Squamous Cell Skin Carcinoma in Systemic Lupus Erythematosus: Case Report and Literature Review
Article Information
Topolyanskaya SV*
I.M. Sechenov First Moscow State Medical University (Sechenov University), RF Health Ministry, Hospital Therapy Department No.2; Moscow, Russia
*Corresponding Author: Svetlana Topolyanskaya, I.M. Sechenov First Moscow State Medical University (Sechenov University), RF Health Ministry, Hospital Therapy Department No.2; 8-2, Trubetskaya str., Moscow, 119991, Russia
Received: 25 August 2020; Accepted: 21 September 2020; Published: 18 December 2020
Citation: Svetlana Topolyanskaya. Squamous Cell Skin Carcinoma in Systemic Lupus Erythematosus: Case Report and Literature Review. Fortune Journal of Rheumatology 2 (2020): 107-117.
View / Download Pdf Share at FacebookAbstract
The case report of squamous cell skin carcinoma diagnosed in a patient with systemic lupus erythematosus 26 years after the onset of rheumatic disease is presented. The features of this case included the absence of skin manifestations of systemic lupus erythematosus, the occurrence of a tumor at the site of ulcers and trophic disorders on the leg, a long period from the ulcer occurrence to the diagnosis of skin cancer (despite multiple biopsies and consultations of various specialists), as well as the occurrence of a cytokine release syndrome, which directly led to the death of the patient after the first use of the immune checkpoint inhibitors. Possible causes of skin cancer in patients with systemic lupus erythematosus, as well as the features of the cytokine release syndrome after immunotherapy for oncological diseases, are discussed.
Keywords
Systemic lupus erythematosus, Squamous cell carcinoma, Skin, Ulcers, Cytokine release syndrome, Immune checkpoint inhibitors
Systemic lupus erythematosus articles Systemic lupus erythematosus Research articles Systemic lupus erythematosus review articles Systemic lupus erythematosus PubMed articles Systemic lupus erythematosus PubMed Central articles Systemic lupus erythematosus 2023 articles Systemic lupus erythematosus 2024 articles Systemic lupus erythematosus Scopus articles Systemic lupus erythematosus impact factor journals Systemic lupus erythematosus Scopus journals Systemic lupus erythematosus PubMed journals Systemic lupus erythematosus medical journals Systemic lupus erythematosus free journals Systemic lupus erythematosus best journals Systemic lupus erythematosus top journals Systemic lupus erythematosus free medical journals Systemic lupus erythematosus famous journals Systemic lupus erythematosus Google Scholar indexed journals Squamous cell carcinoma articles Squamous cell carcinoma Research articles Squamous cell carcinoma review articles Squamous cell carcinoma PubMed articles Squamous cell carcinoma PubMed Central articles Squamous cell carcinoma 2023 articles Squamous cell carcinoma 2024 articles Squamous cell carcinoma Scopus articles Squamous cell carcinoma impact factor journals Squamous cell carcinoma Scopus journals Squamous cell carcinoma PubMed journals Squamous cell carcinoma medical journals Squamous cell carcinoma free journals Squamous cell carcinoma best journals Squamous cell carcinoma top journals Squamous cell carcinoma free medical journals Squamous cell carcinoma famous journals Squamous cell carcinoma Google Scholar indexed journals Skin articles Skin Research articles Skin review articles Skin PubMed articles Skin PubMed Central articles Skin 2023 articles Skin 2024 articles Skin Scopus articles Skin impact factor journals Skin Scopus journals Skin PubMed journals Skin medical journals Skin free journals Skin best journals Skin top journals Skin free medical journals Skin famous journals Skin Google Scholar indexed journals Ulcers articles Ulcers Research articles Ulcers review articles Ulcers PubMed articles Ulcers PubMed Central articles Ulcers 2023 articles Ulcers 2024 articles Ulcers Scopus articles Ulcers impact factor journals Ulcers Scopus journals Ulcers PubMed journals Ulcers medical journals Ulcers free journals Ulcers best journals Ulcers top journals Ulcers free medical journals Ulcers famous journals Ulcers Google Scholar indexed journals Cytokine release syndrome articles Cytokine release syndrome Research articles Cytokine release syndrome review articles Cytokine release syndrome PubMed articles Cytokine release syndrome PubMed Central articles Cytokine release syndrome 2023 articles Cytokine release syndrome 2024 articles Cytokine release syndrome Scopus articles Cytokine release syndrome impact factor journals Cytokine release syndrome Scopus journals Cytokine release syndrome PubMed journals Cytokine release syndrome medical journals Cytokine release syndrome free journals Cytokine release syndrome best journals Cytokine release syndrome top journals Cytokine release syndrome free medical journals Cytokine release syndrome famous journals Cytokine release syndrome Google Scholar indexed journals Immune checkpoint inhibitors articles Immune checkpoint inhibitors Research articles Immune checkpoint inhibitors review articles Immune checkpoint inhibitors PubMed articles Immune checkpoint inhibitors PubMed Central articles Immune checkpoint inhibitors 2023 articles Immune checkpoint inhibitors 2024 articles Immune checkpoint inhibitors Scopus articles Immune checkpoint inhibitors impact factor journals Immune checkpoint inhibitors Scopus journals Immune checkpoint inhibitors PubMed journals Immune checkpoint inhibitors medical journals Immune checkpoint inhibitors free journals Immune checkpoint inhibitors best journals Immune checkpoint inhibitors top journals Immune checkpoint inhibitors free medical journals Immune checkpoint inhibitors famous journals Immune checkpoint inhibitors Google Scholar indexed journals autoimmune rheumatic diseases articles autoimmune rheumatic diseases Research articles autoimmune rheumatic diseases review articles autoimmune rheumatic diseases PubMed articles autoimmune rheumatic diseases PubMed Central articles autoimmune rheumatic diseases 2023 articles autoimmune rheumatic diseases 2024 articles autoimmune rheumatic diseases Scopus articles autoimmune rheumatic diseases impact factor journals autoimmune rheumatic diseases Scopus journals autoimmune rheumatic diseases PubMed journals autoimmune rheumatic diseases medical journals autoimmune rheumatic diseases free journals autoimmune rheumatic diseases best journals autoimmune rheumatic diseases top journals autoimmune rheumatic diseases free medical journals autoimmune rheumatic diseases famous journals autoimmune rheumatic diseases Google Scholar indexed journals systemic glucocorticoids articles systemic glucocorticoids Research articles systemic glucocorticoids review articles systemic glucocorticoids PubMed articles systemic glucocorticoids PubMed Central articles systemic glucocorticoids 2023 articles systemic glucocorticoids 2024 articles systemic glucocorticoids Scopus articles systemic glucocorticoids impact factor journals systemic glucocorticoids Scopus journals systemic glucocorticoids PubMed journals systemic glucocorticoids medical journals systemic glucocorticoids free journals systemic glucocorticoids best journals systemic glucocorticoids top journals systemic glucocorticoids free medical journals systemic glucocorticoids famous journals systemic glucocorticoids Google Scholar indexed journals cytokine articles cytokine Research articles cytokine review articles cytokine PubMed articles cytokine PubMed Central articles cytokine 2023 articles cytokine 2024 articles cytokine Scopus articles cytokine impact factor journals cytokine Scopus journals cytokine PubMed journals cytokine medical journals cytokine free journals cytokine best journals cytokine top journals cytokine free medical journals cytokine famous journals cytokine Google Scholar indexed journals oncological diseases articles oncological diseases Research articles oncological diseases review articles oncological diseases PubMed articles oncological diseases PubMed Central articles oncological diseases 2023 articles oncological diseases 2024 articles oncological diseases Scopus articles oncological diseases impact factor journals oncological diseases Scopus journals oncological diseases PubMed journals oncological diseases medical journals oncological diseases free journals oncological diseases best journals oncological diseases top journals oncological diseases free medical journals oncological diseases famous journals oncological diseases Google Scholar indexed journals
Article Details
1. Introduction
Systemic lupus erythematosus (SLE) is one of the most common autoimmune rheumatic diseases. With this disease, many organs and systems are involved in the pathological process, and its main manifestations include damage to the kidneys, nervous system, cardiovascular system, skin, mucous membranes and serous membranes. SLE therapy includes the mandatory use of systemic glucocorticoids, both as monotherapy and in combination with other immunosuppressants. Despite the significant increase in life expectancy in patients with SLE, the likelihood of death in this disease is still two to five times higher than in the general population. Mortality in SLE is caused not only by the manifestations and complications of the underlying disease, but also by other pathologies, including cancer. In the last decade, a number of cohort studies and meta- analyzes have been carried out to investigate the relationship between SLE and malignant neoplasms, but their results have been quite contradictory [1-4]. Thus, it has been convincingly shown that patients with SLE significantly increase the risk of onco-hematological diseases (especially non-Hodgkin's lymphomas), as well as neoplasms of the reproductive system in women. As for skin cancer, there are few data on this pathology, and they differ; it was found, for example, that the presence of SLE does not increase, but reduces the risk of skin melanoma [1-3]. In our observation, squamous cell skin cancer was diagnosed in a 48-year-old woman
26 years after the onset of systemic lupus erythematosus.
2. Case Report
Patient I., 48 years old, was observed for a long time at the Institute of Rheumatology and at the Department of Hospital Therapy of the First Moscow State Medical University with a diagnosis of Systemic Lupus Erythematosus.
2.1 Medical history
Onset of SLE at 22 years old with the onset of arthritis of the metatarsophalangeal joint. Then migrating arthralgias joined in almost all groups of joints and “flying” arthritis of small joints of the hands, increased hair loss was noted. Subsequently, the appearance of fever, proteinuria, hematuria, edema of the lower extremities and shortness of breath were noted. She was hospitalized at the Institute of Rheumatology, where she was diagnosed with Systemic lupus erythematosus: glomerulonephritis, central nervous system damage, enanthema, leukopenia, immunological disorders, and therapy with glucocorticoids per os in combination with pulse therapy with methylprednisolone and cyclophosphamide was started, plasmapheresis sessions were occasionally carried out. In the next 10 years, the main manifestation of SLE was lupus nephritis. Pulse therapy with glucocorticoids and cyclophosphamide was carried out periodically, as well as intramuscular injections of cyclophosphamide with frequent withdrawal due to poor tolerance. Given the lack of efficacy and poor tolerance of cyclophosphamide, the drug was canceled and other immunosuppressants, cyclosporin A and mycophenolate mofetil, were sequentially prescribed. The patient's state of health remained stable, there were no other signs of SLE activity. Proteinuria (2-3 g/day) and high titers of antinuclear antibodies remained in the analyses. The last exacerbation of SLE 15 years ago, when the patient developed clinical manifestations of nephrotic syndrome, and the level of proteinuria increased to 7.42 g/day. Pulse therapy with glucocorticoids and cyclophosphamide was again carried out, with a good effect. In subsequent years, the patient was not regularly observed, took maintenance doses of methylprednisolone (8 mg/day), occasionally - azathioprine in low doses. Proteinuria decreased to a trace, periodically protein in the urine was not detected at all; there were no changes in urinary sediment. Nitrogen excretory function of the kidneys was relatively preserved, the creatinine level increased to a maximum of 120 μmol/L, the glomerular filtration rate corresponded to chronic kidney disease 2-3a stage. There were no other manifestations of SLE activity. There was a slight increase in blood pressure, dizziness, flies and double vision, and occasionally a short-term narrowing of the visual fields. In addition, the patient was worried about the increased formation of ecchymosis, and with minor trauma, long non-healing wounds appeared on the anterior surface of the legs. Hyperpigmentation and slight induration of the skin of the anterior surface of the legs progressed. When performing Doppler ultrasound of the vessels of the lower extremities, no significant changes in the arteries and veins of the lower extremities were detected.
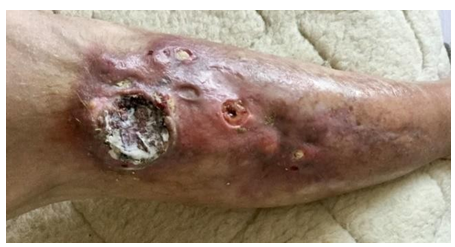
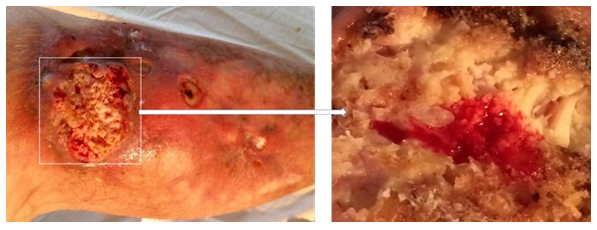
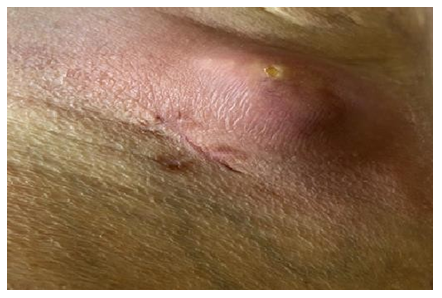
In August 2012, after minimal trauma, a small ulcerative defect appeared on the anterior surface of the upper third of the right leg. In the next 2 years, the size and depth of this ulcer increased. The patient was observed by surgeons at the place of residence, local therapy was prescribed, without effect. Histological examination presumably revealed basal cell skin cancer cells, however, with repeated biopsies (including at the Moscow Oncological Institute), no data for a malignant neoplasm of the skin was obtained. She was repeatedly consulted at the Department of Dermatology and Dermato-oncology, the diagnosis of skin cancer was rejected. Local therapy was continued, the effect of which was not observed. Small ulcers appeared around the main ulcerative defect (Figure 1), as well as caseous-like discharge (Figure 2).
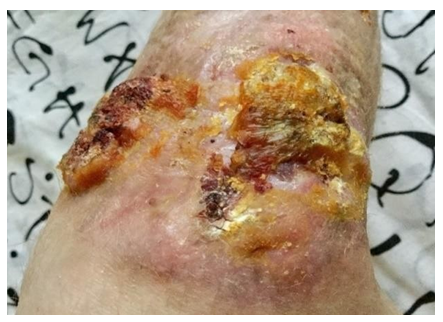
She was consulted again at the Department of Wounds and Wound Infection of the Institute of Surgery. No data were obtained for an active purulent process; additional examination is recommended (exclusion of skin tuberculosis, deep mycoses, pyoderma gangrenosum). In the following months, a comprehensive examination was carried out, the patient was repeatedly consulted by various specialists (from a vascular surgeon to a mycologist), including leading specialists in the field of tuberculosis, mycology and skin vasculitis. The patient was repeatedly hospitalized in leading clinics in Moscow and St. Petersburg, but the exact diagnosis was never made. Only local symptomatic therapy was carried out, without a noticeable effect. For several years, a biopsy of a skin flap and subcutaneous tissue was taken from the patient more than 10 times; data for cancer were not revealed. Morphological changes were regarded as nonspecific and possibly the outcome of chronic inflammation: “The epidermis is moderately hyperplastic, with pronounced vacuolar degeneration of cells of all layers. Dermis with pronounced eosinophilia and homogenization (dermal hyalinosis). The vessels are dilated, in the perivascular spaces there are single lymphocytes, plasma cells and histiocytes. " In the last year, the patient was observed in the department of purulent surgery, periodically excision of the edges of the ulcer and its revision was performed. After each surgical intervention, a histological examination was carried out; data for the neoplasm were not obtained. As a result of surgical interventions, the size of the ulcers decreased, there was no noticeable discharge (Figure 3).
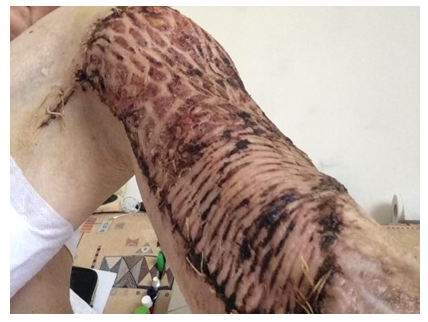
However, during the next histological examination after excision of the ulcer (07.2018), signs of neoplasm were revealed: “At the level of the epidermis and dermis, abnormal layers of squamous epithelium with high differentiation, with areas of hyperkeratosis, foci of necrosis are determined.” When revising the results of histological examination at the Moscow Oncological Research Institute: “Areas of the skin with overgrowths of highly differentiated squamous cell skin cancer with infiltration of the dermis up to all edges of the sites.” Diagnosed with verrucous form of squamous cell keratinizing skin cancer. Results of MRI of the right tibia (08.2018): “The skin of the antero-outer surface of the upper third of the right tibia is deformed over an area of 7 × 8 cm, a solid tissue is determined in its thickness, which forms an exophytic node measuring 6.5 × 5.0 × 1.5 cm. On the inner surface of the distal biceps muscles of the thigh is determined by a node 1.0 × 1.5 × 1.0 cm. A node with a diameter of 1.2 cm is also determined in the subcutaneous fatty tissue of the anterior surface of the right leg. In September 2018, at National Medical Research Center of Oncology, excision of the tumor with a reconstructive-plastic component was performed. (Figure 4) Results of intraoperative biopsy: 7.5 × 6 cm, at a distance of 1 cm from it - the 2nd tumor node (4.5 × 3 cm), and 5 cm from it - the second plaque-like formation (2, ×1.5 cm). Microscopic preparations: tumor nodes have the structure of a moderately differentiated keratinizing squamous cell carcinoma, growing into the subcutaneous fatty tissue and underlying fibrous tissue.” 3 months after tumor resection, metastases of malignant neoplasm were found in the iliac lymph nodes on the right (Figure 5) The diagnosis was “Squamous cell skin cancer T3N3M0”. Given the “borderline” operability and the complex localization of metastatic lesions of the iliac lymph nodes, initial chemotherapy (paclitaxel in combination with carboplatin) is recommended. However, against the background of chemotherapy, further progression of metastases was noted; in addition, poor tolerance to this chemotherapy regimen was observed. PET-CT data (04.2019, 9 months after the diagnosis of “squamous cell carcinoma”): “In the region of the right inguinal canal, a single conglomerate of external iliac and inguinal lymph nodes with dimensions up to 69 × 52.5 × 76 mm is determined, with necrotic changes in the central section and with hyperfixation radiopharmaceutical. Also, lymph nodes are determined along the external, internal and common iliac arteries 8.5 mm in size. In the soft tissues of the lower third of the right thigh, a pathological formation measuring 49 × 46 × 52 mm is determined, with necrotic changes and hyperfixation of the radiopharmaceutical, similar to a conglomerate in the iliac region. Conclusion: a picture of a pathological conglomerate of lymph nodes in the soft tissues of the lower third of the right thigh, similar to the structure of a necrotic conglomerate of the right external iliac and inguinal lymph nodes, most likely of metastatic origin”.
In the Russian Scientific Center for Radiology, an operation was performed to remove the inguinal lymph nodes; during the operation, a necrotic altered conglomerate of lymph nodes was found without a clear border with the surrounding tissues and dissemination. After the operation, a second course of chemotherapy was carried out (carboplatin in combination with 5- fluorouracil), without effect (disease progression). This type of tumor is refractory to both chemotherapy and radiation therapy, so no further chemotherapy attempts have been made. In October 2019, a single infusion of atezolizumab (anti-PD-L1 monoclonal antibody) in combination with dexamethasone was performed as a “despair therapy”. A day after the introduction of atezolizumab, hectic fever (up to 40 degrees), chills, myalgia, a sharp decrease in blood pressure, confusion occurred. Blood tests showed an increase in ESR up to 51 mm/h, leukocytes up to 16 × 109/l, a decrease in hemoglobin levels up to 100 g/l, an increase in C- reactive protein from 28.2 to 120.9 mg/l, an increase in creatinine levels (up to 111 , 6 μmol/l) and urea (up to 11.8 mmol/l), uric acid (up to 539 mmol/l), triglycerides (up to 3.2 mmol/l), potassium (up to 5.53 mmol/l) , gamma-glutamyl transpeptidase (up to 120 U/l); no pathology was revealed in urine analyzes. Against the background of progressive multiple organ failure, a lethal outcome occurred. No autopsy was performed on the patient.
4. Discussion
Squamous cell carcinoma of the skin is a malignant tumor originating from the cells of the epidermis of the skin and/or hair follicles. This is the second most common neoplasm (after basal cell carcinoma) in the group of non-melanoma skin tumors [5]. Risk factors for the development of squamous cell skin cancer include advanced age, exposure to ultraviolet radiation, a certain (light) skin phototype, and immunodeficiency states [2, 5]. One of the features of squamous cell skin cancer is that this tumor is the most common neoplasm that occurs at the site of a long-standing scar or a long- lasting wound; this form of squamous cell carcinoma has a worse prognosis and often recurs after treatment [6]. In our observation, factors that can provoke the onset and progression of skin cancer include long-term immunosuppression while taking various immunosuppressants, the presence of an autoimmune disease with pathological features of the immune system, a long-term non-healing (more than 6 years) ulcerative defect on the anterior surface of the leg, and before that - multiple recurrent wounds on the legs after minimal trauma. Light skin phototype can be considered an additional factor. It is worth noting, however, that due to a long history of SLE, the patient avoided excessive exposure to ultraviolet radiation, so the role of this factor can be excluded. Moreover, the neoplasm appeared in the upper third of the lower leg, in the area that is constantly covered with clothing (trousers).
One of the key features of the described clinical observation was a long (over 26 years) history of systemic lupus erythematosus. According to the literature, skin cancer is a rare but severe complication of SLE [7]. The pathology of the immune system present in SLE and its dysregulation can prevent the removal of tumor cells and, ultimately, increase the risk of neoplasms [1, 2, 7]. Long-term persistent inflammation in patients with lupus skin lesions is considered to be another potential risk factor for the development of skin cancer [7] This type of skin syndrome is characterized by the accumulation of T- regulatory lymphocytes, mast cells, macrophages, as well as a significant increase in the level of transforming growth factor-β1 and interleukin-6, which stimulate carcinogenesis. It is believed that pro- oncogenic immune cells and cytokines in patients with lupus are able to overcome the tumor suppressing effects of Th1 lymphocytes and stimulate the development of skin cancer [7].
It should be emphasized that our patient did not have any manifestations of the skin syndrome caused by SLE throughout the course of the disease. At the same time, in the available literary sources, as a rule, there are descriptions of cases or a series of cases of skin cancer in patients who already have lupus skin lesions in the form of discoid or subacute cutaneous lupus. Based on these data, it is believed that squamous cell carcinoma of the skin is recorded in approximately 2.3-3.3% of patients with discoid lupus. Squamous cell carcinoma of the skin that occurs in patients with discoid lupus is generally more aggressive, as evidenced by the increased rates of recurrence, metastasis, and mortality, compared with other forms of sporadic skin cancer [6- 8].
The interval between the onset of lupus and squamous cell carcinoma of the skin usually varies from 4 to 20 years [6]. The factors that increase the risk of squamous cell skin cancer in lupus are age over 40, male sex, exposure to ultraviolet radiation, skin pigmentation, and chronic inflammation [6]. In our observation, skin cancer was diagnosed in a 48-year-old patient 26 years after the onset of SLE. At the same time, there was a pronounced hyperpigmentation of the skin of the legs, especially on the front surface, and long-term non- healing ulcers on the legs.
Our observation confirms the literature data on the unfavorable prognosis of squamous cell skin cancer arising at the site of a scar or long-term non-healing ulcer. Thus, according to French researchers who have studied the transformation of ulcers on the lower extremities in 80 elderly patients, ulcerative defects usually long (at least 3 years) precede the onset of skin cancer (as in the case of our patient) [9]. Virtually all patients in this study had squamous cell carcinoma of the skin, and findings unusual for "vascular" ulcers included abnormal granulation, abnormal vegetation, lack of healing, and unusual ulcer localization; all these signs were in our patient. Every third patient in this cohort died (against the background of metastasis) and the main cause of death was late diagnosis of the neoplasm. 57% of patients underwent amputation of the lower extremities [9], but our patient categorically refused this intervention.
Immunosuppressive drugs used to treat SLE (cyclosporin A, mycophenolate mycophenolate, tacrolimus and azathioprine) can also promote the development of squamous cell skin cancer by suppressing the anti-tumor immune response in the skin [7, 10]. As noted earlier, our patient, due to the refractory course of lupus nephritis, received cyclosporine A, mycophenolate mofetil, azathioprine, and cyclophosphamide, but over the past 15 years she took only maintenance doses of glucocorticoids, occasionally azathioprine.
Another feature of our patient can be considered the rapid development of death a few days after the introduction of the drug atezolizumab. Atezolizumab is a monoclonal antibody (IgG1) that directly binds to PD- L1 and belongs to a group of modern anti-tumor drugs called check-point inhibitors. The mechanism of action of these drugs is aimed at restoring the normal antitumor immune response by blocking inhibitory receptors of T-lymphocytes, the so-called key points of immunity (in particular, programmed cell death protein (PD-1) and its ligands PD-L1 and PD-L2), allowing tumor cells "escape" from immunological surveillance. By blocking the signaling pathway of the checkpoints of the immune response PD-1 / PD-L1, an increase in the antitumor immune response, restoration of the activity of cytotoxic T-lymphocytes, and a decrease in the number and activity of T-suppressors are achieved.
In recent years, a good efficacy of immune checkpoint inhibitors has been demonstrated in the treatment of various cancers. However, by inhibiting a number of links of the immune system, this class of drugs enhances not only the immune activity against cancer cells, but also against unchanged cells of various organs and systems, which leads to a number of immune-mediated undesirable phenomena. The most severe side effect is cytokine release syndrome, which is a systemic inflammatory disease characterized by massive cytokine release [16]. It can present with a variety of symptoms, from mild to life-threatening and sometimes fatal. Mild manifestations of cytokine release syndrome include fever, general weakness and malaise, nausea, vomiting, headache, rash, arthralgia, and myalgia. More severe cases are characterized by very high fever, arterial hypotension, requiring high doses of vasopressor drugs, and can lead to an uncontrolled systemic inflammatory response with the development of shock, disseminated intravascular coagulation syndrome and multiple organ failure [16-17]. In the syndrome of cytokine release, various laboratory disorders are often recorded, in particular, cytopenia, coagulopathy, increased levels of liver enzymes and creatinine, as well as high levels of C-reactive protein [16-17].
The term "cytokine release syndrome" was first coined in the early 1990s, when antibodies to T lymphocytes were used as an immunosuppressive drug. Subsequently, this syndrome was described after the use of various monoclonal antibodies (eg, rituximab), some chemotherapeutic agents and drugs for immunotherapy, including inhibitors of immune checkpoints [17]. A cytokine "storm" caused by massive stimulation of T- lymphocytes can also develop in severe viral infections, including novel coronavirus and influenza.
The pathogenesis of cytokine release syndrome is primarily based on the activation of T-lymphocytes, leading to an increase in the release of interferon gamma and tumor necrosis factor-alpha. The consequence of this is the activation of macrophages, dendritic cells, and other immune and endothelial cells, additionally releasing pro-inflammatory cytokines. It is important that macrophages and endothelial cells produce large amounts of interleukin-6, which activates T-lymphocytes and other immune cells through a positive feedback mechanism, which, in turn, leads to a cytokine “storm” [17].
The largest series of cases of cytokine release syndrome during treatment with checkpoint inhibitors (including atezolizumab) included 58 patients; the results were published in May 2020 [16]. In this series, the cytokine release syndrome occurred 1-18 weeks after the initiation of therapy with inhibitors of immune checkpoints and was fatal only in 2 cases. According to the authors of this article, the following clinical manifestations of the syndrome of cytokine release were most often recorded: constitutional symptoms (general weakness, fatigue, asthenia, fever (most often), arthralgia, myalgia; skin rash; gastrointestinal tract pathology (nausea, diarrhea); respiratory tract damage (pulmonary edema, ARDS, respiratory failure, pleural effusion, hypoxia); cardiovascular pathology (tachycardia, arterial hypotension); nephropathy (acute renal injury, nephritis); neurological symptoms (headaches, tremors) [16].
In the patient we described, among the signs of the cytokine release syndrome, hectic fever with chills, severe arterial hypotension requiring the administration of vasopressor drugs, tachycardia that was unresponsive to standard β-blockers, severe damage to the central nervous system, a marked increase in the level of C- reactive protein, and others manifestations (Figure 6). In our observation, in contrast to other descriptions of the cytokine release syndrome or cytokine "storm", no cytopenia was noted. However, in the above-described cohort of patients receiving checkpoint inhibitors of the immune response, cytopenia was also recorded extremely rarely - 1 case of anemia, leukopenia and lymphopenia, 2 cases of thrombocytopenia and neutropenia [16].
The management of patients with cytokine release syndrome depends on the severity of this pathological condition. The presence of high fever and a significant increase in C-reactive protein has been suggested as routine prognostic markers of this syndrome (especially when it is impossible to determine the level of interleukin-6, subpopulations of T-lymphocytes and other immune cells) [17]. In mild manifestations of the cytokine release syndrome, only symptomatic therapy (in particular, antihistamines and antipyretic drugs) is used [17].
Patients with a severe course of this syndrome are advised to immediately administer monoclonal antibodies to interleukin-6 (for example, tocilizumab). Glucocorticoids in such cases should not be prescribed as first-line drugs; it is advisable to use them in patients with ineffectiveness of monoclonal antibodies to interleukin-6, as well as with severe damage to the central nervous system. If antibodies to interleukin-6 and glucocorticoids are ineffective, therapy with monoclonal antibodies to tumor necrosis factor-α, to interleukin-1 or the use of immunoadsorption is possible [17]. Unfortunately, our patient did not have the possibility of using the above methods of treating severe cytokine release syndrome (with the exception of glucocorticoids); the patient died on the background of increasing multiple organ failure.
5. Conclusions
Thus, the main features of the described case are as follows:
- The appearance of squamous cell skin cancer 26 years after the onset of systemic lupus erythematosus in a patient who has never had any skin manifestations of SLE.
- The presence of such a risk factor for squamous cell skin cancer, as immunosuppression (associated with the presence of SLE, and with prolonged use of immunosuppressants).
- Development of squamous cell skin cancer at the site of a long-term non-healing (within 6 years) ulcer defect on the anterior surface of the upper third of the right
- Insufficient clarity (and up to the present time) of the etiology of an ulcer defect on the leg, despite numerous consultations in the leading clinical centers of the Russian Federation and many histological studies of the skin and subcutaneous tissue. The ulcerative defect was distinguished by a peculiar localization (the upper third of the anterior surface of the leg), pathological granulation and abnormal vegetation, as well as a lack of healing over the years, despite the ongoing
- Rapid progression of squamous cell skin cancer with the development of metastasis, despite the ongoing surgical and chemotherapy treatment, in contrast to the sporadic forms of this tumor, which responds well to
- The emergence of a probable cytokine release syndrome after the first injection of an inhibitor of immune checkpoints in a patient with autoimmune pathology (SLE).
Conflict of Interests
The author claims no conflict of interest.
References
- Song L, Wang Y, Zhang J, et al. The risks of cancer development in systemic lupus erythematosus (SLE) patients: a systematic review and metanalysis. Arthritis Research and Therapy 20 (2018):
- Cao L, Tong H, Xu G, et al. Systemic Lupus Erythematous and Malignancy Risk: A Meta- PLoS ONE 10 (201): e0122964.
- Björnadal L, Löfström B, Yin L, et al. Increase cancer incidence in a Swedish cohort of patients with systemic lupus Scand J Rheumatol 31(2002): 66-71.
- Chen YJ, Chang YT, Wang CB, et Malignancy in systemic lupus erythematosus: a nationwide cohort study in Taiwan. Am J Med 123 (2010): 1150-1156.
- Bottomley MJ, Thomson J, Harwood C, et al. The role of the immune system in cutaneous squamous cell Int J Mol Sci 20 (2019): 2009.
- Bhat MR, Hulmani M, Dandakeri S, et Disseminated discoid lupus erythematosus leading to squamous cell carcinoma. Indian J Dermatol 57 (2012): 158-161.
- Zaalberg A, Tuchayi SM, Ameri AH, et Chronic inflammation promotes skin carcinogenesis in cancer-prone discoid lupus erythematosus. J Invest Dermatol 139 (2019): 62-70.
- Gamble M, Tocci E, Venna S, et Pooly differentiated squamous cell carcinoma arising within a lesion of discoid lupus erythematosus in an African-American woman. JAAD Case Rep 1 (2015): 138-140.
- Combemale P, Bousquet M, Kanitakis J, et al. Angiodermatology group, French Society of Malignant transformation of leg ulcers: a retrospective study of 85 cases. J Eur Acad Dermatol Venereol 21 (2007): 935-941.
- Wu X, Nguyen BC, Dziunycz P, et Calcineurin and ATF3: opposite roles in squamous skin cancer. Nature 465 (2010): 368- 372.
- Ceschi A, Noseda R, Palin K, et al. Immune Checkpoint Inhibitor-Related Cytokine Release Syndrome: Analysis of WHO Global Pharmacovigilance Database. Front. Pharmacol 11 (2020):
- Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer 6 (2018):