Supervised Self-Collected SARS-Cov-2 Testing in Classroom-Based Summer Camps to Inform Safe In-Person Learning
Article Information
Peter B Cooch1*, Annalisa Watson1, 2, Apryl Olarte1, 2, Emily D Crawford3, 5, Joseph L DeRisi4, 5, Bryan Greenhouse5, 6, Jill Hakim6, Keirstinne Turcios6, Katherine S Pollard5, 7, 8, Lee R Atkinson-McEvoy1, Raphael Hirsch1, Roberta L Keller1, Theodore D Ruel1, Auritte Cohen-Ross9, Araceli Leon10, Naomi S Bardach1, 2
1Department of Pediatrics, University of California San Francisco, CA, USA
2Philip R. Lee Institute for Health Policy Studies, University of California San Francisco, CA, USA
3Department of Microbiology and Immunology, University of California San Francisco, CA, USA
4Department of Biochemistry and Biophysics, University of California San Francisco, CA, USA
5Chan Zuckerberg Biohub, CA, USA
6Department of Medicine, University of California San Francisco, CA, USA
7Department of Epidemiology and Biostatistics, Institute for Human Genetics, and Bakar Computational Health Sciences Institute, University of California San Francisco, CA, USA
8Gladstone Institute of Data Science & Biotechnology, CA, USA
9Celsius and Beyond Science Camps, CA, USA
10Buena Vista Child Care, CA, USA
*Corresponding Author: Peter B Cooch, Department of Pediatrics, University of California San Francisco, 550 16th St, 4th Floor, San Francisco, CA, 94143, USA
Received: 30 March 2021; Accepted: 07 April 2021; Published: 21 April 2021
Citation:
Peter B Cooch, Annalisa Watson, Apryl Olarte, Emily D Crawford, Joseph L DeRisi, Bryan Greenhouse, Jill Hakim, Keirstinne Turcios, Katherine S Pollard, Lee R Atkinson-McEvoy, Raphael Hirsch, Roberta L Keller, Theodore D Ruel, Auritte Cohen-Ross, Araceli Leon, Naomi S Bardach. Supervised Self-Collected SARS-Cov-2 Testing in Classroom-Based Summer Camps to Inform Safe In-Person Learning. Journal of Pediatrics, Perinatology and Child Health 5 (2021): 075-093.
View / Download Pdf Share at FacebookAbstract
Objectives: To evaluate the acceptability and feasibility of serial, self-collected non-nasopharyngeal samples for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing in school-like settings, and describe adherence to infection mitigation strategies.
Methods: We performed a cohort study in classroom-based day camps during summer 2020 in San Francisco, California. We assessed participation rates at two time points among campers, adult household contacts, and camp staff for self-collecting anterior nares samples for reverse transcription polymerase chain reaction (RT-PCR) and saliva samples for antibody testing. We qualitatively assessed sampling feasibility and observed adherence to camp infection mitigation policies.
Results: 76% (186/246) of eligible participants consented; all consented campers and staff present at both time points completed test collection. No virus was detected by RT-PCR; seven participants had antibodies. Testing was feasible to implement, and adherence to stated camp policies was generally high.
Conclusions: Supervised, self-collected serial anterior nasal and saliva-based SARS-CoV-2 testing was acceptable and feasible in school-like setting, including by children ages 5-14. This strategy for testing, and the observed infection mitigation practices, comprise potential components permitting safe in-person learning.
Keywords
Schools, Return to School, Safety, Communicable Disease Control, Feasibility Studies, COVID-19, COVID-19 Testing
Schools articles; Return to School articles; Safety articles; Communicable Disease Control articles; Feasibility Studies articles; COVID-19 articles; COVID-19 Testing articles
Article Details
1. Introduction
Most countries, and all 50 U.S. states, implemented school closures in response to the coronavirus disease 2019 (COVID-19) pandemic. As of March 15, 2021, less than half of American students had returned to full in-person schooling, and 20.8% were still entirely remote [1]. Over the summer of 2020, some states permitted indoor camps to convene over the summer, offering opportunities to study strategies to inform in-person school reopening during the 2020-21 academic year. Closures of schools and other childcare settings are known to adversely impact children and families economically, educationally, and psychologically, while worsening race, gender, and economic disparities [2-5]. While returning to in-person education is a priority for the well-being of children and adolescents, how best to do it safely while the pandemic is ongoing remains unclear. Safe in-person learning likely requires appropriate testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, paired with infection mitigation strategies (e.g., masking, physical distancing, stable cohorts, and hand hygiene) [6]. A major limitation to expanding testing so far, especially in children, has been the acceptability and feasibility of sample collection. Nasopharyngeal (NP) sampling, the primary SARS-CoV-2 test collection modality to date, is uncomfortable, with risk of refusal by both adults and children, requires medical provider collection, confers risk of transmission, and requires extensive personal protective equipment (PPE) including N95 mask, gown, face shield, and gloves, many of which have faced shortages [7, 8].
To address these obstacles, alternate sampling options have been studied, including anterior nares or oral fluid (hereafter “saliva”) collection. Anterior nares collection is more comfortable than NP sampling [11], can be self-performed, is approved by the Centers for Disease Control and Prevention (CDC) for SARS-CoV-2 testing [10], with comparable sensitivity and specificity to provider-performed NP sampling [9], and reduces PPE requirements [7, 8, 11]. Non-invasive antibody testing using saliva is used in HIV screening [12], and is being studied for SARS-CoV-2. Assessing the acceptability and feasibility of non-NP testing strategies for SARS-CoV-2 among children and in educational environments will help determine how best to expand testing to support safe in-person learning. Additionally, there is debate to what degree students are able to adhere to restrictive policies, such as masking or physical distancing. To address these questions, we implemented serial non-NP-specimen SARS-CoV-2 testing in two indoor, classroom-based summer camps. We hypothesized that supervised self-collection of anterior nares and saliva samples for the purpose of SARS-CoV-2 surveillance would be acceptable and feasible for kindergarten through 8th grade children, their household contacts, and camp staff, and that camp staff could assist with collection supervision. We also observed infection mitigation practices and estimated the incidence of SARS-CoV-2 infection using reverse transcription polymerase chain reaction (RT-PCR) testing (anterior nares specimen) and antibody testing (saliva specimen) collected at the beginning and end of camp sessions.
2. Methods
2.1 Study design and setting
We performed a prospective cohort study at two indoor day camps within San Francisco, California: a general camp and a science camp. Both took place in school classrooms and had indoor curricula. The general camp was 5 weeks long and was affiliated with and located in a parochial school, enrolling kindergarten through 5th grade campers (many of whom are students in the school). The general day camp was located in one of the highest prevalence COVID-19 zip codes in San Francisco. The science camp was 3 weeks long, enrolled 2nd through 8th grade campers, and was located in a zip code with below median COVID-19 prevalence. Camps were in session June-July 2020. Both camps were inside the classroom setting for three to six hours per day.
2.2 Participants
Eligible participants were: all children attending the summer camps (“campers”); up to two adults from each camper household (“household contacts”); and camp staff working the entire session (“staff”). Study participant-facing materials encouraged inclusion of household contacts who worked outside the home during the pandemic. We excluded participants from the final cohort who were not present for both test collection sessions
2.3 Recruitment and enrollment
Camp directors sent a recruitment email to parents and staff, including a link to the consent form and baseline survey and a video of a child self-collecting an anterior nasal swab (Supplementary Content). The study team obtained electronic or paper consent in English or Spanish. Enrolling in the study or completing testing was not required for camp entrance, and there was no compensation for participation. We informed participants that they would receive their results from RT-PCR but not antibody testing.
2.4 Co-variates
The baseline survey collected participant characteristics. Adult participants (household contacts and staff) completed the survey on their behalf and on behalf of participating campers. For all participants, we gathered individual and household information, including demographics known to be associated with COVID-19 infection (i.e., cohabitation with confirmed or suspected COVID-19 cases, home zip code, race and ethnicity) [13, 14]. From campers and camp staff we elicited a list of potential COVID-19 symptoms within 14 days prior to testing. Household contacts reported whether they continued to work outside the home during the pandemic (“frontline worker”), and if so, what category of work. From state databases, we obtained participant household zip code-level cumulative incidence of COVID-19 at the time of camp start (June 2020) [15]. For households in a county that did not report zip code incidence (n=8), we used the households’ city cumulative incidence.
2.5 Specimen collection, handling, and testing assays
We collected samples at two time points: within the first three and last two days of the camp session. Collection took place in an outdoor setting or in large classrooms with no more than three participants at a time, following CDC recommendations for distanced collection and specimen handling [11]. Maintaining 6 feet of distance, the research team instructed participants to self-collect an anterior nares swab and a saliva swab (Supplementary Content). We offered camp staff the opportunity to assist with supervision of camper specimen collection. On-site training involved observing and participating in self-collection. Staff demonstrated self-collection to campers and then helped observe and coach them.
We assessed active SARS-CoV-2 infection by RT-PCR and prior exposure by antibody testing. RT-PCR of viral N and E genes and human RNAse P gene was performed on the anterior nares samples. Saliva samples were tested for IgG against SARS-CoV-2 spike and receptor binding domain proteins using a multiplex microsphere assay. Internal validation showed that the saliva antibody assay had an estimated sensitivity of 76% and specificity of 100% based on 51 positive and 41 negative controls using oral fluid reference standards. Test results from RT-PCR were available within two business days and were shared with participants via their preferred method of contact (phone, email, or text).
2.6 Outcome measures
As the primary measure of acceptability, we used the proportion of eligible participants who enrolled in the study, and then subsequently completed self-collection at both time points. The research team qualitatively assessed testing feasibility in the camp setting through observations of proper test self-collection and camp staff willingness and capability to supervise testing. The research team qualitatively observed adherence to written infection mitigation policies for each camp (Supplementary Content) regarding masking, classroom cohorting, symptom screening, ventilation, and physical distancing on testing days. RT-PCR and antibody testing results provide measures of acute incidence of infection and prevalence of prior infection, respectively, in the study population.
2.7 Analysis
We report descriptive statistics of participant demographics, household characteristics, and RT-PCR and antibody test results, comparing proportions using chi-squared tests, means using t-tests, and medians using nonparametric equality-of-medians tests. We did not perform multivariable analyses of associations between participant characteristics and antibody results due to the low number of positive antibody tests. We used Stata 16.1 (StataCorp LLC, College Station, TX). The institutional review board at our institution approved this study as public health surveillance.
3. Results
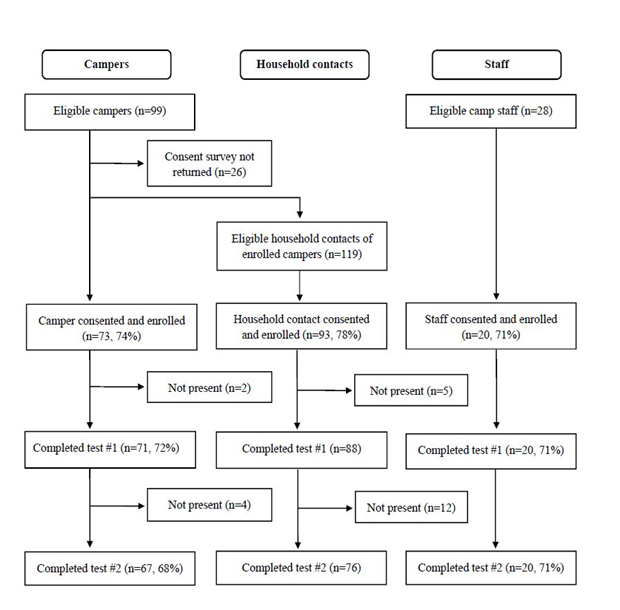
Of 246 eligible participants, 186 (76%) initially consented and enrolled. From the enrolled group, we excluded 6 campers and 17 household contacts from our analysis who were not present on both testing days (Figure 1). Our final cohort comprised 163 participants, including 67 campers, 76 household contacts, and 20 staff (Table 1). Campers in the final cohort were aged 5-14 (mean 9) years. More participants came from the science camp (55%, n=90). The sample was diverse, with Latinx participants comprising almost a third (29%, n=47) and white participants less than half (48%, n=78). Preferred method of contact for test results varied (phone call: 11% (n=6), email: 32% (n=18), text message: 57% (n=32)).
The two camps represented different urban demographic profiles. General camp households lived in areas with higher cumulative incidence of COVID-19 compared to the science camp (mean 53 cases/10,000 people vs. 21 cases/10,000, p<0.001; Table 2). Camp type was also associated with substantial differences in having one or more Latinx household members (n=25 households [76%] at the general day camp vs. n=1 [3%] at the science camp, p<0.001). There were no statistically significant differences between the general day camp and science camp in median total household size (4 vs 5 respectively, p=0.80), nor in presence of at least one household frontline worker (32% vs 39% respectively, p=0.61).
3.1 Acceptability and feasibility of specimen collection
3.2 Infection mitigation policies and observations
Both camps had the following written policies: cohorts with ≤12 campers and 2 camp staff; separate classroom cohorting; staff mask requirement except while eating; and temperature checks on arrival (Table 3). The general day camp had additional policies: daily on-site symptom screening, 6 feet of physical distancing between campers within classroom cohorts, and encouragement of camper masking. The science camp required camper masking except while eating and had a policy of open windows and doors for ventilation (Supplementary Content).
Selection of the study’s final cohort selection, starting with eligible campers age 5-14 years participating in two San Francisco classroom-based camps in summer 2020, up to 2 household contacts per camper household, and camp staff, followed by initial consent and enrollment, and subsequent exclusions.
|
Campers |
Household Contacts |
Staff |
All |
|
|
Participants, N |
67 |
76 |
20 |
163 |
|
Camp, n (%) |
||||
|
General Camp |
30 (45) |
30 (40) |
13 (65) |
73 (45) |
|
Science Camp |
37 (55) |
46 (61) |
7 (35) |
90 (55) |
|
Male, n (%)a |
34 (51) |
30 (40) |
7 (35) |
71 (44) |
|
Age, mean (95% CI)b |
9 (9-10) |
46 (45-48) |
25 (20-29) |
28 (26-31) |
|
Race/Ethnicity, n (%) |
||||
|
Caucasian |
27 (40) |
42 (55) |
9 (45) |
78 (48) |
|
Latinx |
19 (28) |
23 (30) |
5 (25) |
47 (29) |
|
Asian |
8 (12) |
8 (11) |
1 (5) |
17 (10) |
|
Multiracial |
10 (15) |
0 (0) |
4 (20) |
14 (9) |
|
Other, or decline |
3 (5) |
3 (4) |
1 (5) |
7 (4) |
CI, confidence interval; aOne household contact was trans-male (included as male); one household contact did not respond and was not included; bAge range was bi-modal among staff, with most staff 20-30 years of age, and several 40-50 years.
Table 1: Demographics for Participants Completing Both Rounds of SARS-CoV-2 Self-Collected Testing in a Classroom-Based Camp Study, Summer 2020, By Participant Type (N=163).
|
|
All households |
General Camp |
Science Camp |
P-value |
|
Households, n (%) |
56 |
25 |
31 |
-- |
|
Family size, median (IQR) |
4.5 (4-5) |
4 (4-5) |
5 (4-6) |
0.80a |
|
Latinx household, n (%)b |
20 (36) |
19 (76) |
1 (3) |
<0.001c |
|
COVID-19 incidence in household location, mean (95% CI)d |
35 (28 – 42) |
53 (43 – 62) |
21 (15 – 27) |
<0.001e |
|
Frontline worker household f, n (%) |
20 (36) |
8 (32) |
12 (39) |
0.61c |
|
Healthcare |
6 (30) |
2 (25) |
4 (33) |
0.69c |
|
Grocery or pharmacy |
0 (0) |
0 (0) |
0 (0) |
-- |
|
Construction |
4 (20) |
2 (25) |
2 (17) |
0.65c |
|
Other |
12 (60) |
4 (50) |
8 (67) |
0.46c |
|
Highest educational attainment in household, n (%) |
0.001c |
|||
|
Did not complete high school |
3 (5) |
3 (12) |
0 (0) |
0.09c |
|
High school |
4 (7) |
3 (12) |
1 (3) |
0.31c |
|
Some college |
7 (12) |
7 (28) |
0 (0) |
0.004c |
|
College |
13 (23) |
4 (16) |
9 (29) |
0.42c |
|
Graduate study |
26 (46) |
6 (24) |
20 (65) |
0.01c |
|
Decline/unsure |
3 (5) |
2 (8) |
1 (3) |
0.43c |
|
Adult diagnosed with COVID-19 in household before camp, n (%) |
1 (2) |
1 (4) |
0 (0) |
0.26c |
|
Adult suspected to have COVID-19 in household before camp, n (%) |
3 (5) |
1 (4) |
2 (6) |
0.26c |
IQR, interquartile range; CI, confidence interval
aP-value calculated using nonparametric equality-of-medians test;
bHousehold with one or more Latinx participant compared to households without any Latinx participants;
cP-value calculated using chi-squared test;
dCumulative incidence of COVID-19 cases/10,000 people from pandemic start to June 21st, 2020 for household zip code or household city, if zip code data not available (n=8 households);
eP-value calculated using t-test;
fPercentages represent the percent of frontline workers within the category, and add up to over 100% if there are multiple frontline workers in same household. Across all households there were 22 frontline workers, eight from the general camp and 14 from the science camp.
Table 2: Household Characteristics for Camper & Household Contacts Participating in SARS-CoV-2 Testing Study at Two Classroom-Based Camps, Summer 2020 (N=56).
|
Policy |
General Camp |
Science Camp |
|
Staff Masking |
Required |
Required |
|
Camper Masking |
Encouraged |
Required |
|
Staff:camper cohort ratio |
2:12 |
2:12 |
|
Temperature checks on site |
Required |
Required |
|
Verbal symptom screening on site |
Required |
Not required |
|
Physical Distancing in Cohort |
Required |
Not required |
|
Ventilation and Open Windows |
Required |
Required |
|
Hand Hygiene |
Encouraged |
Encouraged |
|
High Touch Surface Disinfecting |
Required |
Required |
The two San Francisco classroom-based camps where the study was performed each had written infection mitigation policies, shared with campers and their households prior to camp start, and summarized here
Table 3: Summary of Camp Infection Mitigation Written Policies at Each Camp.
|
|
Total, n (%) |
Campers, n (%) |
Household contacts, n (%) |
Staff, n (%) |
|
Active infection (RT-PCR) resultsa |
||||
|
Not detected |
161 (100) |
67 (100) |
75 (100) |
19 (100) |
|
Detected |
0 (0) |
(0) |
(0) |
(0) |
|
Prior Exposure (Antibody) results (camp start/camp end) |
||||
|
Negative/Negative |
152 (94) |
61 (91) |
72 (95) |
18 (90) |
|
Positive/Positive |
4 (3) |
2 (3) |
2 (3) |
0 (0) |
|
Negative/Positive |
1 (1) |
0 (0) |
0 (0) |
1 (5) |
|
Positive/Negative |
2 (1) |
2 (3)b |
0 (0) |
0 (0) |
|
Other |
4 (3) |
2 (3)c |
2 (3)d |
0 (0) |
RT-PCR, reverse transcriptase polymerase chain reaction
aTwo tests errors occurred due to tube leaking, one for staff and one for household contact, both from the second testing session at the general day camp
bSecond time point for one camper had low total IgG indicating poor quality sample.
cOne camper had a negative test 1 and missing test 2. One camper had a negative test followed by an indeterminate result, which was just below threshold for response to receptor binding and 2-3x rise in both receptor binding domain and whole spike responses between time points.
dOne household contact had a missing first test followed by a negative second test; one had a negative first test followed by a missing second test.
Table 4: COVID-19 Active Infection and Prior Exposure Self-Testing Results Among Participants in Classroom-Based Camp Study in San Francisco, Summer 2020 (N=163).
On observation, both camps’ cohort sizes were within stated goals. We observed consistent adherence with the science camp’s strict mask policy, and generally high but variable masking adherence at the general camp where masking was encouraged. There was variable consistency observed with classroom cohorting policies at the science camp and consistent adherence at the general camp. There was variable adherence to the general camp’s within-cohort physical distancing policy, especially among younger children.
3.3 Testing results
SARS-CoV-2 was not detected by RT-PCR at either time point among the 163 participants in the final cohort, nor was it detected in the tests of the 16 participants excluded for not being present for one of the testing time points. In all RT-PCR tests, the human RNAse P gene was detected, indicating adequate sample collection. RT-PCR follow-up results were unavailable for two participants whose specimen transport tubes from the second testing day leaked. All participants were asymptomatic at the time of SARS-CoV-2 testing, except one camper who was excluded from camp due to new symptoms, but who underwent self-testing outside the camp before returning home. Within the final cohort, 7/163 participants (4%, 95% confidence interval: 1-7%) had SARS-CoV-2 antibodies at one or more time point (four campers, two household contacts, and one staff) (Table 4, Supplementary Content, Supplementary Table 5). Of these, six (86%) were from a high or moderate incidence zip code, and also of Latinx ethnicity. Four of the subjects with positive antibodies were in two household clusters, each with one camper and one household contact. Three testing samples were lost, and one was indeterminate. One participant had antibodies detected at the end of camp but not the start, suggestive of either seroconversion or an initial false negative test; this participant was general camp staff who lived in a higher incidence zip code. Two participants reverted from positive to negative; both were children aged 7, one from
a low and one from a moderate incidence zip code.
4. Discussion
In this longitudinal study, we found that serial, supervised non-NP self-collection for COVID-19 testing in classroom-based summer day camps was acceptable and feasible for kindergarten through 8th grade campers, their household contacts, and staff. Children as young as 5 years participated in self-collection at two time points, with 100% of consented and present campers and staff completing both rounds. At one camp, staff were able to assist with collection, suggesting the possibility of non-medically-trained school staff assisting with testing, if needed. In addition, in the context of observed adherence to infection mitigation methods, we detected no cases of active infections by RT-PCR. However, we did detect possible seroconversion in one young adult staff member.
This study adds to existing evidence demonstrating promising acceptability and feasibility of less-invasive SARS-CoV-2 testing strategies and self-collection [9]. Multiple studies have demonstrated comparable results with self-collected testing compared to provider-collected NP sampling, including with supervised anterior nares self-collection [7], as well as unsupervised [8] and supervised mid-turbinate self-collection [17]. However, these studies were in symptomatic adults and the findings have not been validated in children. Children age 6-18 years with cystic fibrosis were able to perform unsupervised anterior nares self-collection to test for common respiratory viruses; they reported high acceptability and the approach led to increased yield compared to provider-collected swabs [16]. To our knowledge, ours is the first study to describe self-collected anterior nares SARS-CoV-2 testing among children, or any participants, in a school-like setting.
Our study suggests potential limitations in the feasibility of self-collected saliva testing in younger children, including the need for closer coaching among younger children for adequate saliva sampling. The antibody reversion of two young children based on saliva samples may have been due to variable sample collection, as it is biologically unlikely that they would have reverted in this time frame, and one of the two had evidence of low total antibodies in the second sample. These findings emphasize the importance of feasibility assessments when introducing novel sampling strategies for children. For example, Wyllie et al. have demonstrated self-collected saliva RT-PCR testing SARS-CoV-2 testing to be feasible and to have excellent test characteristics in adults [18]. However, this saliva sampling method requires collection prior to eating or drinking upon waking, and production of 3ml of saliva without bubbles into a cup, which is more complicated than the saliva collection we studied and therefore may be more difficult for children than was found for adults.
Neither indoor camp experienced documented COVID-19 outbreaks in the context of infection mitigation strategies. This occurred in the setting of concurrent community transmission in San Francisco that exceeded the California county “watchlist” criteria (<10 new cases per 10,000 people per 14 days), developed for the purpose of safe school reopening [15]. Our experience aligns with literature demonstrating that school and classroom-based transmission appears rare among younger children in the setting of physical distancing, stable cohorts, and reduced numbers of students, although prior studies had lower community incidence than in our study [19-21]. While we found evidence of possible seroconversion of one young adult staff member, negative testing in other participants suggests that if infection occurred, it was not transmitted to others at camp. In contrast, other studies have described substantial outbreaks amongst those aged 6-19 years in the absence of adequate mitigation policies (specifically: allowing large cohorts, poor ventilation, and no masking) [22, 23].
Findings from this study have implications for testing strategies for school re-opening and in-person learning activities. Our methods suggest a scalable approach for on-site testing. Current testing strategies utilizing provider-collected nasopharyngeal RT-PCR are limited by the need for skilled collectors, discomfort leading to increased likelihood of child and adult refusal, risk of transmission, and high utilization of PPE. We demonstrated a testing strategy that overcame these obstacles to permit serial test sampling in children as young as five years. Furthermore, our results suggest a potential approach for school-based testing of asymptomatic individuals as part of a community surveillance or school-based screening strategy. The CDC notes that schools may perform school-based testing if they have capabilities to do so. At the time of writing, the CDC suggests schools may consider asymptomatic testing in school setting in communities where public health officials are recommending expanded testing on a voluntary basis, especially in areas of moderate to high community transmission [6]. An acceptable and feasible school-based testing strategy could make this approach easier to implement and improve the net benefits.
Our study has some limitations. Our setting was limited to two indoor camps in San Francisco, California, and camp duration was 3-5 weeks. The acceptability and feasibility we observed may not generalize to other areas of the nation, or to the longer duration of the school year. However, our relatively large sample of participants, including campers as young as 5 years, consistent experience across camps with diverse populations, and use of classroom-based camp settings, all support the potential generalizability of our findings to a variety of educational environments including schools. To date, clinical validation of SARS-CoV-2 self-collected testing has been limited to symptomatic adults. We did not compare the anterior nares self-collection in asymptomatic participants or children to the gold standard of healthcare personnel-collected NP swabs. It is possible that our approach had lower sensitivity than the gold standard and might not be able to detect all COVID-19 infections. However, given that viral levels appear to be highest before and during early symptom onset, and the nares have comparatively high viral loads, there is biologic plausibility for anterior nares collection in asymptomatic participants [24, 25]. In addition, the detection of human RNAse P gene in all samples suggests adequate sample collection and supports the validity of the negative test results. In addition, data suggest that frequent testing with short turnaround times, even with lower test sensitivity, is more likely to identify a subject early in COVID-19 infection, even while asymptomatic, than highly sensitive tests performed less frequently [26]. Hence, the acceptability of the anterior nasal collection, allowing for frequent repeated testing, is potentially a key piece to preventing transmission.
In conclusion, we demonstrated excellent feasibility and acceptability of a serial surveillance SARS-CoV-2 testing approach with supervised anterior nares self-collection in 5-14 year-old children, their household contacts, and staff, in an indoor, classroom-based camp setting. The test collection methods and infection mitigation strategies described here may be potential core components to allow school re-opening and safe in-person learning.
Acknowledgements
We thank the staff and leadership of the two summer camps for their close collaboration, with special acknowledgement to the leadership of Rochelle Celedon and Judith Diaz of the Buena Vista Day Camp. We thank Dr. Kelley Meade of Benioff Children’s Hospital Oakland for her contributions to the study design. We offer our gratitude to the families and camp staff for their participation. We thank the laboratory staff at the Chan Zuckerberg Biohub, collectively working as the CLIAhub consortium, who performed RT-PCR testing for our participants.
Funding/Support
This project is supported by funding from the UCSF
COVID-19 Relief Fund. BG and KSP are Chan Zuckerberg Biohub investigators.
Role of Funder/Sponsor
The Chan Zuckerberg Biohub donated proteins used in the antibody assay. There was no additional funder/sponsor participation in the work.
Conflict of Interest Disclosure
The authors have no conflicts of interest relevant to this article to disclose.
Contribution Statement
Dr. Bardach conceptualized, designed, and implemented the study, designed the data collection tools, carried out the analyses, drafted the initial manuscript, and reviewed and revised the manuscript.
Dr. Cooch implemented the study, carried out the analyses, drafted the initial manuscript, and reviewed and revised the manuscript.
Ms. Watson and Ms. Olarte implemented the study, designed the data collection tools, drafted the initial manuscript, and reviewed and revised the manuscript.
Professor Crawford, Professor DeRisi, and Dr. Greenhouse assisted in study design, carried out the laboratory testing, and critically reviewed the manuscript for important intellectual content.
Ms. Hakim and Ms. Turcios carried out the laboratory testing and critically reviewed the manuscript for important intellectual content.
The CLIAhub Consortium carried out the laboratory testing.
Dr. Atkinson-McEvoy, Dr. Hirsch, Dr. Keller, Dr. Pollard, and Dr. Ruel conceptualized and designed the study, and critically reviewed the manuscript for important intellectual content.
Ms. Cohen-Ross and Ms. Leon assisted in implementing the study, and critically reviewed the manuscript for important intellectual content.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
References
- Burbio D. K-12 School Reopening Trends (2021).
- Donohue JM, Miller E. COVID-19 and school closures. JAMA (2020): 1-3.
- Schleicher A. The Impact of Covid-19 on education: insights From Education At a Glance (2020).
- Fuchs-Schündeln N, Krueger D, Ludwig A, et al. The long-term distributional and welfare effects of COVID-19 school closures. The Center for Economic Policy Research (CEPR) Discussion Papers (2020): 47.
- Dorn E, Hancock B, Sarakatsannis J, et al. COVID-19 and student learning in the United States: The hurt could last a lifetime. McKinsey & Company (2020).
- Centers for Disease Control and Prevention. Interim considerations for K-12 school administrators for SARS-CoV-2 testing (2020).
- Tu Y-P, Jennings R, Hart B, et al. Swabs collected by patients or health care workers for SARS-CoV-2 testing. N Engl J Med 5 (2020): 494-496.
- McCulloch DJ, Kim AE, Wilcox NC, et al. Comparison of unsupervised home self-collected midnasal swabs with clinician-collected nasopharyngeal swabs for detection of SARS-CoV-2 infection. JAMA Netw Open 3 (2020): 1-4.
- Frazee BW, Rodríguez-Hoces de la Guardia A, Alter H, et al. Accuracy and discomfort of different types of intranasal specimen collection methods for molecular influenza testing in emergency department patients. Ann Emerg Med 71 (2018): 509-517.
- Centers for Disease Control and Prevention. Overview of testing for SARS-CoV-2 (2020).
- Centers for Disease Control and Prevention. Interim guidelines for collecting, handling, and testing clinical specimens from persons for Coronavirus Disease 2019 (COVID-19) (2020).
- Emmons WW, Paparello SF, Decker CF, et al. A modified elisa and western blot accurately determine anti-human immunodeficiency virus type 1 antibodies in oral fluids obtained with a special collecting device. J Infect Dis 171 (1995): 1406-1410.
- Chamie G, Marquez C, Crawford E, et al. SARS-CoV-2 community transmission disproportionately affects Latinx population during shelter-in-place in San Francisco. Clin Infect Dis (2020) In Press.
- Wadhera RK, Wadhera P, Gaba P, et al. Variation in COVID-19 hospitalizations and deaths across New York city boroughs. JAMA 323 (2020): 2192-2195.
- Los Angeles Times. California Coronavirus Data (2020).
- Emerson J, Cochrane E, McNamara S, et al. Home self-collection of nasal swabs for diagnosis of acute respiratory virus infections in children with cystic fibrosis. J Pediatric Infect Dis Soc 2 (2013): 345-351.
- Wehrhahn MC, Robson J, Brown S, et al. Self-collection: An appropriate alternative during the SARS-CoV-2 pandemic. J Clin Virol 128 (2020).
- Wyllie AL, Fournier J, Campbell ACM, et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N Engl J Med 383 (2020) 1283-1286.
- Carlson J. Covid-19 in schoolchildren: a comparison between Finland and Sweden (2020).
- National Centre for Immunisation Research and Surveillance (NCIRS). COVID-19 in schools – the experience in NSW 9 (2020): 1-5.
- Link-Gelles R, DellaGrotta AL, Molina C, et al. Limited secondary transmission of SARS-CoV-2 in child care programs — Rhode Island, June 1–July 31, 2020. MMWR Recomm Rep 69 (2020): 1170-1172.
- Szablewski CM, Chang KT, Brown MM, et al. SARS-CoV-2 transmission and infection among attendees of an overnight camp-Georgia, June 2020. MMWR Recomm Rep 69 (2020): 2019-2021.
- Stein-Zamir C, Abramson N, Shoob H, et al. A large COVID-19 outbreak in a high school 10 days after schools’ reopening, Israel, May 2020. Euro Surveill 25 (2020): 1-5.
- Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med 382 (2020): 1175-1177.
- He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 26 (2020): 672-675.
- Larremore DB, Wilder B, Lester E, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv 7 (2021): 1-11.
Supplementary Content
Contents:
- 1 Instructional video of anterior nares self-collection
- 2 Standard operating procedures for swab collections
- 3 Written infection control protocols at two camps, distributed to parents and staff
- 4 Description of participants with any positive antibody result
- 5 Table 5: Associations between antibody results and participant demographics
- Instructional video of anterior nares self-collection

Instructional video of camper performing anterior nares self-collection, similar to video provided to families during consent process
- Standard operating procedures for swab collections
Participants performed hand hygiene before and after specimen collection using alcohol-based hand sanitizer. Specimen collectors performed hand hygiene and changed gloves for each participant. Specimen collectors opened swab packages and presented the swab to the participant in the opened package, without handling the swab. Then specimen collectors stepped back to allow 6 feet of distance between themselves and the participant, instructing the participant to remove their mask. For anterior nares self-collection, participants inserted the flocked swab about 0.5 inch deep and rubbed around the inner walls of both nostrils for 10 seconds each (Figure 2, instructional video linked in Supplement A) [13]. Participants then handed the swab back to the specimen collector, who placed the swab into DNA/RNA Shield (Zymo research, Irvine, CA) for stabilization during transport. Saliva was collected using the OraSure Oral Specimen Collection Device (OraSure Technologies, Bethlehem, PA). Participants took the swab and rubbed and then retained the swab against their outer gum line for at least two minutes, timed by the specimen collector. The gum line has higher concentration of antibodies with fewer inhibitory substances compared to other parts of the mouth [14]. Participants then handed the swab back to the specimen collector who placed it in a separate OraSure collection tube.
- Written infection control protocols at two camps, distributed to parents and staff
General day camp
Drop off procedures:
- Strict Drop Off Time: 8:30 a.m. - 8:45 a.m. Arrival time staggered for families. Please arrive promptly.
- Stand in designated marked spot on sidewalk with your child to maintain 6 feet distance
- All children will be screened with health questions and temperature taken
- Sign your child in with your own pen if possible
- Wash your hands and your child’s hands before coming to program
- Do not come into the building. Arrive with a mask on, wait at the entry for a staff member to let your child in
Pick up procedures:
- Children must be picked up on time, between 3:00 p.m - 3:15 p.m
- Sign your child out with your own pen if possible
- Do not enter the school building. A staff member will be outside during dismissal time
- The staff member will call for your child to be escorted to you
Early pick up:
- Notify staff at drop-off if you must have an early pick-up. Staff will coordinate with parents’ arrival and walk the child outside to them.
Classroom (pods):
- Maximum capacity is 12 children and 2 teachers, the same children and teachers everyday, every week
- Pods (classrooms) will not interact with other pods
- Each child will have a designated desk area that is 6 feet away from other children
- Each child is encouraged to wear a mask
- Hand washing/sanitizer will happen often during the day
- Classrooms will be cleaned often when children are not present in the classroom
- Windows opened and fans used for ventilation
Lunch:
- Children will eat lunch at staggered times
- Handwashing before and after eating lunch
- Absolutely no sharing food
Outdoor play:
- Games that do not require contact will be played
- All equipment will be cleaned after each use
Bathroom use:
- One child at a time will be escorted by an adult to the bathroom, adult will wait outside for the child
- Bathrooms will be cleaned after each use by an adult
Illness:
- Children with 100.4°F will be sent home and can return to Summer Camp after not having a fever for 72 hours.
- If a child has COVID-19 or has a household member who has COVID-19 they will not be able to return to camp for 14 days or when the Department of Health has given the OK.
What to do if your child is sick:
- If a child tests positive for COVID-19 we will shut down for 3-5 days to do a thorough cleaning of the camp area.
- The child may not return to camp for 14 days.
- If a child has a common cold with a fever they may not return to camp until they have not had a fever for 72 hours.
- If a child becomes sick during the day they will be kept in a separate but supervised space until they can go home. We will limit the number of staff who take care of sick children.
For staff:
- All staff will be screened with health questions and temperature at arrival.
- If you are sick please stay home. If you have a fever of 100.4 degrees you can not return to work until you have not had a fever for 72 hours
- If you or a member of your household has tested positive for COVID-19 please notify one of the administrators immediately. You will not be able to return to work for a 14 day period
Science camp
Classrooms:
- Campers will be grouped in “pods” according to their session.
- Campers will stay with their same pod for the entire three-week session.
- Pod size will be limited to 10 campers with some up to 12, depending on the size of the room
- Each pod will remain in a separate room and will not interact with kids from other pods.
- Two instructors will lead each pod and will remain with the same pod each week.
- Children and youth must attend the first week of the session. Those who do not attend the first week may not join the camp later and will not receive any refund or credit (SFDPH’s rule)
- Children will wear a facemask in classroom at all times. We will provide one washable three-layer soft cotton mask for each child for free. They are very breathable. Masks are washed every Wednesday and Friday. Masks will bear the name of each child and will be kept in an individual mask holder in the classroom for the duration of the session.
- Campers will take home their mask at the end of the session. We will use encouragement and appeal to reason when asking kids to wear masks.
- If a child manages to lose their mask somehow, a new one will be issued, and your account will be charged $5.
- If you would like your child to use his/her own mask, it needs to be reusable and have at least 2 layers of fabric.
- Children will wash hands for 20 seconds between activities, before eating, after recess, and before leaving for home; approximately 7 times a day.
- Children will sanitize surfaces and objects in the classroom twice a day. Including doorknobs, light switches, classroom sink handles, countertops, desks, chairs, and objects such as PCR machines, keyboards, and polishing machines.
- Staff will sanitize surfaces in the common areas twice daily.
- Classroom windows and doors will remain open as much as possible.
Sign-in rules:
- We ask that you consider dropping off children in grades 2-5 at 8:45-9am, and grades 6-8 at 9-9:15. We know this is not always possible, but please try. This will help minimize movement and interaction.
- Family members and caregivers waiting outside to drop-off or pick-up children must wear face masks per SFDPH’s rules.
- We are sorry, but parents are not allowed to enter the building. If you have a shy small child, you may enter the lobby area only, wearing a face mask.
- Staff should remain 6 feet apart from parents and caregivers. Please show our staff you care and move about with this in mind.
- A staff member will take children’s temperatures with a thermometer upon arrival, using a “non-touch” (infrared) thermometer
- Sign in and out will be done using an app—detailed information to be sent separately.
- During AM drop off, a hygiene station will be located near the entrance for children and staff to use immediately upon their arrival.
- Please try not to have grandparents pick up campers. If you love someone over the age of 60, keep them away from busy places.
- Children with symptoms or fever will be sent home. Information about getting tested can be found here: https://sf.gov/find-out-how-get-tested-coronavirus
Children – Recess:
- All lunch and snacks should be provided by the parents. [The Camp] will not be providing any snacks or lunch this year per regulations. Please make sure you pack enough food for your child.
- Children should not share food. Lunch and snacks will be taken in either the classroom. There will be no cafeteria this year, regrettably.
- Sports with shared equipment or physical contacts, like soccer and baseball, will be played only within the same pod.
- Campers in each pod will wear over their shirts, a Dry-FIT T-shirt with a unique color of their group. Shirts will be laundered by the camp at the end of each week and will be taken home at the end of the session.
- Description of participants with any positive antibody results
Of the seven antibody positive participants, four had positive tests at both time points (sero-positive), one had an initial negative test at camp beginning and subsequent positive at camp end (sero-conversion), two had an initial positive test and a subsequent negative (sero-reversion). Sero-positive: The four with both tests positive comprised two clusters, each with a camper and adult contact in the same household. Both were in Latinx households in high or moderate incidence zip codes (71/10,000 and 47/10,000). Campers were 11 and 8 years old. One cluster lived with a construction worker in the household. Sero-conversion: The participant who sero-converted was a Latinx staff member also in a high incidence zip code (71/10,000). Sero-reversion: The participants who reverted from positive to negative were both children aged 7. We hypothesize they may not have given good samples in the second test day, as it is unlikely that they would have reverted in this period. No household contacts were tested for those who reverted. Both lived in households without any frontline workers, one in a low incidence zip code (13/10,000) and one in a moderate incidence zip code (50/10,000).
|
Antibody test result |
Negative (no positive Ab test) |
Positive (any positive Ab test) |
|
All Participants |
156 |
7 |
|
Participant Type, n (%) |
||
|
Campers |
63 (40) |
4 (57) |
|
Household contacts |
74 (47) |
2 (29) |
|
Staff |
19 (12) |
1 (14) |
|
Camp type, n (%) |
||
|
General Day |
67 (43) |
6 (86) |
|
Science |
89 (57) |
1 (14) |
|
Gender, n (%)a |
||
|
Male |
69 (44) |
2 (29) |
|
Female |
85 (55) |
5 (71) |
|
Age, years |
||
|
0-14 |
63 (40) |
4 (57) |
|
15-29 |
18 (12) |
1 (14) |
|
30+ |
75 (48) |
2 (29) |
|
Race/Ethnicity, n (%) |
||
|
Not Latinx |
107 (69) |
1 (14) |
|
Latinx |
49 (31) |
6 (86) |
|
COVID incidence in household locality, mean (95% CI)b |
36 (32 – 40) |
53 (34 – 72) |
|
Frontline worker in household, n (%) |
52 (33) |
3 (43) |
|
Household member with history of suspected or confirmed COVID-19, n (%) |
8 (5) |
2 (29) |
|
Household member antibody positive, n (%) |
1 (1.28) |
4 (57.1) |
Ab, antibody; CI Confidence Interval
aOne household contact was trans male; one household contact did not respond
bCumulative incidence of COVID-19 cases/10,000 people during June 19th - 21st, 2020 for household zip code (or city, for n=8 households).
Table 5: Description of Participant Demographics by Antibody Results.