Spontaneous Regression of Congenital Cholesteatoma: A Report of Two Cases with Differing Ossicle Involvement
Article Information
Yoshimasa Tsuchiya, Taichi Kan*, Mayuko Kishimoto, Yasue Uchida, Tetsuya Ogawa, Hiromi Ueda
Department of Otorhinolaryngology Aichi Medical University School of Medicine, 1-1 Yazakokarimata, Nagakute, Aichi, Japan
*Corresponding Author: Dr. Taichi Kan, Department of Otorhinolaryngology Aichi Medical University School of Medicine, 1-1 Yazakokarimata, Nagakute, Aichi, 4801195, Japan
Received: 27 Sep 2019; Accepted: 07 October 2019; Published: 29 November 2019
Citation: Yoshimasa Tsuchiya, Taichi Kan, Mayuko Kishimoto, Yasue Uchida, Tetsuya Ogawa, Hiromi Ueda. Spontaneous Regression of Congenital Cholesteatoma: A Report of Two Cases with Differing Ossicle Involvement. Archives of Clinical and Medical Case Reports 3 (2019): 683-691.
View / Download Pdf Share at FacebookAbstract
The mechanism of congenital cholesteatoma has not been clarified yet. Usually, middle ear cholesteatoma (congenital and acquired) is a progressive and destructive disease that requires surgery for complete cure. In recent years, early detection of congenital cholesteatomas has become more frequent due to advances in endoscopy and microscopy. Along with these developments, there have been reports of congenital cholesteatoma regressing spontaneously, as seen by tympanic and computed tomography findings over time. Herein, we report two cases of spontaneous regression of cholesteatoma, both with ossicular chain malformation. One cholesteatoma was close to the ossicles. In the other case, the cholesteatoma and ossicle were separated; however, the ossicular morphology was inconsistent with that of congenital ossicular malformation. Further similarities and differences between these two cases are discussed herein.
Keywords
Congenital cholesteatoma; Spontaneous regression/ reduction; Conductive hearing loss; Ossicular chain malformation
Congenital cholesteatoma articles, Spontaneous regression/ reduction articles, Conductive hearing loss articles, Ossicular chain malformation articles
Congenital cholesteatoma articles Congenital cholesteatoma Research articles Congenital cholesteatoma review articles Congenital cholesteatoma PubMed articles Congenital cholesteatoma PubMed Central articles Congenital cholesteatoma 2023 articles Congenital cholesteatoma 2024 articles Congenital cholesteatoma Scopus articles Congenital cholesteatoma impact factor journals Congenital cholesteatoma Scopus journals Congenital cholesteatoma PubMed journals Congenital cholesteatoma medical journals Congenital cholesteatoma free journals Congenital cholesteatoma best journals Congenital cholesteatoma top journals Congenital cholesteatoma free medical journals Congenital cholesteatoma famous journals Congenital cholesteatoma Google Scholar indexed journals cholesteatoma articles cholesteatoma Research articles cholesteatoma review articles cholesteatoma PubMed articles cholesteatoma PubMed Central articles cholesteatoma 2023 articles cholesteatoma 2024 articles cholesteatoma Scopus articles cholesteatoma impact factor journals cholesteatoma Scopus journals cholesteatoma PubMed journals cholesteatoma medical journals cholesteatoma free journals cholesteatoma best journals cholesteatoma top journals cholesteatoma free medical journals cholesteatoma famous journals cholesteatoma Google Scholar indexed journals Spontaneous regression articles Spontaneous regression Research articles Spontaneous regression review articles Spontaneous regression PubMed articles Spontaneous regression PubMed Central articles Spontaneous regression 2023 articles Spontaneous regression 2024 articles Spontaneous regression Scopus articles Spontaneous regression impact factor journals Spontaneous regression Scopus journals Spontaneous regression PubMed journals Spontaneous regression medical journals Spontaneous regression free journals Spontaneous regression best journals Spontaneous regression top journals Spontaneous regression free medical journals Spontaneous regression famous journals Spontaneous regression Google Scholar indexed journals reduction articles reduction Research articles reduction review articles reduction PubMed articles reduction PubMed Central articles reduction 2023 articles reduction 2024 articles reduction Scopus articles reduction impact factor journals reduction Scopus journals reduction PubMed journals reduction medical journals reduction free journals reduction best journals reduction top journals reduction free medical journals reduction famous journals reduction Google Scholar indexed journals Conductive hearing loss articles Conductive hearing loss Research articles Conductive hearing loss review articles Conductive hearing loss PubMed articles Conductive hearing loss PubMed Central articles Conductive hearing loss 2023 articles Conductive hearing loss 2024 articles Conductive hearing loss Scopus articles Conductive hearing loss impact factor journals Conductive hearing loss Scopus journals Conductive hearing loss PubMed journals Conductive hearing loss medical journals Conductive hearing loss free journals Conductive hearing loss best journals Conductive hearing loss top journals Conductive hearing loss free medical journals Conductive hearing loss famous journals Conductive hearing loss Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals Ossicular chain malformation articles Ossicular chain malformation Research articles Ossicular chain malformation review articles Ossicular chain malformation PubMed articles Ossicular chain malformation PubMed Central articles Ossicular chain malformation 2023 articles Ossicular chain malformation 2024 articles Ossicular chain malformation Scopus articles Ossicular chain malformation impact factor journals Ossicular chain malformation Scopus journals Ossicular chain malformation PubMed journals Ossicular chain malformation medical journals Ossicular chain malformation free journals Ossicular chain malformation best journals Ossicular chain malformation top journals Ossicular chain malformation free medical journals Ossicular chain malformation famous journals Ossicular chain malformation Google Scholar indexed journals chain malformation articles chain malformation Research articles chain malformation review articles chain malformation PubMed articles chain malformation PubMed Central articles chain malformation 2023 articles chain malformation 2024 articles chain malformation Scopus articles chain malformation impact factor journals chain malformation Scopus journals chain malformation PubMed journals chain malformation medical journals chain malformation free journals chain malformation best journals chain malformation top journals chain malformation free medical journals chain malformation famous journals chain malformation Google Scholar indexed journals CT- Computed tomography articles CT- Computed tomography Research articles CT- Computed tomography review articles CT- Computed tomography PubMed articles CT- Computed tomography PubMed Central articles CT- Computed tomography 2023 articles CT- Computed tomography 2024 articles CT- Computed tomography Scopus articles CT- Computed tomography impact factor journals CT- Computed tomography Scopus journals CT- Computed tomography PubMed journals CT- Computed tomography medical journals CT- Computed tomography free journals CT- Computed tomography best journals CT- Computed tomography top journals CT- Computed tomography free medical journals CT- Computed tomography famous journals CT- Computed tomography Google Scholar indexed journals patient articles patient Research articles patient review articles patient PubMed articles patient PubMed Central articles patient 2023 articles patient 2024 articles patient Scopus articles patient impact factor journals patient Scopus journals patient PubMed journals patient medical journals patient free journals patient best journals patient top journals patient free medical journals patient famous journals patient Google Scholar indexed journals
Article Details
Abbreviations:
CT- Computed tomography; OME- Otitis media with effusion
1. Introduction
In congenital cholesteatoma, it is thought that the cholesteatoma epithelium often pre-exists in the middle ear cavity from birth and is subsequently found in childhood. Epithelioma is derived from epidermis cells that have entered the embryonic period, and most epitheliomas are of a cystic form containing keratin (closed type), but there is also a membranous form of epithelioma that does not form a cyst (open type) [1-3]. Tos M. stated the incidence of congenital cholesteatoma is around 0.12 out of 100,000, and it is more common among boys [4]. In recent years, otolaryngologists and pediatricians alike have increased the awareness of this disease, and the number of cases diagnosed at a young age has increased due to the spread of improved screening and diagnostic techniques. In addition, there has been an increase in the number of cases diagnosed coincidentally from symptoms such as hearing loss and otitis media. Furthermore, there are reports that congenital cholesteatoma can spontaneously regress, identified by changes in tympanic and CT findings over time [5]. We examined if there is something with congenital cholesteatoma of spontaneous regression, and it can be used for follow-up. Herein we report two cases of spontaneously regressed cholesteatoma, each with different relationships between the cholesteatoma and ossicles.
2. Case Report
2.1 Case 1
Our first patient was a boy who presented with a history of progressive hearing loss in the left ear. Left hearing loss was first noted during a school checkup at the age of 6 years in 2008, after which he visited a nearby clinic. Pure-tone audiometry showed a conductive hearing loss (Figure 1).
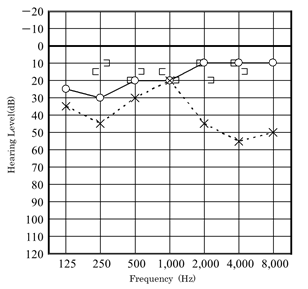
Figure 1: Hearing level at the 6 years old patient of Case 1, tested on his first visit to our hospital.
Magnetic resonance imaging showed no acoustic tumor. Since then, follow-up had been performed at the aforementioned nearby clinic. At the age of 7 years, in February 2010, the patient had no change in hearing loss and was referred to our hospital. Upon admission to our hospital, the eardrum findings were normal (Figure 2).
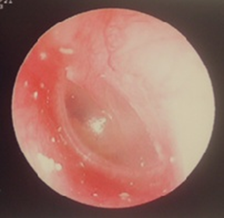
Figure 2: Eardrum condition of patient of Case 1 at the first presentation at our hospital.
Audiometry again showed left conductive hearing loss and acoustic reflex tests were bilaterally positive. At this time, the patient was asked to continue with follow-up at the nearby clinic. In August 2011, computed tomography (CT) at the nearby clinic showed small, soft shadows around the tympanic segment of the facial nerve and the stapes (Figure 3). In December 2011, pure-tone audiometry at the clinic showed deterioration (Figure 4).
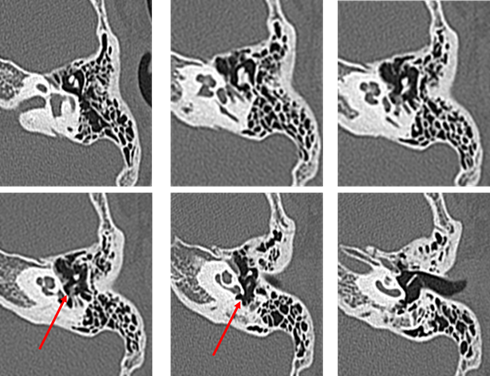
Figure 3: CT performed in August 2011 on the first patient at 8 years old. (The red arrow indicates the soft shadow suspected to be cholesteatoma).
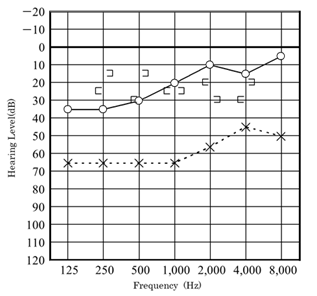
Figure 4: Hearing level of the first patient, tested in December 2011.
The patient visited our hospital again in January 2012 at the age of 9 years. Possible congenital cholesteatoma or ossicular chain malformation were considered, and the patient was scheduled for surgery. In July 2012, preoperative CT showed a reduction in the previously seen shadow (Figure 5); however, the hearing test showed no significant change. Left tympanoplasty was performed in August 2012.
During the operation, the mucosa of the tympanic cavity was normal, but an open type cholesteatoma was found from the tympanic segment of the facial nerve and the long process of the incus to the stapes. The long process of the incus was eroded and the anterior and posterior crura of the stapes were missing, and the stapes superstructure was separated from the footplate. The cholesteatoma was removed; however, because the cholesteatoma had invaginated into the lower surface of the tympanic segment of the facial nerve, we considered the possibility of remaining tumor cells and scheduled a staged operation. The postoperative diagnosis was Potsic classification Stage II congenital cholesteatoma (Figure 6) [6]. In 2013, left tympanoplasty was performed, and there was no subsequent recurrence of cholesteatoma. In August 2018, 5 years after the second operation, no recurrence was found by CT, and follow-up at our hospital was ended.
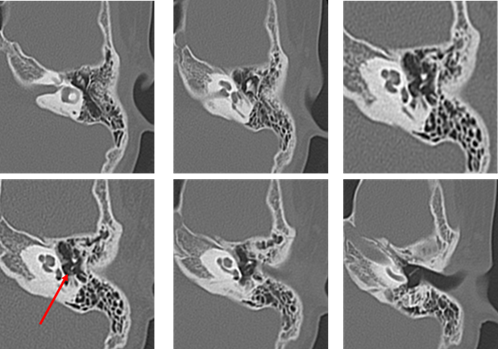
Figure 5: CT performed one month prior to surgery in July 2012 on the first patient at 9 years old. (The red arrow indicates the soft shadow suspected to be cholesteatoma. Compared to the first presentation, the shadow has decreased in size).
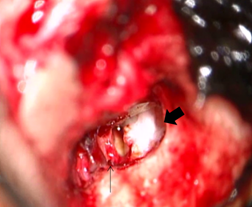
Figure 6: Intraoperative findings in the patient of Case 1. The thin arrow indicates the disfigurement of the crura of the stapes. The thick arrow indicates the open type cholesteatoma on the tympanic segment of the facial nerve.
2.2 Case 2
Our second was a boy diagnosed with hearing loss and left tympanic membrane abnormality at a medical checkup in April 2013. He visited a local doctor and was treated for left otitis media with effusion (OME). His left OME resolved, but there was no improvement in hearing, and the patient was thus referred to our hospital in August 2013. Examination revealed small perforations in the posterosuperior quadrant of the left eardrum (Figure 7). CT revealed a soft shadow from the epitympanum to the mastoid cavity, and the long process of the incus and the superstructure of the stapes appeared to be missing. However, CT showed discontinuity of these two lesions. Therefore, we suspected left pars tensa cholesteatoma or congenital cholesteatoma and ossicular chain malformation (Figure 8). In March 2014, preoperative CT showed a reduction in the previously seen soft shadow (Figure 9).
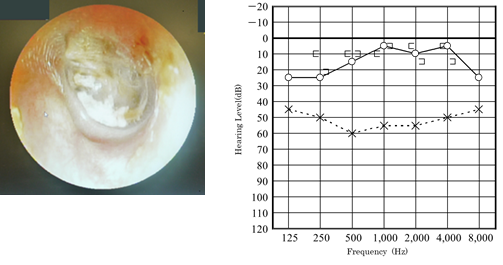
Figure 7: Eardrum condition and hearing level of the patient of Case 2 in April 2013.
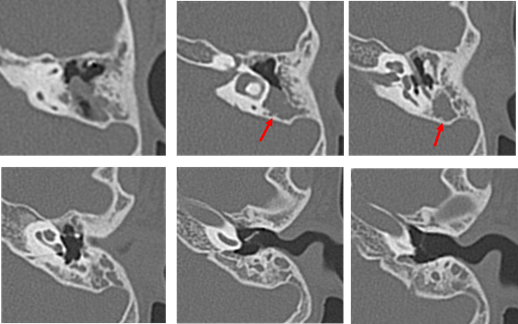
Figure 8: Computed tomography performed in August 2013 on the patient of Case 2 aged 7 years. The long process of the incus and stapes are missing. Suspected cholesteatoma from the soft shadow (indicated the red arrow) in the epitympanic attic and mastoid sinus. There are no suspicious findings around the ossicles.
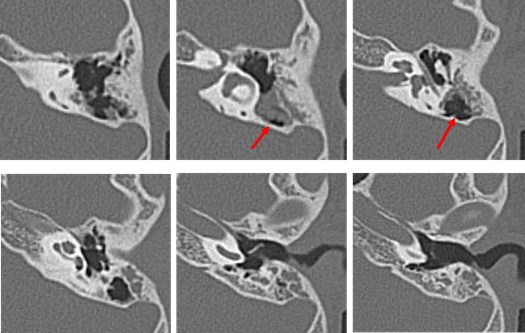
Figure 9: CT performed in April 2014 on the patient of Case 2 at 8 years old. There is no change in the findings around the ossicles. The soft shadow in the epitympanic attic and mastoid sinus (indicated by the red arrow) has decreased in size.
Additionally, the small perforations in the eardrum had closed. Surgery was performed in April 2014, and open type cholesteatoma and cholesterol granuloma were found in the attic and mastoid sinus, and the lesions were removed. The malleus was intact, the long process of the incus was defective, and the stapes were displaced backward in the structure, but no cholesteatoma was found around the ossicle. The cholesteatoma was evaluated as Potsic classification Stage II. At the time of this writing, the patient continues to follow up at the outpatient clinic (Figure 10).
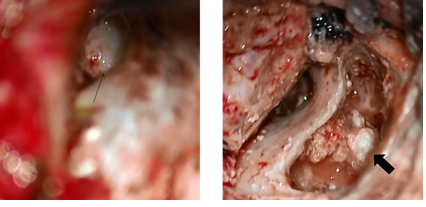
Figure 10: Intraoperative findings in the patient of Case 2.
The thin arrow indicates that there is no cholesteatoma in tympanic cavity. The long process of the incus and the superstructure of the stapes were disfigured. The thick arrow indicates the cholesteatoma in the mastoid cavity.
3. Discussion
There have been reports on the spontaneous regression of congenital cholesteatomas [6]. There are several possibilities for the mechanism of spontaneous regression or reduction of congenital cholesteatoma. First, because closed type cholesteatoma may spontaneously morph to an open type, and the eustachian tube function of most congenital cholesteatoma patients is normal, debris excreted from the eustachian tube may move slowly, and the cholesteatoma may disappear. Second, ectodermal tissue improperly in the embryonic stage may resolve through apoptosis [7]. In early-childhood congenital cholesteatoma, the reasons for spontaneous regression are not clear, and the current standard of care for cholesteatoma remains surgical treatment. However, in infants and children, the eustachian tube function, the middle ear and the surrounding bone are still developing, and it may be difficult to predict changes in the external auditory canal and middle ear morphology several years after surgery. They may also have difficulty cooperating with postoperative procedures, which may be a reason for undesirable effects on surgical outcomes. Therefore, if the characteristics and cause of spontaneously regressed congenital cholesteatoma are clarified, it may be possible to select surgery after waiting for physical and mental growth.
In these two cases reported here, we confirmed that cholesteatoma spontaneously regressed over time. In Case 1, the patient’s hearing loss deteriorated gradually and there was a reduction in the soft shadow seen by CT; however, the possibility of congenital cholesteatoma was high, and thus surgery was performed. During the operation, open type cholesteatoma was found around the stapes, and both crura were missing. This ossicular malformation was clearly attributed to cholesteatoma. In Case 2, small perforations were observed in the eardrum at first and, after CT, we suspected pars tensa cholesteatoma that had progressed to the mastoid cavity. However, the mastoid cavity lesion appeared to be separated from the ossicular defect, which was thought to be the cause of hearing loss. During surgery, the separation between the cholesteatoma and the defect was confirmed. Interestingly, the ossicle abnormalities were different from the congenital ossicular malformations that we usually experience, and thus we suspected that there had initially been a cholesteatoma surrounding the ossicles that had spontaneously regressed, leaving behind the ossicular transection. The differences between the two cases are 1. the locations of the cholesteatomas, 2. the separation between the cholesteatoma and the ossicle defect in case 2, and 3. the sizes of the cholesteatomas. Accordingly, the common points of these two cases are as follows: 1) the cholesteatomas were both open type, 2) the long process of the incus was damaged and 3) the crura of the stapes were disconnected from the footplate in both patients. In the report of two cases by Kodama et al., one of them CT showed the soft shadow suspected congenital cholesteatoma around the incudostapedial joint. It was operated on, and the cholesteatoma had regressed completely, so the type was unknown, but the long process of the incus was damaged.
If preoperative exams demonstrate regression of cholesteatoma, and there is no infection, bone destruction, impairment to ventilation in the middle ear, or deteoration of hearing loss, watchful waiting may be indicated. There were similarities and differences between our two cases. Future retrospective analyses should be done on the spontaneous regression of congenital cholesteatoma to identify independent factors that indicate “watchful waiting” for these patients.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Conflicts of Interest
There are no conflicts of interest to declare.
References
- Aimi K. Role of the tympanic ring in the pathogenesis o congenital cholesteatoma. Laryngoscope 93 (1983): 1140-1146.
- Michaels L. An epidermoid formation in the developing middle ear; possible source of cholesteatoma. Journal of Otolaryngology-Head and Neck Surgery 15 (1986): 169-174.
- Michaels L. Origin of congenital cholesteatoma from a normally occurring epidermoid rest in the developing middle ear. International Journal of Pediatric Otorhinolaryngology 15 (1988): 51-
- Tos M. A new pathogenesis of mesotympanic (congenital) cholesteatoma. Laryngoscope 110 (2000): 1890-
- Kodama K, Hara M, Matsuzawa S, et al. Two cases of spontaneous regression of congenital cholesteatoma. International Journal of Pediatric Otorhinolaryngology 76 (2012): 142-
- Potsic WP, Samadi DS, Marsh RR, et al. A staging system for congenital cholesteatoma. Archives of Otolaryngology-Head & Neck Surgery128 (2002): 1009-
- Kojima H, Miyazaki H, Shiwa M, et al. Molecular Biological Diagnosis of Congenital and Acquired Cholesteatoma on the Basis of Differences in Telomere Length. Laryngoscope 111 (2001): 867-
