin, manuka honey and their combinations. The least square mean estimates were plotted against time. Statistical analyses were performed using SAS version 9.4.
3. Results and Discussion
Using the disc diffusion method all tested treatments exhibited significant (p<0.0001) antibacterial activity against E. coli (Figure 1). By Day 1, the antibacterial effects exerted by ampicillin were more potent, as demonstrated by larger inhibition zones, compared to the effects of penicillin (p<0.0001) or manuka honey (p<0.0001), while penicillin and manuka honey had comparable efficacy. On Day 2, ampicillin and manuka honey had comparable antibacterial activities which were significantly higher than penicillin (p=0.0103 and p=0.0011, respectively). Whereas, on Day 3, Manuka honey was most potent, followed by ampicillin and penicillin.
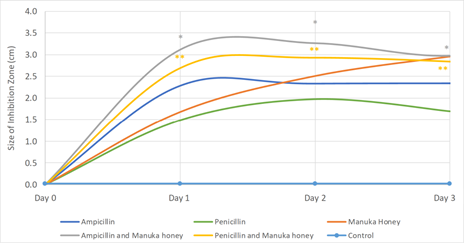
Figure 1: Antimicrobial activity of manuka honey, synthetic antibiotics and combinations thereof.
*p<0.0001 compared to ampicillin alone; **p<0.0001 compared to penicillin alone
In regard to combination treatments, on both Days 1 and 2, combination of manuka honey with ampicillin (manuka honey + ampicillin) or with penicillin (manuka honey + penicillin) resulted in significantly greater inhibition of bacterial growth compared to ampicillin alone (p<0.0001), penicillin alone (p<0.0001), or manuka honey (both p<0.0001). By Day 3, the antibiotic activity of all treatments had reached a plateau with maximal effects observed with manuka honey alone, manuka honey + ampicillin, and manuka honey + penicillin which were comparable. Overall, these results suggest a synergistic effect of manuka honey with ampicillin and penicillin, particularly in early phases of treatment. Similar synergistic effects in vitro were previously reported for manuka honey with antibiotics from the same class (oxacillin) and different classes (tetracycline, imipenem, mupirocin, rifampicin) against Staphylococcus aureus increasing the external validity of our findings [14, 15]. Further investigation will be needed to confirm whether the combinations identified here are effective against clinical isolates and biofilms. In the context of recommended antibiotic stewardship programs, aimed at improving antibiotic use to conserve effectiveness and reduce emergence of resistant strains [16], the results of our study suggest that use of manuka honey either alone or in combination with synthetic agents to lower their therapeutic dose in E. coli-related infections, merits further consideration.
Acknowledgments
Not applicable
Declaration of Interest Statement
None declared
Appendices
Not applicable
References
- Bogdanov S, Jurendic T, Sieber R, et al. Honey for nutrition and health: a review. J Am Coll Nutr 27 (2008): 677-689.
- Cornara L, Biagi M, Xiao J, et al. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front Pharmacol 8 (2017): 412.
- Adams CJ, Boult CH, Deadman BJ, et al. Isolation by HPLC and characterisation of the bioactive fraction of New Zealand manuka (Leptospermum scoparium) honey. Carbohydr Res 343 (2008): 651-659.
- Anthimidou E, Mossialos D. Antibacterial activity of Greek and Cypriot honeys against Staphylococcus aureus and Pseudomonas aeruginosa in comparison to manuka honey. J Med Food 16 (2013): 42-47.
- Brudzynski K, Abubaker K, Wang T. Powerful bacterial killing by buckwheat honeys is concentration-dependent, involves complete DNA degradation and requires hydrogen peroxide. Front Microbiol 3 (2012): 242.
- Mavric E, Wittmann S, Barth G, et al. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol Nutr Food Res 52 (2008): 483-489.
- Carter DA, Blair SE, Cokcetin NN, et al. Therapeutic Manuka Honey: No Longer So Alternative. Front Microbiol 7 (2016): 569.
- Cooper RA, Jenkins L, Henriques AF, et al. Absence of bacterial resistance to medical-grade manuka honey. Eur J Clin Microbiol Infect Dis 29 (2010): 1237-1241.
- George NM, Cutting KF. Antibacterial Honey (Medihoney): in-vitro Activity Against Clinical Isolates of MRSA, VRE, and Other Multiresistant Gram-negative Organisms Including Pseudomonas aeruginosa. Wounds 19 (2007): 231-236.
- Global Antimicrobial Resistance Surveillance System (GLASS) Report Early Implementation (2017).
- Pejin B, Iodice C, Tommonaro G, et al. Further in vitro evaluation of antimicrobial activity of the marine sesquiterpene hydroquinone avarol. Curr Pharm Biotechnol 15 (2014): 583-588.
- Pejin B, Savic A, Sokovic M, et al. Further in vitro evaluation of antiradical and antimicrobial activities of phytol. Nat Prod Res 28 (2014): 372-376.
- Bauer AW, Kirby WM, Sherris JC, et al. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45 (1966): 493-496.
- Jenkins R, Cooper R. Improving antibiotic activity against wound pathogens with manuka honey in vitro. PLoS One 7 (2012): e45600.
- Muller P, Alber DG, Turnbull L, et al. Synergism between Medihoney and rifampicin against methicillin-resistant Staphylococcus aureus (MRSA). PLoS One 8 (2013): e57679.
- Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 62 (2016): 51-77.
