Successful Use of Interferon-Gamma for Refractory Disseminated Candida Tropicalis Infection in a Pediatric Patient with Acute Myeloid Leukemia and Heterozygous CARD9 Mutation
Article Information
Katharina Kleinschmidt1*#, Anja Troeger1#, Juergen Foell1, Marcus Jakob1, Selim Corbacioglu1
1Department of Pediatric Hematology, Oncology and Stem Cell Transplantation, University of Regensburg, Regensburg, Germany
#Authors contributed equally
*Corresponding Author: Katharina Kleinschmidt, Department of Pediatric Hematology, Oncology and Stem Cell Transplantation, University Hospital of Regensburg, Franz-Josef-Strauss-Allee 11, 93053 Regensburg, Germany.
Received: 10 July 2022; Accepted: 12 August 2022; Published: 16 September 2022
Citation: Katharina Kleinschmidt, Anja Troeger, Juergen Foell, Marcus Jakob, Selim Corbacioglu. Successful Use of Interferon-Gamma for Refractory Disseminated Candida Tropicalis Infection in a Pediatric Patient with Acute Myeloid Leukemia and Heterozygous CARD9 Mutation. Archives of Clinical and Medical Case Reports 6 (2022): 626-629.
View / Download Pdf Share at FacebookAbstract
Invasive fungal infections remain among the most frequent severe and fatal complications of oncology, despite effective antifungal agents. Genetic aberrations may additionally increase the individual patient’s susceptibility to invasive mycosis, with the need for an intensified treatment, such as adjunctive immunotherapy combined with standard antifungal medication. Interferon-gamma (IFN-γ) as an immunostimulatory agent may represent a promising surrogate to reactivate the cytotoxic activity of macrophages in patients with ‘blind spots’. We report the case of a pediatric patient with AML and disseminated candida tropicalis infection, bearing a heterozygous CARD9 mutation, who was treated successfully with combined IFN-γ and conventional antifungal therapy.
Keywords
AML; Infections in Immunocompromised Hosts; Immunology; Immunotherapy; Molecular Genetics; Stem Cell Transplantation
AML article; Infections in Immunocompromised Hosts article; Immunology article; Immunotherapy article; Molecular Genetics article; Stem Cell Transplantation article
AML articles AML Research articles AML review articles AML PubMed articles AML PubMed Central articles AML 2023 articles AML 2024 articles AML Scopus articles AML impact factor journals AML Scopus journals AML PubMed journals AML medical journals AML free journals AML best journals AML top journals AML free medical journals AML famous journals AML Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Infections in Immunocompromised Hosts articles Infections in Immunocompromised Hosts Research articles Infections in Immunocompromised Hosts review articles Infections in Immunocompromised Hosts PubMed articles Infections in Immunocompromised Hosts PubMed Central articles Infections in Immunocompromised Hosts 2023 articles Infections in Immunocompromised Hosts 2024 articles Infections in Immunocompromised Hosts Scopus articles Infections in Immunocompromised Hosts impact factor journals Infections in Immunocompromised Hosts Scopus journals Infections in Immunocompromised Hosts PubMed journals Infections in Immunocompromised Hosts medical journals Infections in Immunocompromised Hosts free journals Infections in Immunocompromised Hosts best journals Infections in Immunocompromised Hosts top journals Infections in Immunocompromised Hosts free medical journals Infections in Immunocompromised Hosts famous journals Infections in Immunocompromised Hosts Google Scholar indexed journals Immunology articles Immunology Research articles Immunology review articles Immunology PubMed articles Immunology PubMed Central articles Immunology 2023 articles Immunology 2024 articles Immunology Scopus articles Immunology impact factor journals Immunology Scopus journals Immunology PubMed journals Immunology medical journals Immunology free journals Immunology best journals Immunology top journals Immunology free medical journals Immunology famous journals Immunology Google Scholar indexed journals Immunotherapy articles Immunotherapy Research articles Immunotherapy review articles Immunotherapy PubMed articles Immunotherapy PubMed Central articles Immunotherapy 2023 articles Immunotherapy 2024 articles Immunotherapy Scopus articles Immunotherapy impact factor journals Immunotherapy Scopus journals Immunotherapy PubMed journals Immunotherapy medical journals Immunotherapy free journals Immunotherapy best journals Immunotherapy top journals Immunotherapy free medical journals Immunotherapy famous journals Immunotherapy Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Breast Cancer articles Breast Cancer Research articles Breast Cancer review articles Breast Cancer PubMed articles Breast Cancer PubMed Central articles Breast Cancer 2023 articles Breast Cancer 2024 articles Breast Cancer Scopus articles Breast Cancer impact factor journals Breast Cancer Scopus journals Breast Cancer PubMed journals Breast Cancer medical journals Breast Cancer free journals Breast Cancer best journals Breast Cancer top journals Breast Cancer free medical journals Breast Cancer famous journals Breast Cancer Google Scholar indexed journals Molecular Genetics articles Molecular Genetics Research articles Molecular Genetics review articles Molecular Genetics PubMed articles Molecular Genetics PubMed Central articles Molecular Genetics 2023 articles Molecular Genetics 2024 articles Molecular Genetics Scopus articles Molecular Genetics impact factor journals Molecular Genetics Scopus journals Molecular Genetics PubMed journals Molecular Genetics medical journals Molecular Genetics free journals Molecular Genetics best journals Molecular Genetics top journals Molecular Genetics free medical journals Molecular Genetics famous journals Molecular Genetics Google Scholar indexed journals Stem Cell Transplantation articles Stem Cell Transplantation Research articles Stem Cell Transplantation review articles Stem Cell Transplantation PubMed articles Stem Cell Transplantation PubMed Central articles Stem Cell Transplantation 2023 articles Stem Cell Transplantation 2024 articles Stem Cell Transplantation Scopus articles Stem Cell Transplantation impact factor journals Stem Cell Transplantation Scopus journals Stem Cell Transplantation PubMed journals Stem Cell Transplantation medical journals Stem Cell Transplantation free journals Stem Cell Transplantation best journals Stem Cell Transplantation top journals Stem Cell Transplantation free medical journals Stem Cell Transplantation famous journals Stem Cell Transplantation Google Scholar indexed journals
Article Details
1. Introduction
Infectious complications remain major causes of treatment-related morbidity and mortality in children with acute myelogenous leukemia (AML) due to the intensive chemotherapeutic regimens needed to treat this disease. Invasive fungal infections (IFI) represent a particularly severe condition that affects 5-13% of AML patients [1,2] with a persistently unacceptable mortality rate of 40% [3]. However, even though IFI are not uncommon in pediatric AML patients, unusual presentations such as very early onset and severe, refractory disease courses might be indicative for an underlying immunodeficiency. Numerous genetic aberrations have been identified in the last years that confer particular susceptibility to fungal infections [4]. In the innate immune system, myeloid cells express C-type lectin receptors (CLRs) in their extracellular region, which belong to a class of transmembrane pattern recognition receptors (PRRs). Dectin-1, Dectin-2 and Mincle are three specific PRRs for beta-glucans and alpha-mannans, which are components of fungal cell wall [5]. Upon recognition of these carbohydrates, the host innate immune system is activated against these invading microbes. Downstream the CLR activation, all signaling pathways converge on an adaptor protein named caspase-associated recruitment domain 9 (CARD9) that is crucial in the signaling of CLRs for a successful antifungal response, leading finally to Th1 and Th17 differentiation and to the initiation of the inflammatory cytokine cascade in myeloid cells [5,6]. Particularly interferon-gamma (IFN-g) increases the cytotoxic activity of macrophages and plays therefore a pivotal role in the immune response to fungal invasion [7,8]. Patients with homozygous or compound heterozygous mutations of CARD9 are known to have a significantly increased susceptibility to life-threatening systemic candidiasis [9–11]. However, some sequence variants may lead to increased susceptibility for fungal infections even in heterozygosity, e.g. under immunosuppression. Identification of these patients is of crucial importance, as standard antimycotic treatment might not be sufficient for those high-risk patients who might benefit from adjunctive immunotherapy added to standard antifungal therapy [12,13]. Several case reports have indicated IFN-g as an additive treatment option in patients with IFI, where it seems to have an immune-stimulating effect with the potential to improve the cellular immune response in a situation of immunological “blindness” [3,13,14]. Here, we report the clinical case of an 11-year old boy with AML FAB M6 who suffered from a severe systemic candida tropicalis (c. tropicalis) infection, unresponsive to multimodal antimycotic treatment regimen, leading to multi-organ failure (MOF). Genetic analysis revealed a heterozygous CARD9 mutation. IFN-g as additive immunotherapy lead to complete resolution of this treatment refractory and disseminated fungal disease in a severely immunodeficient patient.
2. Case Description
A previously healthy 11-year-old boy was diagnosed with AML M6 according to FAB classification. He started induction chemotherapy according to the AML BFM 2004 protocol. After one week, he developed septic fever, for which antibiotic treatment with ceftazidime and vancomycin was started. Unexpectedly, candida tropicalis was confirmed in the blood culture within 24 hours. Liposomal amphotericin B (LAB) was started immediately, however the patient’s conditions deteriorated rapidly towards MOF with involvement of heart, lungs, kidneys, liver and Central Nervous System (CNS), necessitating the transfer to the pediatric intensive care unit. Despite extended antifungal regimens including fluconazole, voriconazole, caspofungin, micafungin and posaconazole in changing combinations and negative blood cultures for candida after five days, radiological imaging documented a progressive dissemination of candida infection with bilateral pulmonary infiltrates, epidural spinal cord affection, bilateral renal and liver involvement as well as sinusitis, suspicious for further mycotic foci (Figure 1 a, c, e). A massive splenic infiltration with extensive areas of infarction required splenectomy (Figure 1 g, h): microbiologic analysis of the tissue showed positive results for c. tropicalis, with histology exhibiting severe epithelial granulomatous necrotic inflammation. Due to the unusual early, extensive and treatment-refractory fungal infection, genetic testing for predisposition for mycosis was performed and revealed a heterozygous mutation in the CARD 9 gene, inherited from the father where the same mutation could be detected (c.809a>T(p.Glu270Val)). After the identification of this a priori unsuspicious genetic aberration as the only possible reason for the fungal infections, an experimental treatment with recombinant IFN-g (rIFN-g; 100 µg subcutaneously, 3 times per week) was initiated on a compassionate use basis, with the idea to bypass the immunological ‘blindness’. This led to an almost complete resolution of the disseminated infections (Figure 1 b, d, f). During treatment with IFN-g, the only antimycotic therapy consisted in daily oral fluconazole. Due to the severe infectious complications, the patient’s chemotherapy was interrupted after only one course. However, the bone marrow remained in complete hematological remission. One year after initial diagnosis, the patient relapsed with a mixed phenotype acute leukemia (MPAL). IFN-g in combination with LAB was restarted as prophylaxis during relapse chemotherapy according to the AML BFM 2004 protocol. After achieving a second complete remission, he was transplanted from a matched unrelated donor. Despite the myeloablative and highly immunosuppressive conditioning regimen consisting of fludarabine, thiotepa, treosulfan and anti-human T-lymphocyte immuneglobulin (ATG Grafalonâ), he did not experience any mycotic complication while on IFN-g and LAB prophylaxis. 2.5 years after hematopoietic stem cell transplantation (HSCT), the patient experienced a second relapse with the previous MPAL clones. After remission-induction with cytarabine only (AML BFM 2004 induction modified: cytarabine 20 mg/m2 day (d) 1, 2x20 mg m2 d 2-3, 250 mg/m2 d 4-5), he was eligible for a second HSCT. The conditioning consisted of gemtuzumab ozogamicin, 12 Gy total body irradiation and etoposide, followed by transplantation with a T-repleted bone marrow graft from a haploidentical donor with administration of post-transplant cyclophosphamide. Despite the extensive and prolonged immunosuppression, no further mycosis was observed under IFN-g/LAB. To date the patient is well and in remission for more than 2 years.
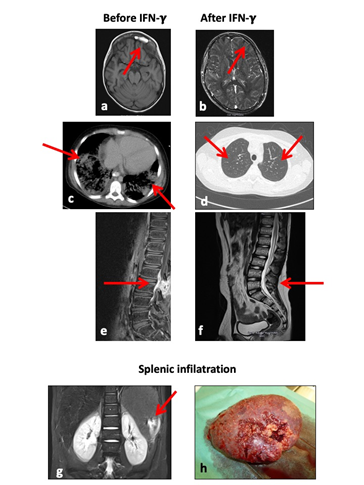
Figure 1: Fungal infiltration of a) lungs, c) sinus frontalis, e) epidural space, and resolution respectively of b) lungs, d) sinus frontalis, f) epidural space after therapy with IFN-g. Splenic infiltration by c. tropicalis g) on MRI, h) macroscopically after splenectomy.
3. Discussion
We report a very unusual case of a pediatric AML patient with a life-threatening, rapidly progressive disseminated invasive candidiasis, leading to MOF due to mycotic infiltration of almost all vital organs. Although invasive fungal infections in AML patients are common [1], such a serious, ubiquitously spread and refractory infection in a presumably immunocompetent individual should raise the suspicion for an underlying immunodeficiency. In our case, the previously healthy child developed an unexpected early occurrence of a completely treatment-refractory candida-sepsis, which lead to genetic testing for predisposition for mycosis and consecutively to the identification of a heterozygous CARD9 mutation. While IFN-g is used in adults to stimulate antifungal immune activity [13,15,16], little data are available for this kind of treatment in children. Based on the experience in children with chronic granulomatous disease [17], few case reports describe its use in children with invasive fungal infection and oncological diseases [18,19]. To our knowledge, this is the first case of a heterozygous CARD9 mutation with such a clinically relevant presentation and a successful IFN-g treatment in a pediatric patient with disseminated candida sepsis and AML. Given the increased mortality rate in oncological patients with IFI, and the apparently safe and well-tolerated application of IFN-g in children, awareness for potentially curable, molecularly proven IFI, warrants increased awareness and rewards molecular confirmation for an effective adjuvant treatment option in refractory patients. Most remarkably, this previously healthy child unmasked his predisposition only in the extreme situation of a massive immunosuppression so that, considering the wide heterogeneity of genetic mutations involved in susceptibility to fungal infections, genome-wide expression profiling might play an increasing role in the future in pediatric cancer patients, as knowledge of defective immune pathways might help to identify patients at risk early on [20].
Conflict of Interest
The authors have no conflict of interest to disclose.
References
- Johnston DL, Lewis V, Yanofsky R, et al. Invasive fungal infections in paediatric acute myeloid leukaemia. Mycoses 56 (2013): 482-487.
- Lehrnbecher T, Tramsen L, Koehl U, et al. Immunotherapy against Invasive Fungal Diseases in Stem Cell Transplant Recipients. Immunol Invest 40 (2011): 839-852
- Davidson L, Netea MG, Kullberg BJ. Patient Susceptibility to Candidiasis—A Potential for Adjunctive Immunotherapy. J Fungi 9 (2018): 4.
- Lionakis MS. Genetic Susceptibility to Fungal Infections in Humans. Curr Fungal Infect Rep 6 (2012): 11-22.
- Drummond ÃRA, Saijo ÃS, Iwakura Y, et al. The role of Syk/CARD9 coupled C-type lectins in antifungal immunity. Eur J Immunol 41 (2011): 276-281.
- Zhong X, Chen B, Yang L, et al. Molecular and physiological roles of the adaptor protein CARD9 in immunity. Cell Death Dis 9 (2018): 52.
- Yamamoto H, Nakamura Y, Sato K, et al. Defect of CARD9 Leads to Impaired Accumulation of Gamma Interferon-Producing Memory Phenotype T Cells in Lungs and Increased Susceptibility to Pulmonary Infection with Cryptococcus neoformans. Infect Immun 82 (2014): 1606-1615.
- Hung CY, Castro-Lopez N, Cole GT. Card9-and MyD88-Mediated Gamma Interferon and Nitric Oxide Production Is Essential for Resistance to Subcutaneous Coccidioides posadasii Infection. Infect Immun 84 (2016): 1166-1175.
- Glocker EO, Hennigs A, Nabavi M, et al. A Homozygous CARD9 Mutation in a Family with Susceptibility to Fungal Infections. N Engl J Med 361 (2009): 1727-1735.
- Herbst M, Gazendam R, Reimnitz D, et al. Chronic Candida albicans Meningitis in a 4-Year-Old Girl with a Homozygous Mutation in the CARD9 Gene (Q295X). Pediatr Infect Dis J 34 (2015): 999-1002.
- Gavino C, Cotter A, Lichtenstein D, et al. CARD9 Deficiency and Spontaneous Central Nervous System Candidiasis: Complete Clinical Remission With GM-CSF Therapy. Clin Infect Dis 59 (2014): 81-84.
- Kullberg BJ, Van De Veerdonk F, Netea MG. Immunotherapy: a potential adjunctive treatment for fungal infection. Current Opinion in Infectious Diseases 27 (2014): 511-516.
- Delsing CE, Gresnigt MS, Leentjens J, et al. Interferon-gamma as adjunctive immunotherapy for invasive fungal infections: a case series. BMC Infect Dis 14 (2014): 166.
- Kullberg BJ, van’t Wout JW, Hoogstraten C, et al. Recombinant interferon-gamma enhances resistance to acute disseminated Candida albicans infection in mice. J Infect Dis 168 (1993): 436-443.
- Dignani MC, Rex JH, Chan KW, et al. Immunomodulation with interferon-gamma and colony-stimulating factors for refractory fungal infections in patients with leukemia. Cancer 104 (2005): 199-204.
- Leentjens J, Kox M, van der Hoeven JG, et al. Immunotherapy for the Adjunctive Treatment of Sepsis: From Immunosuppression to Immunostimulation. Time for a Paradigm Change? Am J Respir Crit Care Med 187 (2013) : 1287-1293.
- Marciano BE, Wesley R, De Carlo ES, et al. Long-Term Interferon-g Therapy for Patients with Chronic Granulomatous Disease. Clin Infect Dis 39 (2004): 692-699.
- Buddingh EP, Leentjens J, van der Lugt J, et al. Interferon-gamma Immunotherapy in a Patient With Refractory Disseminated Candidiasis. Pediatr Infect Dis J 34 (2015): 1391-1394.
- Assendorp EL, Gresnigt MS, Sprenkeler EGG, et al. Adjunctive interferon-γ immunotherapy in a pediatric case of Aspergillus terreus infection. Eur J Clin Microbiol Infect Dis 37 (2018): 1915-1922.
- Lee GE, Sung L, Fisher BT, et al. Gene Expression Profiling to Predict Viridans Group Streptococcal and Invasive Fungal Infection in Pediatric Acute Myeloid Leukemia: A Brief Report from the Children’s Oncology Group. Acta Haematol 131 (2014): 167-169.
