Stem Cell Therapy: A Green Expectation of Advanced Knee Osteoarthritis Treatment in Bangladesh
Article Information
Mohiuddin AKM*, Lewis P, Mia Md. Manik, Rahman Iqram, Anowar Raihan
Department of Regenerative Orthopedic, Surecell Medical [BD] Ltd. Dhaka, Bangladesh
*Corresponding authors: Mohiuddin AKM, Department of Regenerative Orthopedic, Surecell Medical [BD] Ltd. Dhaka, Bangladesh.
Received: 11 February 2024; Accepted: 15 February 2024; Published: 06 March 2024
Citation: Mohiuddin AKM, Lewis P, Mia Md. Manik, Rahman Iqram, Anowar Raihan. Stem Cell Therapy: A Green Expectation of Advanced Knee Osteoarthritis Treatment in Bangladesh. Journal of Biotechnology and Biomedicine. 7 (2024): 160-165.
View / Download Pdf Share at FacebookAbstract
Background: Treatment of knee osteoarthritis (OA) is very challenging. Sometimes, it can be resistant to medications, procedures, and surgery. Surgery may not be an option for some patients due to old age, obesity, and co-morbidities. On the other hand, stem cell therapy is an effective method to regenerate damaged tissue in regenerative medicine because adult stem cells are self-renewing, clonogenic, and multi-potent by nature. Their principal role is to maintain tissue homeostasis. Due to their multi-potent character, stem cells can be activated to proliferate and differentiate into the required types of cells upon cell loss or injury to the tissue. This clinical study has demonstrated the structural and functional regeneration capabilities of Small Blood Stem Cells (SBSC) derived from autologous peripheral blood and adipose tissue-derived stem cells (ADSCs) to regenerate cartilage damage. Aim of the study: This study has been conducted to observe the effectiveness of autologous adult stem cell therapy on pain, function, and severity in OA Knee for Bangladeshi patients.
Methods: This retrospective study was conducted at the Department of Regenerative Orthopedics in Surecell Medical [BD] Ltd, Dhaka, Bangladesh. The study duration was two years, from July 2021 to June 2023. A total of 100 patients were enrolled and analyzed in this study. All of them were present till the end of the treatment. Before all actions, patients were taken to a general practitioner, who noted their medical history and referred them to an orthopedic surgeon. The orthopedic surgeon selected osteoarthritis knee patients for stem cell therapy. Pre-treatment and post-treatment osteoarthritis knee conditions were measured using the (WOMAC) score. The post-treatment outcome has been compared based on the pre-treatment WOMAC score by the descriptive data analysis system of SPSS software.
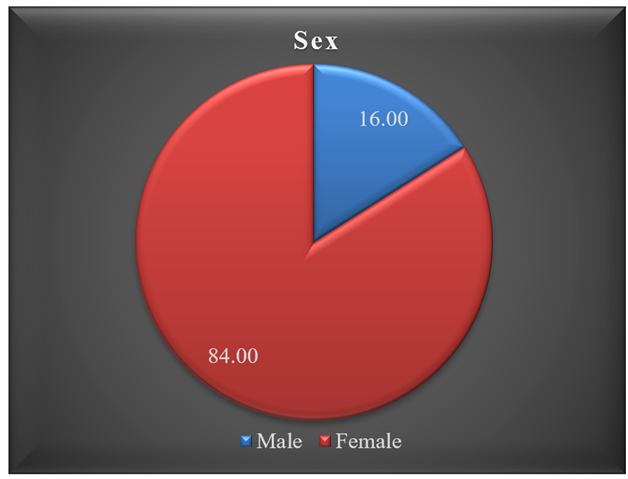
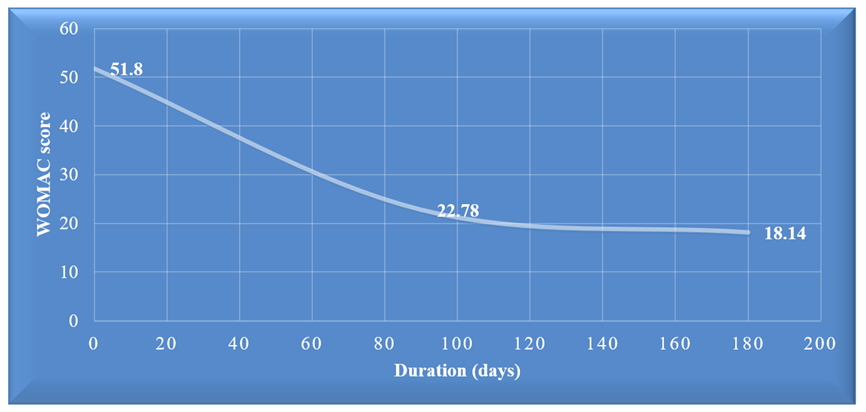
Result: The study analyzes a population's age, BMI, sex distribution, and co-morbidities. The majority (68%) of patients are aged 51-70, with a mean age of 64.47±8.40. The mean BMI is 31.04±5.55. Females constitute 84% of the population. Pre-treatment WOMAC score averages 51.8±10.42, dropping to 22.78±10.04 at 90 days post-treatment and 18.14±7.20 at 180 days. Co-morbidity rates include 45.00% for DM, 38.00% for HTN, 12.00% for Cardiac, 4.00% for renal, 2.00% for breast cancer, and 26.00% with no co-morbidities. These findings comprehensively overview the study population's demographics, health outcomes, and co-existing conditions.
Conclusion: The study, following detailed data analysis, affirms the safety and efficacy of administering fresh, autologous small blood stem cells and ADSCs, for treating advanced osteoarthritis knee. This gradual improvement is not an immediate remedy for pain relief, stiffness, or recovery. Despite a few limitations, Stem cell therapy offers a sustainable and clinically significant outcome for joint function in elderly patients with Osteoarthritis in Bangladesh, surpassing conventional treatments in safety and efficacy.
Keywords
Stem cell therapy; Small Blood Stem Cell (SBSC); adipose tissue-derived stem cells (ADSCs); Osteoarthritis (OA); Knee joint; WOMAC score
Article Details
Introduction
Osteoarthritis (OA) of the knee is a chronic, indolent disease that affects all genders, ages, and races but is known to be most common in the elderly and obese people in Bangladesh and even all over the world [1]. A degenerative connective tissue disease, it mainly affects the articular cartilage [2]. The definition of knee OA varies in reported studies. It includes self-reported knee OA (obtained from a questionnaire), radiographic definitions of knee osteoarthritis, and symptomatic knee OA (self-reported joint pain and radiographic evidence of OA) [3]. Symptoms may include joint pain, stiffness, and tenderness. Furthermore, as the cartilage substance decreases, the bone surface may also become affected, developing osteophytes (bone spurs) and direct bone-bone contact. In addition to the stiffness of the joint, the patient tries to avoid pain by minimizing joint movement, which leads to muscle atrophy and laxity of the ligaments [2-5]. The pathogenesis of knee OA has been linked to biomechanical and biochemical changes in the cartilage of the knee joint, e.g., inability to withstand everyday mechanical stresses, limited supply of nutrients and oxygen, inadequate synthesis of extracellular matrix components, increased synthesis of tissue-destructive proteinases (matrix metalloproteinase and aggrecanases) and overall apoptosis of chondrocytes [2,5-7]. Synovial inflammation has recently been accredited as a factor limiting knee cartilage repair. Moreover, it correlates to clinical signs of knee OA, such as swelling and inflammatory pain [7,8]. Synovial inflammation is believed to be a response of synovial macrophages to cartilage debris and catabolic mediators entering the synovial cavity [8,9]. During OA knee treatment by stem cells, we have to know that there is a need for more standardization in the isolation methods to be overcome to eliminate the significant variability in cell quality. The subsets have also hampered limited knowledge of ADSCs research progress due to the need for unique markers for their isolation. In addition, good manufacturing practices (GMP) and quality isolation protocols are needed to use ASCs in clinical trials [10]. Various other questions need to be answered before ASCs can be used in standard clinical usages: bio-safety (tumour capacity), reproducibility, and efficiency of transplanted ASCs. Moreover, a trained biotechnologist or specialist in molecular cell biology and related fields should operate the SOP of Cell isolation, culture, and application.
Literature
In adults, stem cells can be found in various tissues, such as the bone marrow, peripheral blood, adipose tissue, intestine, and many others. However, in most cases, they are characterized as multipotent, giving rise to limited types of different cells. Hematopoietic, mesenchymal, and epithelial stem cells are the three central multipotent stem cells in adult tissues. Although most multipotent stem cells can be quickly isolated and cultured, their use in regenerative medicine is limited due to their restricted differentiation capacity. The aim of using stem cells is to support the self-healing process of the knee joint cartilage, which results in relief from OA symptoms [11-17]. This treatment should be used with additional treatment to improve patients’ functional status and quality of life. However, osteoarthritis cannot be cured by any radical treatment at the moment. Small Blood Stem Cells (SBSCs) are potential candidates for stem cell therapies. A distinct variety of stem cells referred to as SBSCs has been documented in previous research [18]. These cells can be obtained from human adult blood, bone marrow, or fetal cord blood. SBSCs share certain features with embryonic stem cells, exhibiting superior plasticity and the capability to differentiate into various mesodermal cell types, such as osteocytes, chondrocytes, adipocytes, as well as ectodermal cells like neurons, endodermal cells like liver cells, and cardiomyocytes. Other stem cell types with similar characteristics to SBSCs include VSELs (very small embryonic-like stem cells; CD133+) [19], MSCs (mesenchymal stem cells; Stro-1+) [20], and BLSCs (blastomere-like stem cells; CEA+) [21]. The SBSCs obtained from human adult blood are precisely termed small blood stem cells, offering additional advantages in regenerative medicine, such as easy accessibility for harvesting, distinguishable surface markers, and the ability for self-renewal [18]. Moreover, the development of methods was required to develop the cartilage phenotype without hypertrophy, fibrinogenesis, or ossification. In addition, a delivery system was devised to target cells in a lesion without inhibiting their chondrogenic differentiation or the integrity of repaired tissue [17]. The Adipose tissue contains a large number of multipotent stem cells, which is an essential prerequisite for stem-cell-based therapies. It has been described that stem and progenitor cells in the uncultured stromal-vascular fraction (SVF) from adipose tissue usually amount to up to 3% of the whole cells, which is 2,500-fold more than the frequency of stem cells in the bone marrow. Others have also described that adipose tissue provides large numbers of stem cells compared to bone marrow. A bone marrow transplant contains approximately 6×106 nucleated cells per mL, of which only 0.001-0.01% are stem cells [22]. ASCs can easily be isolated by tissue digestion and centrifugation steps, followed by the outgrowth of the plastic adherent fraction from the primarily isolated cell mixture (the so-called SVF). SVF is a highly heterogeneous cell population comprising the non-adherent cell population. The composition of the SVF has been reported with significant variability among authors. Cell populations within the SVF could be roughly distinguished by cell size and granularity in flow cytometry by forward and sideward scatter diagrams and by their characteristic expression pattern. Miranville and coworkers described some stem cell markers (CD34, CD133, ABCG2) in the SVF from different anatomic sources. They first described that freshly harvested SVF contains large numbers of CD34+ cells and showed two subpopulations of CD34+ cells. ADSCs are considered ideal for application in regenerative therapies for several reasons. Namely, they can be harvested, handled, and expanded minimally invasive, quickly, and effectively. They have a high potential for differentiation into mature cells along the mesodermal, ectodermal, and endodermal lineage [22].
Objectives
Many research studies have shown much positive feedback for different diseases through stem cell therapy. Stem cell therapy is increasingly used for knee osteoarthritis (OA). This study has been conducted to observe the effectiveness of autologous adult stem cell therapy on pain, function, and severity in OA Knee for Bangladeshi patients.
Methodology and Materials
This retrospective investigation occurred at the Regenerative Orthopedics Department in Surecell Medical BD Ltd, Dhaka, Bangladesh. The study spanned two years, starting from July 2021 to June 2023. A cohort of 100 patients was recruited and thoroughly examined as part of this study, with all participants remaining actively engaged in the treatment until its conclusion. Before any interventions, individuals sought consultation from a general practitioner, who meticulously documented their medical histories before directing them to an orthopedic surgeon. The orthopedic surgeon specifically identified patients suffering from knee osteoarthritis as candidates for stem cell therapy.
Inclusion criteria:
- Males and females
- Aged 18-90 years
- Hemoglobin level >10 gm/dL
- CRP levels <10 mg/L
- All Cancer Markers should be Negative
- LFT should be in the normal range.
- Kellgren and Lawrence grade 4 knee Osteoarthritis.
- Patients are required to stay on a stable drug regime throughout the study.
Pre-treatment and post-treatment osteoarthritis knee conditions were measured using the (WOMAC) score. The post-treatment outcome has been compared based on the pre-treatment WOMAC score by the descriptive data analysis system of SPSS software. The Western Ontario and McMaster Universities Arthritis Index (WOMAC) is widely used to evaluate Hip and Knee Osteoarthritis. WOMAC Index was developed in 1982 at Western Ontario and McMaster Universities. WOMAC has been linguistically validated in over 65 languages [31, 32].
It is a self-administered questionnaire consisting of 24 items divided into three subscales:
- Pain (5 items):during walking, using stairs, in bed, sitting or lying, and standing upright
- Stiffness (2 items):after first waking and later in the day
- Physical Function (17 items):using stairs, rising from sitting, standing, bending, walking, getting in/out of a car, shopping, putting on / taking off socks, rising from bed, lying in bed, getting in/out of bath, sitting, getting on / off toilet, heavy domestic duties, light domestic duties.
Materials
According to GMP protocol, highly protected and high-tech technology support has been provided by Surecell Medical [BD] Ltd and were used Laminar Air Flow (MN120, Vera Medical, Turkey), High-Speed Centrifuge (5810 R, Eppendorf, Fisher Scientific, Finland), Fat Microlyser (2400micron, 1200Micron, 600Micron, T-Lab, Turkey), Falcon Tube (Thermo Scientific), Stem Cell Extraction Tube (Adistem, USA), Petaka G3 Cell Culture late, Vortex Mixture (Fisherbrand ™ Analog Vortex Mixer), Syringe filter (Millex, Germany), Photo Activation Machine (Adistem, USA). Ultrasonic Bath, -80°C Freeze (Thermo Scientific), Cell Counter (TC-20, Biorad, USA).
Administration Protocol
Administration of stem-rich injection is a simple procedure like an injection into intra-articular space. The surface area of the knee joint is cleaned and sterile by a medical-grade cleaning agent. The local anesthetic agent is also used to ensure a less painful procedure. Before stem cell-rich injection, a photo-activation machine (Adi-stem, USA) activates cells, and cell number and cell viability are check by a particular cell counter (TC-20, Bio-Rad, USA). Science, the stem cell administration regenerative orthopedic doctor suggests to the patients 24 hours of bed rest and to avoid heavy physical activity for a week.
Results
Table 1 provides an overview of age and BMI descriptive statistics for the study population. The majority of patients (68.00%) were in the 51-70 age group, followed by 28.00% in the 71-90 age group, and only 4% were between 31-50 years old. The study reports a mean age of 64.47±8.40 and a mean BMI of 31.04±5.55. Figure 1 illustrates the sex distribution, with 84.00% of the population female and the remaining 16% male. Over the 180 days of treatment, WOMAC scores were recorded at three intervals: pre-treatment, 90 days post-treatment, and 180 days post-treatment. Descriptive statistics for patient improvement based on the WOMAC Score reveal a pre-treatment WOMAC score mean of 51.8±10.42, ranging from 22 to 71. The 90 days post-treatment WOMAC score has a mean of 22.78±10.04, ranging from 8 to 53. Furthermore, the 180 days post-treatment WOMAC score has a mean of 18.14±7.20, ranging from 5 to 42 (Table 2). Figures 2 display average of the pre-treatment WOMAC score, post-treatment WOMAC score after 90 days and 180 days. Table 3 outlines the co-morbidities in the study population, indicating high percentages of 45.00% for diabetes (DM) and 38.00% for hypertension (HTN), while 12.00% of cases had cardiac problem, and renal is at low percentages of 4.00%. Breast cancer is present in 2.00% of cases, and 26% of patients have no co-morbidities.
|
Age range (in years) |
Frequency (n) |
Percentage (%) |
|
31-50 |
4 |
4.00 |
|
51-70 |
68 |
68.00 |
|
71-90 |
28 |
28.00 |
|
Mean±SD |
64.47±8.40 |
|
|
BMI |
||
|
Mean±SD |
31.04±5.55 |
|
Table 1: Descriptive Statistics of Age and BMI of the study population (N=100).
|
Follow up |
Mean±SD |
Minimum |
Maximum |
|
Pre-treatment WOMAC score |
51.8±10.42 |
22 |
71 |
|
Post-treatment WOMAC score (after 90 days) |
22.78±10.04 |
8 |
53 |
|
Post-treatment WOMAC score (after 180 days) |
18.14±7.20 |
5 |
42 |
Table 2: Descriptive Statistics of Patient Improvement by WOMAC Score.
|
Co-morbidities |
Frequency (n) |
Percentage (%) |
|
None |
26 |
26.00 |
|
DM |
45 |
45.00 |
|
HTN |
38 |
38.00 |
|
Renal |
4 |
4.00 |
|
Cardiac |
12 |
12.00 |
|
Breast cancer |
2 |
2.00 |
Table 3: Co-morbidities of the study population (N=100).
Discussion
The research data has been analyzed by SPSS software, and descriptive analysis was used to determine the research outcome. The Post-treatment WOMAC scores are compared with the pre-treatment WOMAC scores, and the effectiveness of autologous stem cell therapy in knee osteoarthritis treatment was observed over six months and 12 months follow-up. Table 1 provides an overview of the study population's age and descriptive statistics for BMI. The majority of patients, 68(68.00%), fell within the 51-70 age group, followed by 28(28.00%) patients in the 71-90 age group, while only 4% were between 31-50 years old. The study reports a mean age of 64.47±8.40 and a mean BMI of 31.04±5.55. Figure 1 illustrates the sex distribution, with 84.00% of the population female and the remaining 16% male. They are elderly Bangladeshi patients suffering from OA knee for a few years and with other co-morbidities; some of them are advised for knee replacement by an Orthopedic Surgeon as they are at an advanced stage. They convinced Stem Cell therapy to avoid surgery, as some of them are not fit for pre-operative conditions. Except for OA knee, other diseases like DM, HT, Obesity, and other disease conditions are also considered before stem cell treatment and related medication are advised. Figure 2 outlines the co-morbidities in the study population, indicating high percentages of 45.00% for DM and 38.00% for HTN, 12.00% for cardiac and 4.00% renal. Breast cancer is present in 2.00% of cases, and 26.00% of patients have no co-morbidities. We applied the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scorning system for measuring illness due to OA knee, measured five parameters for pain, two parameters for stiffness, and seventeen parameters for physical function and scoring by number (0=None, 1=slight, 2= Moderate, 3=Very, 4=Extremely illness) of 96 height illness score. The OA knee illness measuring value (by WOMAC score) is 96. After counting the value of the parameters, the total scores convert into a percentage. In 180 days of treatment duration, the WOMAC score was taken in three steps: Pre-treatment WOMAC score, WOMAC score after 90 days, and 180 days post-treatment. Descriptive statistics regarding patient improvement based on the WOMAC Score reveal a pre-treatment WOMAC score mean of 51.8±10.42, ranging from 22-71. The 90 days post-treatment WOMAC score has a mean of 22.78±10.04, ranging from 8 to 53. Furthermore, the 180 days post-treatment WOMAC score has a mean of 18.14±7.20, ranging from 5 to 42 (Table 2). That means values of each parameter of OA knee illness decreased gradually rather than improving the disease condition. There is evidence of the effectiveness of stem cell treatment (Statistical data and histograms mentioned above).
Limitations of the study
There is no post-therapy risk in autologous stem cell therapy except for cross-contamination and contact with infectious microorganisms. Though our study outcome shows some positive effects of stem cell treatment, but not established treatment, this treatment is clinically under research. OA knee conditions are evaluated (by WOMAC score) from the self-information of patients and clinical observation, so a silly mistake can badly impact the study result. X-ray, MRI, Ultrasound, and blood investigation may be the alternative ways to cross-check the post-therapy knee condition. Stem cell therapy is an under-research treatment and costly high, so it is difficult to convince an elderly patient for this treatment.
Conclusion and Recommendations
Autologous ADSCs and Small Blood Stem Cells (SBSCs) are safe and highly effective in osteoarthritis knee. It is a gradual improvement process. Despite a few limitations, stem cell therapy is more sustainable than conventional treatment and gives a strong safety profile and clinically significant efficacy outcome for joint function in elderly Patients. Further research would be conducted to establish the efficacy of stem cell therapy in improving regenerative treatment.
Funding
No funding sources.
Conflict of interest
None declared.
References
- Uth K, Trifonov D. Stem cell application for osteoarthritis in the knee joint: A minireview. World journal of stem cells 6 (2014): 629.
- Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. The Lancet 377 (2011): 2115-2126.
- Chaganti RK, Lane NE. Risk factors for incident osteoarthritis of the hip and knee. Current reviews in musculoskeletal medicine 4 (2011): 99-104.
- Hoffmann C, Rockstroh JK. HIV medicine 2006. (2006).
- Chen X, Rao W, Shi Y, et al. Minimally Invasive Injectable Thermochemical Ablation Therapy of Malignant Tumor via Alkali Metal Fluid. Biomedical Materials & Devices 25 (2022): 1-7.
- Michael JW, Schlüter-Brust KU, Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Deutsches Arzteblatt International 107 (2010): 152.
- Lories RJ, Luyten FP. The bone–cartilage unit in osteoarthritis. Nature Reviews Rheumatology 7 (2011): 43-49.
- Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nature Reviews Rheumatology 6 (2010): 625-635.
- Heinegård D, Saxne T. The role of the cartilage matrix in osteoarthritis. Nature Reviews Rheumatology 7 (2011): 50-56.
- Sensebé L, Bourin P. Producing MSC according GMP: process and controls. Bio-Medical Materials and Engineering 18 (2008): 173-177.
- Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. The international journal of biochemistry & cell biology 36 (2004): 568-584.
- Davatchi F, Abdollahi BS, Mohyeddin M, et al. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. International journal of rheumatic diseases 14 (2011): 211-215.
- Vinatier C, Bouffi C, Merceron C, et al. Cartilage tissue engineering: towards a biomaterial-assisted mesenchymal stem cell therapy. Current stem cell research & therapy 4 (2009): 318-329.
- Murphy JM, Dixon K, Beck S, et al. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis & Rheumatism 46 (2002): 704-713.
- Koelling S, Miosge N. Stem cell therapy for cartilage regeneration in osteoarthritis. Expert opinion on biological therapy 9 (2009): 1399-1405.
- Mobasheri A, Csaki C, Clutterbuck AL, et al. Mesenchymal stem cells in connective tissue engineering and regenerative medicine: applications in cartilage repair and osteoarthritis therapy. Histology and histopathology (2009).
- Nöth U, Steinert AF, Tuan RS. Technology insight: adult mesenchymal stem cells for osteoarthritis therapy. Nature clinical practice Rheumatology 4 (2008): 371-380.
- Wang J, Guo X, Lui M, et al. Identification of a distinct small cell population from human bone marrow reveals its multipotency in vivo and in vitro. PLoS One 9 (2014): e85112.
- Sovalat H, Scrofani M, Eidenschenk A, et al. Identification and isolation from either adult human bone marrow or GCSF-mobilized peripheral blood of CD34(+)/CD133(+)/CXCR4(+)/ Lin(-)CD45(-) cells, featuring morphological, molecular, and phenotypic characteristics of very small embryonic-like (VSEL) stem cells. Exp Hematol 39 (2011): 495-505.
- Ning H, Lin G, Lue TF, et al. Mesenchymal Stem Cell Marker Stro-1 is a 75kd Endothelial Antigen. Biochem Biophys Res Commun 413 (2011): 353-357.
- Young HE, Lochner F, Lochner D, et al. Primitive Stem Cells in Adult Human Peripheral Blood. Stem Cells Regen Med 1 (2017): 1-8.
- Baer PC, Geiger H. Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem cells international 2012 (2012).
- McConnell S, Kolopack P, Davis AM. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): a review of its utility and measurement properties. Arthritis Care & Research: Official Journal of the American College of Rheumatology 45 (2001): 453-461.
WOMAC Osteoarthritis Index.http://www.womac.org/womac/index.htm