Successful Laparoscopic and Endoscopic Cooperative Surgery for a Duodenal Neuroendocrine Tumor: A Case Report
Article Information
Manabu Yamamoto MD1,4*, Hiroko Yano MD1, Yukiko Kosai-Fujimoto MD1, Naoya Kubokura MD2, Hiroyuki Kobayashi MD2, Masafumi Ohya MD3, Masazumi Tuneyoshi MD3, Tetsuhide Ito MD4
1Department of Surgery, Fukuoka Sanno Hospital, Momochi-hama 3-6-45, Fukuoka 814-0001, Japan
2Department of Gastroenterological Internal Medicine, Fukuoka Sanno Hospital, Momochi-hama 3-6-45, Fukuoka 814-0001, Japan
3Department of Pathology, Fukuoka Sanno Hospital, Momochi-hama 3-6-45, Fukuoka 814-0001, Japan
4Center of Neuroendocrine Tumor, Fukuoka Sanno Hospital, Momochi-hama 3-6-45, Fukuoka 814-0001, Japan
*Corresponding Author: Dr. Manabu Yamamoto, Department of Surgery, Fukuoka Sanno Hospital, Momochi-hama 3-6-45, Fukuoka 814-0001, Japan
Received: 27 July 2020; Accepted: 10 August 2020; Published: 10 September 2020
Citation: Manabu Yamamoto, Hiroko Yano, Yukiko Kosai-Fujimoto, Naoya Kubokura, Hiroyuki Kobayashi, Masafumi Ohya, Masazumi Tuneyoshi, Tetsuhide Ito. Successful Laparoscopic and Endoscopic Cooperative Surgery for a Duodenal Neuroendocrine Tumor: A Case Report. Archives of Clinical and Medical Case Reports 4 (2020): 844-852.
View / Download Pdf Share at FacebookAbstract
Neuroendocrine tumors (NET) that develop at the second portion of the duodenum are rare, although most of NET at the duodenum is the first portion. We resected a duodenal NET by using a laparoscopic and endoscopic cooperative surgery (LECS) technique. A 33-year-old man underwent esophagogastroduodenoscopy that revealed a 6 mm, gently raising tumor on the opposite side of the ampulla of vater at the second portion of the duodenum. We performed a full-thickness local excision using the LECS technique. The tumor was confirmed and endoscopically marked along the resection line. After a full-thickness excision, using endoscopic and laparoscopy, closure using a liner stapler was performed laparoscopically. We demonstrated that LECS is a safe and feasible procedure for duodenal G1 NET at the second portion of the duodenum.
Keywords
Duodenum; Second portion; Neuroendocrine tumor; LECS
Duodenum articles, Second portion articles, Neuroendocrine tumor articles, LECS articles
Duodenum articles Duodenum Research articles Duodenum review articles Duodenum PubMed articles Duodenum PubMed Central articles Duodenum 2023 articles Duodenum 2024 articles Duodenum Scopus articles Duodenum impact factor journals Duodenum Scopus journals Duodenum PubMed journals Duodenum medical journals Duodenum free journals Duodenum best journals Duodenum top journals Duodenum free medical journals Duodenum famous journals Duodenum Google Scholar indexed journals Second portion articles Second portion Research articles Second portion review articles Second portion PubMed articles Second portion PubMed Central articles Second portion 2023 articles Second portion 2024 articles Second portion Scopus articles Second portion impact factor journals Second portion Scopus journals Second portion PubMed journals Second portion medical journals Second portion free journals Second portion best journals Second portion top journals Second portion free medical journals Second portion famous journals Second portion Google Scholar indexed journals Neuroendocrine tumor articles Neuroendocrine tumor Research articles Neuroendocrine tumor review articles Neuroendocrine tumor PubMed articles Neuroendocrine tumor PubMed Central articles Neuroendocrine tumor 2023 articles Neuroendocrine tumor 2024 articles Neuroendocrine tumor Scopus articles Neuroendocrine tumor impact factor journals Neuroendocrine tumor Scopus journals Neuroendocrine tumor PubMed journals Neuroendocrine tumor medical journals Neuroendocrine tumor free journals Neuroendocrine tumor best journals Neuroendocrine tumor top journals Neuroendocrine tumor free medical journals Neuroendocrine tumor famous journals Neuroendocrine tumor Google Scholar indexed journals tumor articles tumor Research articles tumor review articles tumor PubMed articles tumor PubMed Central articles tumor 2023 articles tumor 2024 articles tumor Scopus articles tumor impact factor journals tumor Scopus journals tumor PubMed journals tumor medical journals tumor free journals tumor best journals tumor top journals tumor free medical journals tumor famous journals tumor Google Scholar indexed journals EMR articles EMR Research articles EMR review articles EMR PubMed articles EMR PubMed Central articles EMR 2023 articles EMR 2024 articles EMR Scopus articles EMR impact factor journals EMR Scopus journals EMR PubMed journals EMR medical journals EMR free journals EMR best journals EMR top journals EMR free medical journals EMR famous journals EMR Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals laparoscopy articles laparoscopy Research articles laparoscopy review articles laparoscopy PubMed articles laparoscopy PubMed Central articles laparoscopy 2023 articles laparoscopy 2024 articles laparoscopy Scopus articles laparoscopy impact factor journals laparoscopy Scopus journals laparoscopy PubMed journals laparoscopy medical journals laparoscopy free journals laparoscopy best journals laparoscopy top journals laparoscopy free medical journals laparoscopy famous journals laparoscopy Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals radiograph articles radiograph Research articles radiograph review articles radiograph PubMed articles radiograph PubMed Central articles radiograph 2023 articles radiograph 2024 articles radiograph Scopus articles radiograph impact factor journals radiograph Scopus journals radiograph PubMed journals radiograph medical journals radiograph free journals radiograph best journals radiograph top journals radiograph free medical journals radiograph famous journals radiograph Google Scholar indexed journals Electrocardiography articles Electrocardiography Research articles Electrocardiography review articles Electrocardiography PubMed articles Electrocardiography PubMed Central articles Electrocardiography 2023 articles Electrocardiography 2024 articles Electrocardiography Scopus articles Electrocardiography impact factor journals Electrocardiography Scopus journals Electrocardiography PubMed journals Electrocardiography medical journals Electrocardiography free journals Electrocardiography best journals Electrocardiography top journals Electrocardiography free medical journals Electrocardiography famous journals Electrocardiography Google Scholar indexed journals
Article Details
Abbreviations:
EMR- endoscopic mucosal resection; ESD- endoscopic submucosal dissection; GIST- gastrointestinal stromal tumor; LECS- laparoscopic and endoscopic cooperative surgery; NEC- neuroendocrine carcinoma; NET- neuroendocrine tumor; SMT- submucosal tumor
1. Introduction
Gastroenteropancreatic neuroendocrine tumors (NETs) are graded as G1, G2, or neuroendocrine carcinomas (NEC), based on their proliferative activity determined using mitotic counts or the Ki-67 index and the World Health Organization (WHO) 2010 classification [1]. G1 NETs are synonymously referred to as carcinoid tumors. In Japan, the duodenum is the second most common site for G1 NETs, after the rectum [2]. Endoscopic treatment is generally recommended for G1 NETs < 10 mm in diameter and those that extend only to the submucosal layer [3]. However, endoscopic treatments, endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) for duodenal tumors are technically difficult for endoscopists, due to the anatomical features of the duodenum, such as the narrow lumen and thin wall. In fact, several reports have demonstrated that severe complications, such as perforation and bleeding, frequently occur during and after endoscopic treatments [4, 5].
Pancreatoduodenectomy is considered the standard approach for the duodenal tumor resection [6, 7]. This conventional surgery approach, however, is still invasive especially for patients with such early duodenal tumors. Partial duodenal resection has also been attempted as an alternative treatment option for duodenal tumors such as mucosal adenocarcinomas and small submucosal tumors (SMT), which do not require lymph node dissection [8-10]. Though partial resection is not invasive, it is difficult to determinate the proper extent of resected lines. Recently, Hiki et al. [11, 12] reported on laparoscopic and endoscopic co-operative surgery (LECS) for gastrointestinal stromal tumors (GIST). This revolutionary procedure enables the precise assessment of tumor boundaries, thereby facilitating adequate tumor resection.
We thus report a rare case of NET at the second portion of the duodenum, resected by LECS.
2. Case Presentation
A 33-year-old Japanese man was admitted to our hospital in December 2019, without gastrointestinal symptoms. Upper GI endoscopy revealed a IIc-types tumorous lesion at the second portion of the duodenum (Figure 1a). Endoscopic ultrasound showed a 6 mm tumorous submucosal lesion at the second portion of the duodenum. Barium double tomography showed a 6-mm tumor on the opposite side of the ampulla of vater at the second portion of the duodenum (Figure 1b). The tumor was identified as a NET by biopsy. Computed tomography showed no metastasis to the lymph nodes, liver or other organs.
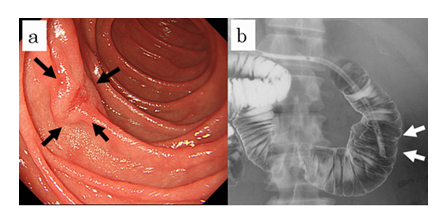
Figure 1: Preoperative examinations. Gastrointestinal endoscopy revealed the tumor at the second portion of the duodenum (a). Barium double tomography revealed the tumor on the opposite side of the ampulla of vater at the second portion of the duodenum (b).
Under general anesthesia, a camera port was inserted into the inferior umbilical lesion. Four additional ports (three 5 mm ports and one 12 mm port) were inserted into the left upper, left lower, right upper, and right lower quadrants (12 mm port), respectively. This was performed under pneumoperitoneum using a CO2 insufflation system (UHI-4; Olympus Medical Systems Corp., Tokyo, Japan). Small intestine clamping was performed during the ESD procedure. The laparoscopic location of the tumor was confirmed by maneuvering the duodenal wall from the mucosal side, using the ESD-knife through the endoscope. After confirmation of the tumor location, both mucosal and submucosal layers around the tumor were dissected circumferentially with a minimum margin, achieved by using the ESD technique (Figure 2a).
Subsequently, a small artificial perforation of the seromuscular layer was created by the endoscopist using a needle knife (KD-1L-1; Olympus Medical Co., Tokyo, Japan) at a laparoscopically appropriate site under the surgeon’s instruction. The optimal perforation site represents the best site for the approach of laparoscopic devices insertion. Subsequently, the tip of the ultrasonically activated device was inserted into the perforation hole laparoscopically and the remaining seromuscular layer was dissected extremely easily along the incision line that was made by the ESD procedure (Figure 2b). After complete resection of the tumor, closure of the defect in the duodenal wall was done by the liner stapler after a few suturing techniques (Figure 2c). Finally, the LECS procedure was completed, and of the absence of air leakage and free passage of the endoscope past the repaired defect was confirmed endoscopically (Figure 2d).
With this approach, a curative operation was performed. A postoperative contrast study did not reveal stasis or stenosis at the suture site. Pathological examinations revealed a proliferative of atypical cells with hyperchromatic round to oval nuclei and eosinophilic cytoplasms arranged in cords or nests, invading the submucosa. Immunostaining for snaptophysin was positive, and the mitotic count was < 2% (Figure 3abc). The patient was discharged on the 10th postoperative day. At the time of this writing, he is doing well without recurrence at 3 months postoperatively.
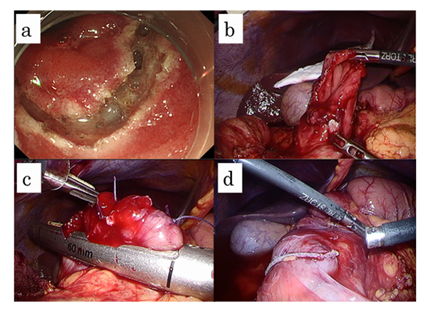
Figure 2: The laparoscopic and endoscopic cooperative surgical procedure performed for the duodenal neuroendocrine tumor. Full-thickness excision was performed along the marked dissection line using a needle knife (a). A post-excisional defect in the duodenal wall is shown (b). Closure of the defect in the duodenal wall was done by the liner stapler after a few suturing techniques (c). The endoscope passes the liner stapler site easily after the curative operation was performed (d).
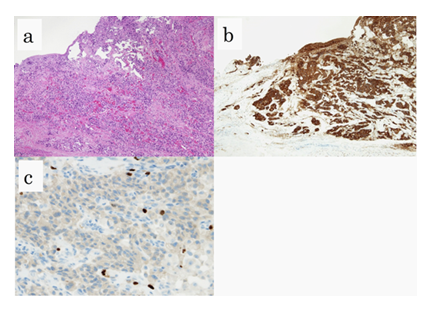
Figure 3: Pathological examinations. HE stains of tumor specimens (a). Positive immunostaining for snaptophysin (b). The immunostaining of Ki-67 indicated an index less than 2% (c).
2. Discussion
Although duodenal NETs are commonly at the first portion of the duodenum in Japanese patients, those found at the second portion are rare. We thus reported on patients who presented with a 6.0 mm duodenal NET at the second portion of the duodenum [13], who was successfully treated by LECS resection. It has been reported that eight patients with duodenal NET have previously been treated by LECS (shown in Table 1) [14-19].
Gastrointestinal tumors, composed of neuroendocrine cells, and showing characteristic histologies have been defined as “carcinoid tumors” since 1917. Oberndorfer et al. described these slow-growing, small, intestinal tumors and reported that patients generally have good prognoses [20]. Burke et al. reviewed 99 cases of duodenal carcinoid tumors and reported that these tumors had no mitotic figures when located in the submucosal layer and were < 1cm in size [21]. In 2010, the WHO classified NETs as G1, G2 or NEC, according to their mitotic count and Ki-67 index. Many cases of conventionally defined carcinoid tumors are equivalent to low proliferative potential G1 NETs. The European Neuroendocrine Tumor Society and Japan Neuroendocrine Tumor Society recommend endoscopic treatment for G1 NETs < 10 mm that do not extend beyond the submucosal layer and do not demonstrate lymph node metastasis. Patients with tumors > 20 mm, with lymph node metastasis, are indicated for surgical treatment [3, 22]. The treatment strategy for tumors 10-20 mm in size remains controversial.
ESD is wildly accepted for early intestinal stromal tumors, including NET G1 < 10 mm. ESD has the advantage of a higher probability of success for larger and more consistent lesion resections of lesions just above the muscle layer than that compared with EMR. However, the duodenal wall at the second or third portion is generally thinner than that of the stomach and ESD for duodenal tumors is associated with an increased risk of early and delated perforation [4, 5]. In addition, maneuvering the flexible endoscope is also technically difficult in the tiny duodenal lumen, and it was very hard to perform inverted scope maneuvers in the narrow working space of the duodenum [14]. However, conventional surgical operations may be excessively invasive for early duodenal tumors, and determination of the proper extent of resection for duodenal lesions is difficult.
LECS was first reported in 2008 by Hiki et al. [11] and has the advantage that the minimum extent of resection is marked and the full- thickness cuts, along the designated margins, are performed endoscopically. Additionally, the defect formed by the resection is also closed in a less invasive manner. There are many reports on the use of LECS for gastric lesions, including early gastric cancers, or GIST [11, 23]. Recently, modified LECS had been reported to prevent the peritoneal infections or scattering of tumor cells in the abdominal cavity for ulcerative SMTs or cancer lesions [24]. Only nine cases, including present case, have been reported on duodenal NETs treated by LECS. Five of nine cases underwent the LECS technique at the second or third portion of the duodenum. This surgical procedure can be performed safely without postoperative complications (Table 1). LECS is a safe and feasible treatment for duodenal tumor resection, in particular at the second or third portion of the duodenum, with sufficient margins and no postoperative complications.
Table 1: Nine cases of duodenal neuroendocrine tumor resected a LECS.
Competing Interests
The authors declared that they have no competing interests.
Consent for Publication
The informed consent for publication and presentation was obtained from the patient.
Funding
This work was no supported from any funding.
Authors’ Contribution
MY drafted the manuscript. HY, YK-F, NK, and HK acquired data, and MO, MT diagnosed pathological examinations. We added the special comments for TI as a specialist. All authors have read and approved the final manuscript.
Acknowledgment
We thank Mr. Fuminori Sakanashi for his technical assistances in immunohistochemical stainings.
References
- Rindi G, Arnold R, Bosman T. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT, editor. WHO classification of tumors of the digestive system. 4th ed. Lyon: IARC (2010).
- Ito T, Sasano H, Tanaka M, et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastrotenterol 45 (2010): 234-243.
- Kloppel G, Couvelard A, Parron A, et al. ENETS Consensus Guidelines for Standards of Care in Neuroendocrine Tumors: towards a standardized approach to the diagnosis of gastroenteropancreatic neuroendocrine tumors and their prognostic stratification. Neuroendocrinology 90 (2009): 162-166.
- Suzuki S, Ishi N, Uemura M, et al. Endoscopic submucosal dissection (ESD) for gastrointestinal carcinoid tumors. Surg Endosc 26 (2012): 759-763.
- Yamamoto Y, Yoshizuka N, Tomida H, et al. Therapeutic outcomes of endoscopic resection for superficial non-ampullary duodenal tumor. Dig Endosc 26 (2014): 50-56.
- Chok AV, Koh YX, Ow MY, et al. A systemic review and meta-analysis comparing panreaticoduodenectomy versus limited resection for duodenal gastrointestinal stromal tumors. Ann Surg Oncol 21 (2014): 3429-3438.
- Cloyd JM, George E, Visser BC. Duodenal adenocarcinoma: Advanced in diagnosis and surgical management. World J Gastrointest Surg 8 (2016): 212-221.
- Stauffer JA, Raimondo M, Woodward TA, et al. Laparoscopic partial sleeve duodenectomy (PSD) for nonampullary duodenal neoplasms: avoiding a whipple by separating the duodenum from the pancreatic head. Pancreas 42 (2013): 461-466.
- Tanaka E, Kim M, Lim JS, et al. Usefulness of laparoscopic side-to-side duodenojejunostomy for gastrointestinal stromal tumors located at the duodenojejunal junction. J Gastrointest Surg 19 (2015): 313-318.
- Chung JC, Kim HC, Hur SM. Limited resections for duodenal gastrointestinal stromal tumors and their oncologic outcomes. Surg Today 46 (2016): 110-116.
- Hiki N, Yamamoto Y, Fukunaga T, et al. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc 22 (2008): 1729-1735.
- Hiki N, Nunobe S, Matsuda T, et al. Laparoscopic endoscopic cooperative surgery. Dig Endosc 27 (2015): 197-204.
- Asaumi Y, Kaizaki Y, Hosokawa O, et al. Duodenal carcinoid tumor-strategy of treatment. Stomach and Intestine 46 (2011):1626-1633.
- Tsushimi T, Mori H, Harada T, et al. Laparoscopic and endoscopic cooperative surgery for duodenal neuroendocrine tumor (NET) G1: Report of a case. International J Surg Case Rep 5 (2014): 1021-1024.
- Tsujimoto H, Ichikura T, Nagao S, et al. Minimally invasive surgery for resection of duodenal carcinoid tumors: endoscopic full-thickness resection under laparoscopic observation. Surg Endosc 24 (2010): 471-475.
- Ishikawa D, Komatsu S, Dohi O, et al. Laparoscopic and endoscopic co-operative surgery for non-ampullary duodenal tumors. World J Gastroenterol 22 (2016): 10424-10431.
- Nagasawa Y, Okauchi H, Kojima M, et al. Laparoscopic-endoscopic cooperative surgery for a duodenal neuroendocrine tumor: a case report. Asian J Endosc Surg 10 (2017): 183-186.
- Nakashima S, Hikawa S, Izumiya Y, et al. A case of laparoscopic-endoscopic cooperative surgery for duodenal neutroendocrine tumor (NET G1). Jpn J Cancer Chemother 44 (2017): 1865-1867.
- Ohi M, Yasuda H, Ishino Y, et al. Single-incision laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor arising from the duodenum. Asian J Endosc Surg 6 (2013): 307-310.
- Oberndorfer S. Karzinoide Tumoren des Dunndarm. Frankf Z Pathol 1 (1907): 426-432.
- Burke AP, Sobin LH, Federspiel BH, et al. Carcinoid tumors of the duodenum. A clinicopathologic study of 99 cases. Arch Pathol Lab Med 12 (1990): 1085-1087.
- Japan Neuroendocrine Tumor Society: Clinical Practice Guildlines for Gastroetneropancreatic Neuroendocrine Neoplasms (GEP-NEN) 2019, Kanehara, Tokyo (2019).
- Dong HY, Wang YL, Li J, et al. New-style laparoscopic and endoscopic cooperative surgery for gastric stromal tumors. World J Gastroenterol 19 (2013): 2550-2556.
- Kikuchi S, Nishizaki M, Kuroda S, et al. Nonexposure laparoscopic cooperative surgery (closed laparoscopic cooperative surgery) for gastric submucosal tumor. Gastric Cancer 20 (2017): 553-557.
