Successfully Treatment of Dermatomyositis with high-dose Intravenous Immunoglobulin Therapy
Article Information
Vanessa Müller, Julia K. Winkler^, Alexander H. Enk^,*
University Hospital Heidelberg Department of Dermatology, Heidelberg, Germany
^Both authors contributed equally
*Corresponding author: Alexander H. Enk, University Hospital Heidelberg Department of Dermatology, Heidelberg, Germany.
Received: 30 July 2025; Accepted: 07 August 2025; Published: 20 August 2025
Citation: Vanessa Müller, Julia K. Winkler, Alexander H. Enk. Successfully Treatment of Dermatomyositis with high-dose Intravenous Immunoglobulin Therapy. Archives of Microbiology and Immunology. 9 (2025): 230-234.
View / Download Pdf Share at FacebookAbstract
We report on three patients with severe dermatomyositis and varying clinical symptoms who draw significant benefit from treatment with high- dose intravenous immunoglobulins (IVIgs). Modern IVIg products demonstrate high therapeutic efficacy at a good tolerability. The safety profile is favorable in particular with regard to a lower risk for thromboembolic events. Challenges in the treatment of dermatomyositis include severe myositis with calcinosis cutis and paraneoplastic occurrence. All three patients achieved significant relief from muscle and skin-related dermatomyositis symptoms under IVIg treatment at a dose of 2g/kg body weight every four weeks. Hence, our case series supports the role of IVIg as a favorable therapeutic option in patients with severe dermatomyositis, including those not responding or not suitable for conventional immunosuppressive strategies.
Keywords
dermatomyositis, IVIg, high-dose intravenous immunoglobulins, autoimmune disease, Yimmugo
dermatomyositis articles, IVIg articles, high-dose intravenous immunoglobulins articles, autoimmune disease articles, Yimmugo articles
Article Details
1. Introduction
Dermatomyositis (DM) belongs to the heterogeneous group of idiopathic inflammatory myopathies (IIM) and is characterized by pathognomonic skin manifestations and a progredient weakness of the (proximal) muscles [1], which can lead to significant functional impairment and patient burden [2]. Characteristic cutaneous features include a violaceous discoloration of the eyelids (heliotrope rash), flat-topped, violaceous papules over the finger joints (Gottron’s papules), erythema over the chest (V-sign), shoulders and upper back (shawl sign), and thighs (holster sign). Additional findings often involve dilated capillaries at the nailfolds (periungual telangiectasia) and a scaly erythema of the scalp [3]. In severe cases dermatomyositis may be accompanied by calcinosis cutis which refers to painful subcutaneous calcium deposits predominantly observed in patients with anti-NXP2-positive DM [4]. Muscle involvement may be objectified by magnetic resonance imaging (MRI) and supported by muscle biopsy and/or electromyography (EMG) [1]. In addition, serological testing for myositis-specific autoantibodies helps verify the diagnosis and identify individual risks. Anti-TIF1γ antibodies are strongly associated with the presence/occurrence of malignancy, anti-NXP2 antibodies are associated with severe muscle symptoms and/or calcinosis, and anti-MDA5 antibodies are typically found in amyopathic variants and carry a risk to develop rapidly progressive interstitial lung disease [5]. Due to the increased risk of accompanying malignancies, a thorough tumor screening is recommended especially in the first two years following diagnosis of dermatomyositis [6].
Treatment choices need to consider disease severity, organ involvement, and concomitant diseases. First-line therapy involves oral prednisone at a dose of 1–1.5 mg/kg/day, which is gradually tapered over the following months to years. To avoid prolonged treatment with high-dose steroids and related side effects, steroid-sparing agents such as azathioprine, mycophenolate mofetil, or methotrexate may be added. In case of disease progression or non-response to standard treatment, high-dose intravenous immunoglobulins (IVIgs) at a dose of 2 g/kg body weight administered every four weeks over two to five days, have proven to be an effective (additional) therapy [7, 8]. Based on the results of the ProDerm study, IVIg therapy has been approved for the treatment of dermatomyositis in Europe [9] and efficacy and safety have been supported by further reports [10]. IVIgs are derived from pooled plasma of up to 10,000 healthy donors and undergo a multistep purification and stabilization process [11]. The final product mainly contains IgG (especially IgG1), with traces of other immunoglobulins such as IgA and IgM. Composition may vary slightly depending on the manufacturing process [12]. Here, we present three patients with severe dermatomyositis who received treatment with a novel IVIg product.
Case 1
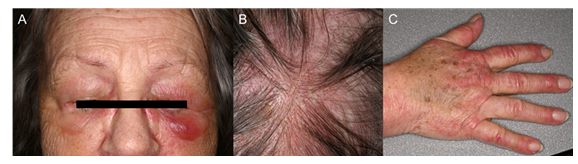
We report on a 71-year-old female patient who presented in February 2024 with weakness and pain in the muscles of shoulder and pelvic region. Since September 2023, she had experienced difficulty getting up from a chair or lifting her arms. Examination revealed purple papules on the extensor sides of the fingers, periorbital erythema with eyelid oedema and erythematous itching skin lesions on the thighs and cheeks. She reported dysphagia as well as redness and scaling of the scalp. MRI of the shoulder and muscle biopsy confirmed acute myositis. Serological analysis revealed anti-TIF1-γ antibodies. The patient had undergone a partial lung resection for squamous cell carcinoma in curative intention four weeks prior to the onset of dermatomyositis symptoms. An extended tumor screening, including computed tomography, positron emission tomography–computed tomography, cranial MRI, esophagogastroduodenoscopy and serum electrophoresis revealed no further pathological findings. The initial treatment included immunosuppressive therapy with prednisolone (80 mg daily) and azathioprine (initially 50 mg, later increased to 100 mg daily). The patient developed side effects such as tremor, insomnia and dizziness. Given the side effects, the unsuccessful immunosuppressive therapy and paraneoplastic origin, in May 2024 an additional IVIg therapy (Yimmugo, 2 g/kg body weight over two days every four weeks) was initiated. Prednisone was tapered and therapy with azathioprine discontinued. During the first IVIg cycle the patient experienced a feeling of intense heat and facial flushing after about 20 minutes, rapid symptoms relief was achieved after treatment with intravenous clemastine 1mg. Following the first infusion, she reported a headache, but all subsequent cycles were well tolerated and led to a complete relief from all symptoms associated with dermatomyositis allowing to reduce dose of oral prednisone to 5 mg/day. Currently, no signs of tumor recurrence have been detected and the patient’s well-being significantly improved.
Case 2
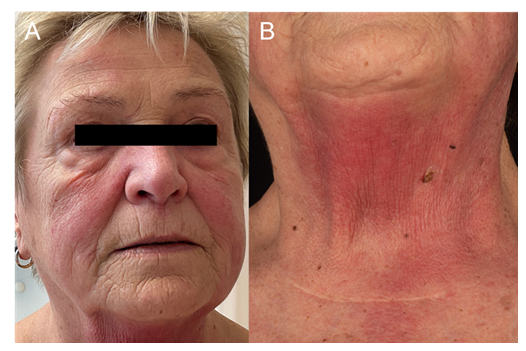
A 75-year-old female patient first presented in March 2023 with characteristic cutaneous manifestations of dermatomyositis including erythematous rash in the anterior cervical region (V-sign), heliotrope erythema, erythema of the cheeks, Gottron’s papules, and “mechanic’s hands.” In addition, enlarged nailfold capillaries were noted. The patient reported dysphagia and myalgia-like pain in the neck and shoulder region. In her medical history she had suffered from ovarian carcinoma (1980), renal cell carcinoma (1990), and breast cancer (2014). Serological testing revealed anti-MDA5 antibodies. Histopathology revealed an interface dermatitis, consistent with dermatomyositis. Initial treatment with oral prednisone (15mg/day) over 10 days showed an insufficient therapeutic response. Due to progressive muscular weakness and pain, imaging of the lower extremities was repeated, revealing symmetrical myositis of the proximal pelvic muscles. Tumor screening revealed an indeterminate mass in the right adrenal gland. In consideration of the high surgical risk, no invasive diagnostic procedure was performed, and follow-up imaging was initiated. Due to progressive muscle weakness, high-dose steroid therapy with prednisolone 80mg/day was started. In June 2023, additional high-dose intravenous immunoglobulin therapy (Octagam) 2g/kg body weight over two days every four weeks was initiated After three cycles, the patient presented with severe dyspnea and pulmonary embolism and a deep vein thrombosis of the right femoral vein were diagnosed. Therapeutic anticoagulation was initiated and IVIg therapy continued with modifications. IVIgs were applied over four instead of two days and the infusion rate was reduced from 200 ml/h to 100 ml/h. Additionally, 500 ml of 0.9 % sodium chloride was administered. In April 2024, the patient reported muscle pain in the hips and an increase in creatine kinase (CK) to >1000 U/l at a prednisolone dose of 7.5 mg/day was found. The MRI showed new inflammatory involvement of the right adductor magnus muscle and part of the gluteal and thigh muscles. Prednisone dose was increased to 20 mg/day. In July 2024, IVIg therapy was switched to Yimmugo (Biotest). From August 2024, IVIgs were applied over three instead of four days and tolerated well by the patient. Meanwhile, prednisone dose was successfully reduced to 15 mg/day.
Case 3
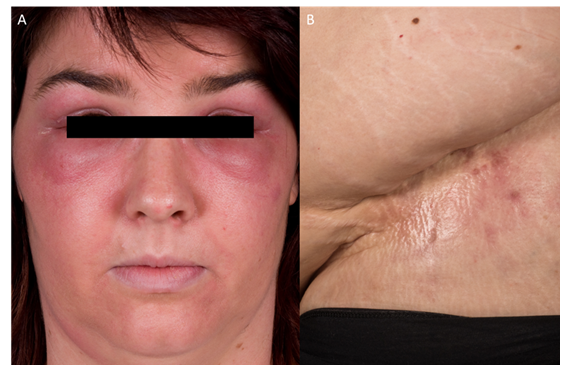
We report on a 32-year-old female patient who first presented in September 2009 with a heliotrope rash accompanied by periorbital swelling. In addition, the patient had proximal muscle weakness affecting both upper and lower extremities. Cutaneous findings also involved erythema on the décolleté and back, thickened nailfold capillaries with telangiectasias, as well as Gottron’s papules. MRI of the thigh muscles revealed symmetrical, bilateral inflammation consistent with myositis. CK levels were significantly elevated at 8,797U/L. While muscle biopsy showed non-specific findings, EMG revealed a characteristic myopathic pattern. ANA titer was 1:320 and anti-NXP2 antibodies were detected. Tumor screening was without pathological findings. Oral prednisone therapy at a dose of 1mg/kg body weight was initiated. In addition, the patient received subcutaneous methotrexate (MTX) at a weekly dose of 15mg. Further on, prednisolone was gradually tapered to 5mg/day. Since December 2013, the patient suffered from progressive calcinosis cutis. It initially presented as a 10×10cm subcutaneous consolidation. By 2016 involvement of the gluteal muscle was observed and in 2018 further deposits appeared over the right tibia and left ventral thigh. Due to worsening of myopathic symptoms and impaired movement caused by calcinosis, the patient received treatment with rituximab (2 × 1000 mg intravenously within two weeks) in May and June 2019, which led to a stabilization of calcinosis and pain relief. Approximately four years later, she experienced another flare-up of the disease accompanied by an increase of CK values up to 1200 U/l and severe pain in the pelvic and sacral region. An MRI scan confirmed active myositis. Therefore, intravenous immunoglobulin therapy with Intratect (2 g/kg body weight over two days, every four weeks) was initiated in October 2023. In August 2024, treatment was switched to Yimmugo, which was well tolerated. Meanwhile, prednisolone was reduced to 5 mg/day with methotrexate being continued. CK values declined to normal and the patient is without muscle pain anymore with calcinosis cutis being stable. She was able to return to her normal life and no side effects were observed during IVIg therapy.
Discussion
Our case series reports on three patients with severe dermatomyositis treated with the IVIg Yimmugo at a dose of 2g/kg body weight administered every four weeks. All three patients had minimal to transient response to standard immunosuppression with prednisolone and/or steroid-sparing agents such as methotrexate and azathioprine before IVIg therapy. Yimmugo is a modern IVIg preparation produced by an innovative manufacturing process. By “vibromixing” for gentle protein handling and a dedicated purification step to remove components such as properdin, it shows very low anticomplementary activity, a low risk of clotting, mostly monomeric (functionally active) antibodies, and very few aggregates or impurities [13]. All patients had individual therapeutic challenges, e.g. association with an underlying malignancy or severe muscle involvement with calcinosis cutis. Our first case illustrates therapeutic management of paraneoplastic dermatomyositis. An association of dermatomyositis and underlying malignancies is well documented [14, 15], particularly in individuals tested positive for anti–TIF1-γ antibodies [16]. Here, therapy with steroids combined with steroid-sparing immunosuppressants may negatively impact long-term survival and tumor progression [17]. We managed to taper steroid therapy more quickly and discontinue azathioprine by initiating IVIg therapy. Our case adds to the limited number of reports on the use of intravenous immunoglobulins for the treatment of paraneoplastic dermatomyositis [18]. So far no progression or recurrence of malignancy was observed and no serious adverse events occurred during treatment. Our second patient was diagnosed with a severe thromboembolic event during IVIg therapy (Octagam 10%). This is a rare but serious adverse event, especially in high-risk individuals [19]. Nevertheless, IVIg therapy could be continued by reducing the infusion rate, administering therapy over four days together with additional intravenous fluids [20]. We switched to Yimmugo, as data indicate a lower risk for thromboembolic events [13].
Our third patient revealed a prolonged disease history with progressive muscle weakness and calcinosis cutis, and had already been treated with steroids, methotrexate and rituximab before IVIg therapy. Rituximab may be used in cases of DM refractory to conventional immunosuppressive therapy [21, 22]. The patient responded well to rituximab with clinical improvement of muscle weakness and stabilization of calcinosis cutis over a four-year period. Currently, there is no standardized treatment protocol for calcinosis cutis. In recent years, IVIgs have been explored as a therapeutic option in calcinosis cutis and cases reported partial regression of calcified lesions after IVIg administration, especially in juvenile dermatomyositis [23, 24]. Hence, IVIgs have become an emerging therapeutic option for clinicians treating calcinosis cutis associated with dermatomyositis [25].
Conclusion
Yimmugo shows lasting clinical efficacy and excellent tolerability in patients with severe dermatomyositis, including paraneoplastic dermatomyositis and calcinosis cutis. Safety profile is favorable, which makes it a valuable therapeutic option also in severely sick patients.
Acknowledgments
Author Contributions
Alexander H. Enk and Julia K. Winkler revised and reviewed the case series. Vanessa Müller prepared the case series.
Funding
This study was not supported by any sponsor or funder.
Data Availability
All data generated or analyzed during this study are included in this article. Further enquiries can be directed at the corresponding author.
Ethic/ Ethical approval
Ethical approval was not required for this case series, in accordance with local and national guidelines. Written informed consent was obtained from patients for publication of their case details and publication of their photographs within your manuscript.
Conflict of interest
Alexander H. Enk received advisory board honoraria and consultancy fees from Biotest AG. Julia K. Winkler received travel expenses and honoraria from Biotest AG. Vanessa Müller has no competing interests.
Thanks
We thank the patients for their consent to publication.
References
- Cassard L, Seraly N, Riegert M, et al. Dermatomyositis: Practical Guidance and Unmet Needs. ImmunoTargets Ther 13 (2024): 151–72.
- Yoshida A, Li Y, Maroufy V, et al. Impaired health-related quality of life in idiopathic inflammatory myopathies: a cross-sectional analysis from the COVAD-2 e-survey. Rheumatol Adv Pract. 4 März 8 (2024): rkae028.
- Callen JP. Cutaneous manifestations of dermatomyositis and their management. Curr Rheumatol Rep. Juni 12 (2010): 192–7.
- Reiter N, El-Shabrawi L, Leinweber B, et al. Calcinosis cutis: part I. Diagnostic pathway. J Am Acad Dermatol. Juli 65 (2011): 1–12.
- Selva-O’Callaghan A, Pinal-Fernandez I, Trallero-Araguás E, et al. Classification and management of adult inflammatory myopathies. Lancet Neurol. September 17 (2018): 816–28.
- Qiang JK, Kim WB, Baibergenova A, et al. Risk of Malignancy in Dermatomyositis and Polymyositis: A Systematic Review and Meta-Analysis. J Cutan Med Surg. 1. März 21 (2017): 131–6.
- Kamperman RG, van der Kooi AJ, de Visser M, et al. Pathophysiological Mechanisms and Treatment of Dermatomyositis and Immune Mediated Necrotizing Myopathies: A Focused Review. Int J Mol Sci 13 (2022): 4301.
- Aggarwal R, Charles-Schoeman C, Schessl J, et al. Trial of Intravenous Immune Globulin in Dermatomyositis. N Engl J Med 387 (2022): 1264–78.
- European and German authorities approve octagam® 10% for adults with dermatomyositis (2025).
- Xiong A, Qiang Y, Cao Y, et al. The therapeutic efficacy and safety of intravenous immunoglobulin in dermatomyositis and polymyositis: A systematic review and meta-analysis. Mod Rheumatol 33 (2023): 533–42.
- Radosevich M, Burnouf T. Intravenous immunoglobulin G: trends in production methods, quality control and quality assurance. Vox Sang 98 (2010): 12–28.
- Danieli MG, Antonelli E, Gammeri L, et al. Intravenous immunoglobulin as a therapy for autoimmune conditions. Autoimmun Rev 24 (2025): 103710.
- Duellberg C, Hannappel A, Kistner S, et al. Biochemical Characterization of a New 10% IVIG Preparation [IgG Next Generation (BT595)/Yimmugo®] Obtained from a Manufacturing Process Preserving IgA/IgM Potential of Human Plasma. Drugs RD 23 (2023): 245–55.
- Cancer risk in dermatomyositis: a meta-analysis of cohort studies – PubMed (2025).
- Buchbinder R, Forbes A, Hall S, et al. Incidence of Malignant Disease in Biopsy-Proven Inflammatory Myopathy: A Population-Based Cohort Study. Ann Intern Med 134 (2001): 1087–95.
- Best M, Molinari N, Chasset F, et al. Use of Anti-transcriptional Intermediary Factor-1 Gamma Autoantibody in Identifying Adult Dermatomyositis Patients with Cancer: A Systematic Review and Meta-analysis. Acta Derm Venereol 99 (2019): 256–62.
- Martinez P, Sabatier JM. Rethinking corticosteroids use in oncology. Front Pharmacol 16 (2025): 1551111.
- Kälber KA, Enk AH, Winkler JK. Successful Treatment of Paraneoplastic Dermatomyositis using a Novel IVIg Preparation - A Case Report. Arch Clin Med Case Rep 09 (2025): 01–4.
- Aggarwal R, Schessl J, Charles-Schoeman C, et al. Safety and tolerability of intravenous immunoglobulin in patients with active dermatomyositis: results from the randomised, placebo-controlled ProDERM study. Arthritis Res Ther 26 (2024): 27.
- Cherin P, Marie I, Michallet M, et al. Management of adverse events in the treatment of patients with immunoglobulin therapy: A review of evidence. Autoimmun Rev 15 (2016): 71–81.
- Levine TD. Rituximab in the treatment of dermatomyositis: an open-label pilot study. Arthritis Rheum. Februar 52 (2005): 601–7.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum 65 (2013): 314–24.
- Shahani L. Refractory calcinosis in a patient with dermatomyositis: response to intravenous immune globulin. BMJ Case Rep (2012): bcr2012006629.
- Touimy M, Janani S, Rachidi W, et al. Calcinosis universalis complicating juvenile dermatomyositis: Improvement after intravenous immunoglobulin therapy. Joint Bone Spine 80 (2013): 108–9.
- Orandi AB, Baszis KW, Dharnidharka VR, et al. Assessment, classification and treatment of calcinosis as a complication of juvenile dermatomyositis: a survey of pediatric rheumatologists by the childhood arthritis and rheumatology research alliance (CARRA). Pediatr Rheumatol Online J 15 (2017): 71.