Study of Kinetic Model for the Adsorption of β-carotene on Activated Bleaching Earth in the Bleaching of Cambodian Soybean Oil
Article Information
Monyneath Bunthan1, Laysreng Pov2, Sela Kong1,2, Manit Say1, Yukleav Nat1,2, Chin Ping Tan3, Reasmey Tan1,2*
1Research and Innovation Center, Institute of Technology of Cambodia, Russian Federation Blvd., P.O. Box 86, Phnom Penh, Cambodia
2Faculty of Chemical and Food Engineering, Institute of Technology of Cambodia, Russian Federation Blvd., P.O. Box 86, Phnom Penh, Cambodia
3Department of Food Technology, Faculty of Food Science and Technology, Universiti Putra Malaysia, 43400 UPM Serdang Selangor, Malaysia
*Corresponding Author: Reasmey Tan, Institute of Technology of Cambodia, Russian Federation Blvd., P.O. Box 86, Phnom Penh, Cambodia
Received: 09 December 2023; Accepted: 21 December 2023; Published: 02 February 2024
Citation: Monyneath Bunthan, Laysreng Pov, Sela Kong, Manit Say, Yukleav Nat, Chin Ping Tan, Reasmey Tan. Study of Kinetic Model for the Adsorption of β-carotene on Activated Bleaching Earth in the Bleaching of Cambodian Soybean Oil. Journal of Food Science and Nutrition Research. 7 (2024): 37-43.
View / Download Pdf Share at FacebookAbstract
Soybean oil is a popular vegetable oil that can be obtained using mechanical or chemical extraction methods. Among these approaches, the hydraulic press is a popular mechanical extraction method that has benefits such as minimal starting cost, no solvent usage, and a high level of operating safety. When the soybean oil has been extracted, it is typically sent to a refinery for further processing which includes degumming, neutralization, bleaching, and deodorization. However, during the bleaching process, there is a lack of comprehensive understanding and a specific kinetic model for the adsorption of β-carotene, a prominent colorant, on activated bleaching earth in soybean oil obtained from a hydraulic press. This research aims to investigate the effect of three parameters, including adsorbent dosage, temperature, and contact time on the bleaching efficiency of soybean oil bleaching. Moreover, three different kinetics models such as pseudofirst order, pseudo-second order, and intraparticle diffusion model for adsorption of β-carotene on activated bleaching earth were studied. According to the result, the physicochemical properties such as acid value, peroxide value, iodine value, and p-anisidine value of soybean oil met the standards set by the Codex Alimentarius standard, indicating its goodness for further processing. Additionally, the color of the soybean oil appeared dark-yellowish. The optimum bleaching process parameters applied were 10 wt.% absorbent dosage to oil, 110°C for temperature, and 60 mins for contact time. The adsorption kinetics of β-carotene of the bleaching process was best fit with the pseudo-second order with R2 values of 0.9903. To sum up, the study contributes to the understanding of β-carotene adsorption behavior in crude soybean oil bleaching using a hydraulic press. Moreover, the kinetic model helps optimize the process, predict adsorption capacity and rate constants, and design cost-effective, safe bleaching strategies for refined soybean oil with reduced colorants.
Keywords
Adsorbent dosage, Bleaching efficiency, Contact time, Pseudosecond order, Temperature
Adsorbent dosage articles; Bleaching efficiency articles; Contact time articles; Pseudo-second order articles; Temperature articles
Article Details
Introduction
Soybean, scientifically known as Glycine max L., is highly valued for its protein and oil content, making it an important source of plant-based nutrients, with protein content ranging from 35-55% and oil content ranging from 13-26% based on the weight of dry soybean seeds [1]. Soybean oil is one of the most commonly consumed vegetable oils and is known for its nutritional benefits due to its large amount of essential fatty acids, including polyunsaturated fatty acids, which are important for overall health, as well as a variety of bioactive compounds such as β-carotene, a precursor to vitamin A [2]. Chlorophylls and carotenoids are key pigments present in soybean oil. Carotenoids are yellow-red pigments that can be categorized into carotenes, with β-carotene being the most prevalent carotenoid found in unrefined vegetable oils. While removing carotenoids from the oil improves its visual look for consumers, their presence improves the oil's oxidative stability. The primary color reduction step occurs during the bleaching process, which is required in the treatment of soybean oil to remove unwanted colors such as β-carotene [3,4]. Bleaching plays a vital role in the refining of oils and is typically carried out after degumming, and neutralization [5]. Bleaching, an intricate process involving both physical and chemical aspects, is an integral part of vegetable oil refining. Its primary purpose is to minimize the presence of colored pigments, such as carotenoids and chlorophylls, in the oil. Additionally, bleaching effectively eliminates residual traces of phosphatides, soaps, phospholipid impurities, lipid peroxidation by-products, and other unwanted contaminants [6,7]. To carry out the bleaching operation, adsorption bleaching clays, activated carbon, specific silica, or a mix of these substances are used [8]. The use of bleaching earth as an adsorbent for oil decolorization is popular in the oil sector because of its low cost and substantially superior adsorption capability [5,8,9].
The aim of this work was therefore to investigate the effect of three parameters including adsorbent dosage, temperature, and contact time on the bleaching efficiency of Cambodian soybean oil. Three different kinetic models such as pseudo-first order, pseudo-second order, and intraparticle diffusion model for adsorption of β-carotene on activated bleaching earth were also evaluated for a good fitting model. The crude soybean oil obtained from the hydraulic press was transferred into brown glass containers and stored in a refrigerator at a temperature of -18°C. Subsequently, the physicochemical properties of crude soybean oil were determined by following AOAC (2012) official method, including acid value, peroxide value, iodine value, p-Anisidine value, and color [10].
Materials and Methods
Sample preparation
In this study, the dried soybean seeds were purchased from a farmer in Samlout district, Battambang province, Cambodia. Following that, the soybean seeds were cleaned to remove the impurities such as dust, wood, and stones. They were then finely processed into soybean powder using a grinding machine (SL-30, Thailand). The soybean powder was then properly stored at room temperature in airtight polyethylene bags for future experiments.
Extraction of crude soybean oil
In this experiment, approximate 350 g of soybean powder was wrapped in press-cloth and inserted into a metallic cylinder of the hydraulic machine (BY-180, China) at 60°C for pressing. Next, the pressing pressure of 50 MPa, and 25 mins of pressing time were applied.
Analysis of physicochemical properties of crude soybean oil
The crude soybean oil obtained from the hydraulic press was transferred into brown glass containers and stored in a refrigerator at a temperature of -18°C. Subsequently, the physicochemical properties of crude soybean oil were determined by following AOAC (2012) official method, including acid value, peroxide value, iodine value, p-Anisidine value, and color [10].
Bleaching process
A 60 g of crude soybean oil was poured into a 250ml round bottom flask, and then heated up to 100°'C on a magnetic hot plate. 3 wt.% of activated bleaching earth was added to the hot oil. The temperature was maintained at 100°'C for 30 mins. After the mixture was centrifuged at 4000 rpm for 15 mins to separate the oil from the activated bleaching earth. Finally, the mixture was filtered through the Whatman filter paper. This process was repeated with the absorbent dosage (4-12 wt.%) in order to determine the effect of dosage. The effect of temperature (90-120°'C) was studied. The effect of time was done by varying the time (15-90 min). The evaluation of the bleaching efficiency was conducted using a UV-Vis Spectrophotometer. To begin, a sample weighing 0.1 g of bleached soybean oil was carefully measured and added to a test tube containing 7.5 ml of petroleum ether. The mixture was then transferred into a cuvette, and the absorbance value was measured at a wavelength of 445 nm using petroleum ether. The percentage of bleached oil was determined using the following formula:
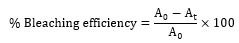
Where:
A0 = Absorbance of unbleached soybean oil
At = Absorbance of bleached oil at a time
Kinetic models
The kinetics of adsorption of β-carotene was determined at 110°C and using 10wt% of activated bleaching earth with the contact time from 15 to 90 mins of 15 mins increments. To determine this value, the preliminary experiments were performed using the activated bleaching earth in a range of 3–12wt% and the temperature from 90-120°C. The relative amounts of β-carotene adsorbed at equilibrium (Qe) and at time t (Qt) were calculated using formula:
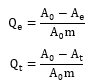
Where:
A0 = Absorbance of the crude soybean oil
Ae = Bleached soybean oil at equilibrium
At = Bleached soybean oil at time t
Pseudo-first order kinetic model
The linear form of the pseudo-first order kinetic modeling [11,12,13].
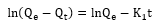
Where:
K1 = Kinetic constant of the first order (min-1)
t = Contact time (mins)
Qe = β-Carotene adsorbed at equilibrium (mg/g)
Qt = β-Carotene adsorbed at time t (mg/g)
Pseudo-second order kinetic model
The linear form of the pseudo-second order kinetic modeling [12,13].
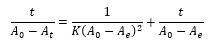
Where:
K = Second order kinetic constant (mg-1.min)
t = Contact time (min)
A0 = Absorbance of the crude soybean oil
Ae = Absorbance of bleached soybean oil at equilibrium
At = Absorbance of bleached soybean oil at time t
Intra-particle diffusion kinetic model
The expression of the intraparticle diffusion model proposed by Weber and Morris [13].
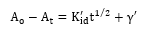
Where:
Kidˆ' = Intra-particle diffusion rate constant (mg/min0.5)
γˆ' = Associated to the boundary layer thickness (mg)
If intra-particle diffusion is the limiting step of adsorption process, the plot (A0-At) against t1/2 is a straight line to express the intra-particle diffusion at different contact time from where Kidˆ' and R2 were evaluated from the slope and intercept respectively [14].
Results and Discussion
Physicochemical properties of crude soybean oil
The results of the physicochemical analysis for crude soybean oil are presented in table 1. The acid value is an important parameter that is used to assess the quality of crude soybean oil [15]. The obtained acid value of 1.25 ± 0.03 mg KOH/g oil is significantly lower than the Codex Alimentarius standard of 4 mg KOH/g oil. Another study also reported the lower acid value which was 1.81 ± 0.11 mg KOH/g oil, it is evident that the crude soybean oil meets the quality requirements specified by the standard [16]. This indicates that the oil has a good quality and has a lower risk of rancidity [17]. The low acid value suggests a low level of free fatty acids in the oil, which is desirable for human consumption. The presence of high levels of free fatty acids can contribute to off-flavors and odors in the oil, negatively impacting its sensory attributes and overall quality [18]. The amount of peroxides present indicates the degree of oil or fat oxidation. The rancidity of oil is due to peroxides. These are the primary compounds that result from the oxidation of unsaturated fatty acids [19,20]. The peroxide values of the crude soybean oil (1.28 ± 0.23 meq/Kg oil) in this study were lower than the maximum permissible limit of 15 meq/Kg oil specified by the Codex Alimentarius standard. In addition, the peroxide value was equal to 1.79 ± 0.09 meq/Kg oil, which is approximately around 0.5 meq/Kg oil different from ours. The peroxide values for the crude soybean oil studied were less than the maximum limit, this indicates that the oils will be stable to oxidative rancidity [21].
|
Parameters |
Mean ± Standard deviation |
Codex Standard (Amended, 2023) |
|
|
Acid value (mg KOH/g oil) |
1.25 ± 0.03 |
4 |
|
|
Peroxide value (meq/Kg oil) |
1.28 ± 0.23 |
15 |
|
|
Iodine value (g I2/ g oil) |
132.96 ± 1.28 |
124-139 |
|
|
p-Anisidine value |
1.35 ± 0.00 |
- |
|
|
Color |
L* |
28.14 ± 0.42 |
- |
|
a* |
-2.55 ± 0.05 |
- |
|
|
b* |
12.69 ± 0.07 |
- |
|
Table 1: Physicochemical properties of crude soybean oil
The iodine value shows the degree of unsaturation in oil and is a useful indicator for quantifying the number of double bonds present in the oil, which reflects its oxidation susceptibility [22]. The iodine values obtained were 132.96 ± 1.28 g I2/ g oil which is in the range of the Codex Alimentarius standard as shown in table 1. Moreover, the iodine value from the previous study was 127.55 ± 0.74 g I2/ g oil that is lower than our study [23]. The high iodine value in the oil samples indicates the presence of high fraction of unsaturated fatty acids in the oil samples while low iodine value indicates lesser number of unsaturated bonds and lower susceptibility of such oil to oxidative rancidity [24].
p-Anisidine value indicates the level of peroxide decomposition into short-chain carbonyls, aldehydes, and ketones [25,26]. At this stage of oxidation, the oil develops a rancid odor and taste [25]. The p-Anisidine values in our study obtained were 1.35 ± 0.00 by using hydraulic press machine. Another study reported the p-Anisidine value of 4.83 ± 0.40 which is higher than that in our study [27]. For p-Anisidine value of 2 is accepted for fresh soybean oil with excellent stability [28]. A high p-Anisidine value in crude soybean oil may result in undesirable flavors [29].
Lastly, the color of soybean oil is an additional quality metric. L* was used to describe the relative luminance, which ranged from 0 (absolute black) to 100. CIELAB was used to describe the precise 3-D location of color at this stage (absolute white). a* represents the redness-greenness ratio, with positive a* indicating redness and negative a* indicating greenness. A positive value for b* indicates yellowness and a negative value indicates blueness. The results indicated that L*= 28.14 ± 0.42 indicates a relatively moderate level of brightness, a*= -2.55 ± 0.05 suggests a slight greenish hue, and b*= 12.69 ± 0.07 indicates an obvious yellowish hue. According to the previous study, their soybean oil was much lighter (higher L*) and yellower (higher b*) than ours. In addition, the obtained oil had a significantly lower a* value (-2.55 ± 0.05) than theirs (33.303 ± 0.30) and thus had less "redness" The higher values of 83.31 ± 0.97, 33.30 ± 0.30, and 70.32 ± 0.62 for L* (brightness), a* (redness), and b* (yellowness) gave their oil a bright, golden-yellow color, whereas the color of crude soybean oil extracted via hydraulic pressing was dark-yellowish or can be described as moderately light with a slight greenish hue and a prominent yellowish hue [23].
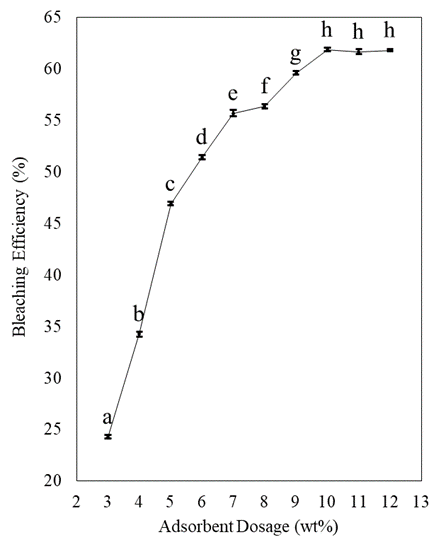
Effect of activated bleaching earth dosage on bleaching efficiency
The bleaching efficiency versus adsorbent dosage is illustrated in figure 1. It turned out that varying adsorbent dosage has a direct effect on the bleaching process as an increase in the adsorbent dosage resulted in an increase in the bleaching efficiency of oil increased from 24.29-61.84%. The adsorbent dosage was increased from 3-12wt% while the temperature of 100°'C) and contact time of 30 mins were kept constant. The results also obviously indicated that the bleaching efficiency reached the highest value at an adsorbent dosage of 10wt%, after which further increase in adsorbent dosage had no significant effect (p<0.05) on the bleaching efficiency, and the value remained constant. These observations are consistent with the adsorption mechanism of the bleaching process, indicating that it is mainly controlled by the availability of active sites for the adsorption [30]. Their number increases with the increasing mass of adsorbent during the adsorption process but reaches a constant state because of saturation. Saturation of sites is a common occurrence in adsorption; it resembles an equilibrium state [13]. This could be due to the fact that adsorption equilibrium has been reached between the adsorbent/oil mixtures, preventing further pigment removal by the excessive adsorbent dosage [31]. From the previous study found that their optimum bleaching condition was 2wt% clay which was less than our study due to the differences in the specification of the clay [32]. The different bleaching clays have varying absorption capacities. From this result, 6 g is the optimal amount of adsorbent for 60 g of oil, which corresponds to a mass ratio of 10wt% adsorbent to oil [33].
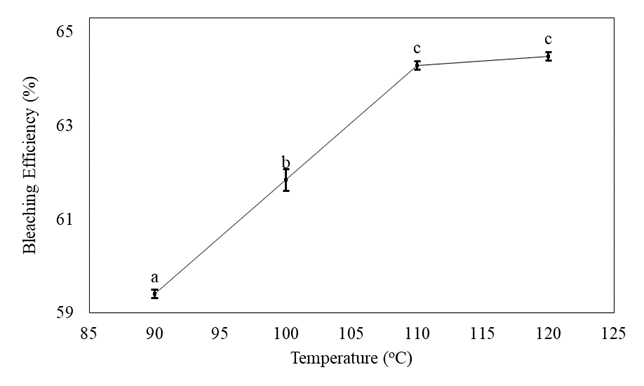
Effect of temperature on bleaching efficiency
As illustrated in figure 2, the bleaching efficiency increased from 59.40 - 64.48% as the temperature increased from 90-120°C while the absorbent of 10wt% and contact time of 30 mins were kept constant. The highest bleaching efficiency is achieved at a temperature of 120°C for obtained activated bleaching earth but the bleaching efficiency at 110°C was no significant difference (p<0.05) from the values at 120°C. The bleaching temperature generally ranges from 90 to 125°C and it was also reported that the viscosity of the adsorbate is reduced as the temperature rises, allowing for improved adsorbent particle dispersion and a rise in clay-oil interactions, and flowability [34]. The optimum bleaching temperature from the previous study was specific for a particular adsorbent and oil which is between 90-120°C for soybean oil. Obviously, the optimum temperature of this result was 110°C [32].
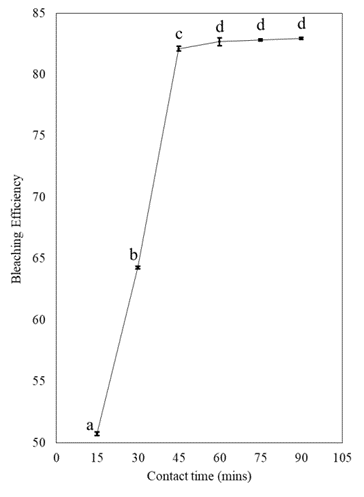
Effect of time on bleaching efficiency
The bleached percentage increased from 50.76-82.93% as the bleaching duration increased from 15-90 mins while the absorbent of 10wt%) and temperature of 110°C were kept constant, as illustrated in figure 3. At 60 mins, the bleaching efficiency was achieved which was 82.67%, the bleaching efficiency was not significantly affected (p<0.05) by the next increase in temperature, and it remained relatively constant afterward. Similar results, the equilibrium time for β-carotene elimination was approximately 60 mins [35]. Other authors observed a shorter time for equilibrium, with the typical range being 15 to 45 mins, with 20 to 30 mins being most common [36]. From the results obtained, the optimum contact time was 60 mins.
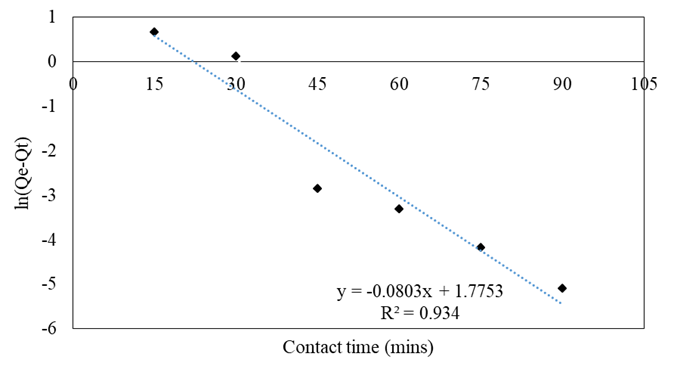
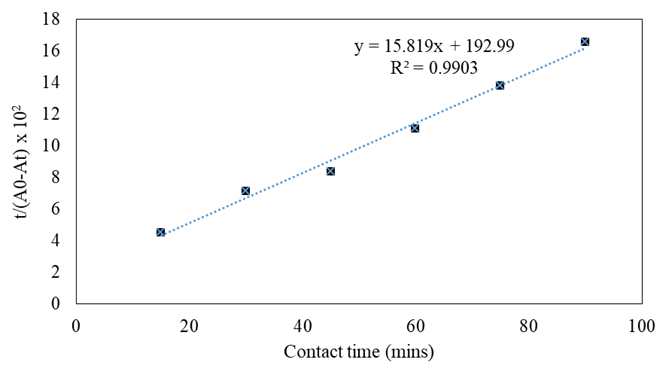
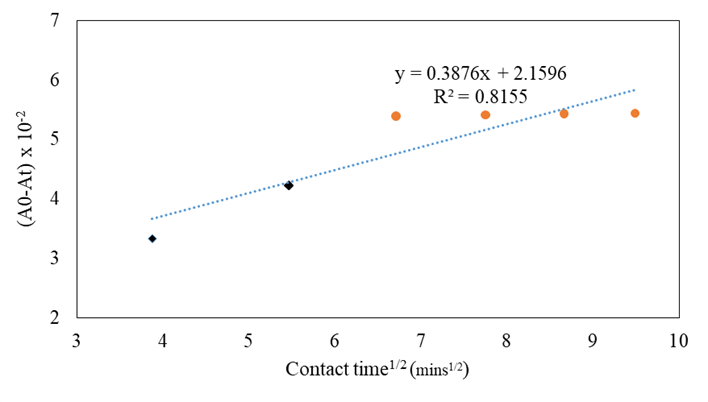
Kinetic models
The contact time is a fundamental adsorption process parameter, particularly for the β-carotene adsorption of soybean oil bleaching [4]. For a better understanding of the kinetic, from which the adsorbent uptake rate can be better explained, thereby facilitating the appreciation of the adsorption efficiency, figures 4, 5, and 6 represent three different kinetic models, include pseudo-1st order, pseudo-second order, and intraparticle diffusion models in the range of 15-90 mins and table 2 summarizes the values of the correlation coefficient R2 and constant rate. It can be seen from the figures 4, 5, and 6 that the pseudo-second order model describes the adsorption of β-carotene onto activated bleaching earth quite well, and its R2 value for the adsorption of β-carotene onto activated bleaching earth is greater than those calculated by pseudo-first order model and intraparticle diffusion model (R2 > 0.99) as shown in table 2. The correlation coefficient R2 of the pseudo-second order model for the adsorbent has extremely high values greater than 0.99. This indicates that the bleaching process and these adsorbents have a very strong correlation [12]. A similar result was reported in previous research for β-carotene removal from palm oil, which showed that the kinetics adsorption followed pseudo-second order model [11].
Figure 6 displays the separation of plots into two linear regions. The first stage represents a curve that is typically attributed to boundary layer diffusion effects or external mass transfer effects. The second stage corresponds to a gradual adsorption period where intraparticle diffusion controls the process. Moreover, the deviation of the straight line from the origin indicates that pore diffusion alone does not solely control the rate of the beta-carotene adsorption process.
|
Pseudo-first Order |
Pseudo-second Order |
Intraparticle diffusion |
|||
|
K1 (min1/2) |
R2 |
K (mg-1.min-1) |
R2 |
K'id (mg.min-1/2) |
R2 |
|
0.0803 |
0.934 |
1.2967 |
0.9903 |
0.03876 |
0.8155 |
Table 2: Values of the correlation coefficients R2 and rate constants for the different kinetic models
Conclusion
The crude soybean oil obtained from the hydraulic press had a good quality due to its physicochemical properties complied with the Codex Standard, and its color was dark-yellowish. The effects of the variables such as dosage, temperature, and time on the bleaching of soybean oil were studied in this work and had a great effect on the bleaching efficiency as each parameter increased the percentage of bleaching efficiency also increased but each of the parameters exhibited a steady state. The highest processing parameters for the bleaching process were 10wt% for activated bleaching earth content, 110°C for bleaching temperature, and 60 mins for contact time. In addition, the study findings showed that the pseudo-second order kinetic model was well described for the adsorption of β-carotene on activated bleaching earth of the bleaching of soybean oil.
Acknowledgement
The work was funded by Cambodia Higher Education Improvement Project (Credit No. 6221-KH).
References
- Choi YM, Yoon H, Shin MJ, et al. Change in protein, oil and fatty acid contents in soybeans (Glycine max (L.) Merr.) of different seed coat colors and seed weight 19 (2021): 430-590.
- Ahmed S, Arefin P, Mondal K, et al. Comparison of Nutritional Quality and Storage Stability of β-Carotene Fortified Soybean Oil and Olive Oil. Tropical Journal of Natural Product Research 7 (2023): 2487-2491.
- Proctor A, Brooks D. Adsorptive Separation of Oils. Sixth edition (2005).
- Abedi E, Amiri MJ, Sahari MA. Kinetic, isotherm and thermodynamic investigations on adsorption of trace elements and pigments from soybean oil using high voltage electric field-assisted bleaching: A comparative study. Process Biochemistry 91 (2020): 208-222.
- Liu Y, Huang J, Wang X. Adsorption isotherms for bleaching soybean oil with activated attapulgite. Journal of the American Oil Chemists’ Society 85 (2008): 979-984.
- Gotor A. Undefined. Effects of refining process on sunflower oil minor components: ocl-journal.org (2016).
- Technology WZ. Bleaching of edible fats and oils. Wiley Online Library (2001).
- Monte ML, Pohndorf RS, Crexi VT, et al. Bleaching with blends of bleaching earth and activated carbon reduces color and oxidation products of carp oil. European Journal of Lipid Science and Technology 117 (2015): 829-836.
- Sabah E, Cinar M, Onsekiz Ç, et al. Decolorization of vegetable oils: Adsorption mechanism of β-carotene on acid-activated sepiolite. Elsevier (2007).
- AOAC Official methods of analysis of AOAC International 1 (2012): 121-130.
- Ahmad AL, Chan CY, Shukor SR, et al. Adsorption kinetics and thermodynamics of β-carotene on silica-based adsorbent. Chemical Engineering Journal 148 (2009): 378-384.
- Joseph N, Chijioke EO, Okwudili CN. Equilibrium, kinetics and thermodynamics of the bleaching of palm oil using activated nando clay. Journal of Engineering Research and Reports 1 (2018): 1-13.
- Kepdieu JM, Djangang CN, Tchanang G, et al. Kinetic and Mechanism of the Adsorption of ß-Carotene Rich-Palm Oil onto Smectite Clay Activated by Fe (II) Lewis Acid Ion. Journal of Chemistry 1 (2022): 1-21.
- Vidoca LP, Almeida ES, Cardoso MF, et al. Extraction of carotene from crude hybrid palm oil using polymeric resin. Journal of Food Engineering 278 (2020): 193-208.
- Nasrullah N. Quality assessment and safety measurement of different industrial processing stages of soybean oil. Turkish Journal of Food and Agriculture Sciences 1 (2020): 28-33.
- Mohdaly AA, Seliem KA, Hassan AE, et al. Effect of refining process on the quality characteristics of soybean and cotton seed oils. International Journal of Current Microbiology and Applied Sciences 6 (2017): 207-222.
- Esfarjani F, Khoshtinat K, Zargaraan A, et al. Evaluating the rancidity and quality of discarded oils in fast food restaurants. Food Science and Nutrition 7 (2019): 2302.
- House KA, Acree TE. Sensory impact of free fatty acids on the aroma of a model Cheddar cheese. Food Quality and Preference 13 (2002): 481-488.
- Mohdaly AAA, Sarhan MA, Mahmoud A, et al. Antioxidant efficacy of potato peels and sugar beet pulp extracts in vegetable oils protection. Food Chemistry 123 (2010): 1019-1026.
- Gomathi R, Anusuya N, Chitravadivu C, et al. Antioxidant activity of lettuce tree (Pisonia morindifolia R.Br.) and tamarind tree (Tamarindus indica L.) and their efficacy in peanut oil stability. Food Science and Biotechnology 20 (2011): 1669-1677.
- Bachari Z, Ezzatpanah H, Aminafshar M, et al. The effect of refining process on the conjugated dienes in soybean oil. Journal of Agricultural Science and Technology 15 (2013): 1185-1193.
- Ladan Z, Okonkwo EM, Amupitan JO, et al. Physicochemical properties and fatty acid profile of Hyptis spicigera seed oil. Research Journal of Applied Sciences 5 (2010): 123-125.
- Makeri MU, Karim R, Abdulkarim MS, et al. Comparative analysis of the physico-chemical, thermal, and oxidative properties of winged bean and soybean oils. International Journal of Food Properties 19 (2016): 2769-2787.
- Eke-Ejiofor J, Beleya EA, Allen JE. Effect of variety on the quality parameters of crude soybean oil. American Journal of Food Science and Technology 9 (2021): 69-75.
- Chong YM, Chang SK, Sia WCM, et al. Antioxidant efficacy of mangosteen (Garcinia mangostana Linn.) peel extracts in sunflower oil during accelerated storage. Food Bioscience 12 (2015): 18–25.
- Drinic Z, Mudric J, Zdunic G, et al. Effect of pomegranate peel extract on the oxidative stability of pomegranate seed oil. Food chemistry 333 (2020).
- Mansour HMM, El-Sohaimy SA, Zeitoun AM, et al. Effect of natural antioxidants from fruit leaves on the oxidative stability of soybean oil during accelerated storage. Antioxidants 11 (2022).
- Medina-Juárez LA, González-Díaz P, Gámez-Meza N, et al. Effects of processing on the oxidative stability of soybean oil produced in Mexico. Journal of the American Oil Chemists’ Society. 75 (1998): 1729-1733.
- Tompkins C, Perkins EG. The evaluation of frying oils with the p-Anisidine value. Journal of the American Oil Chemists’ Society 76 (1999): 945-947.
- Valenzuela D, Francisco R, Souza P. Studies on the acid activation of Brazilian smectitic clays 24 (2001): 345-353.
- Ajemba RO, Onukwuli OD. Adsorptive removal of colour pigment from palm oil using acid activated Nteje clay. Kinetics, equilibrium and thermodynamics. Physicochemical Problems of Mineral Processing 49 (2013): 369-381.
- Henache Z, Boukerroui A, Kashi I. Modeling of the soybean oil bleaching and optimization of its conditions in the refining process for environmental interest. Nova Biotechnologica et Chimical 17 (2018): 48-57.
- Nde-Aga BJ, Kamga R, Nguetnkam JP. Adsorption of palm oil carotene and free fatty acids onto acid activated cameroonian clays. Journal of Applied Sciences 7 (2007): 2462-2467.
- Roberto R. Achieving Optimal Bleaching Performance. Scientific Research Publishing 112 (2006): 2-6.
- Obiageli R, Dominic O, Ajemba RO, et al. Evaluation of the effects of acid activation on adsorptive properties of clay from Ukpor in bleaching palm oil material science. View project Evaluation of International Journal of Multidisciplinary Sciences and Engineering 3 (2012).
- Nwabanne JT, Ekwu FC. Decolourization of palm oil by Nigerian local clay: A study of adsorption isotherms and bleaching kinetics. International Journal of Multidisciplinary Sciences and Engineering 4 (2013): 20-25.