Spinal Intramedullary Ewing Sarcoma with Meningeal Carcinomatosis: A Complete Response to Chemotherapy and Neuroaxis Irradiation. Case Report and Review of Literature
Article Information
Víctor Albarrán1*, María Luisa Villamayor1, Ignacio Ruz2, Jesús Chamorro1, Diana Isabel Rosero1, Javier Pozas1, María San Román1, Pablo Álvarez Ballesteros1, María Ángeles Vaz1
1Medical Oncology Department, Ramon y Cajal University Hospital (Madrid), Spain
2Anatomical Pathology Department, Ramon y Cajal University Hospital (Madrid), Spain
*Corresponding Author: Víctor Albarrán, Medical Oncology Department, Ramon y Cajal University Hospital (Madrid), Spain.
Received: 22 January 2023; Accepted: 17 February 2023; Published: 15 March 2023
Citation: Víctor Albarrán, María Luisa Villamayor, Ignacio Ruz, Jesús Chamorro, Diana Isabel Rosero, Javier Pozas, María San Román, Pablo Álvarez Ballesteros, María Ángeles Vaz. Spinal Intramedullary Ewing Sarcoma with Meningeal Carcinomatosis: A Complete Response to Chemotherapy and Neuroaxis Irradiation. Case Report and Review of Literature. Journal of Cancer Science and Clinical Therapeutics. 7 (2023): 75-81.
View / Download Pdf Share at FacebookAbstract
Intramedullary Ewing sarcoma of the spinal cord is an extremely rare form of extraosseous Ewing sarcoma, with few cases reported in the literature. Given its rarity and the great difficulty in generating scientific evidence, there are no specific recommendations for its management, which is usually extrapolated from the guidelines for bone Ewing sarcomas. Aspects such as the preferred chemotherapy scheme and the indication of whole neuroaxis irradiation remain unanswered. We report the case of an 18-year-old patient with a spinal intramedullary Ewing sarcoma and a leptomeningeal relapse after surgery, who achieved a complete clinical and radiological response to chemotherapy with VDC/IE protocol and craniospinal irradiation.
Keywords
<p>Craniospinal Irradiation; Ewing Sarcoma; Intramedullary; Meningeal Carcinomatosis</p>
Craniospinal Irradiation articles; Ewing Sarcoma articles; Intramedullary articles; Meningeal Carcinomatosis articles
Craniospinal Irradiation articles Craniospinal Irradiation Research articles Craniospinal Irradiation review articles Craniospinal Irradiation PubMed articles Craniospinal Irradiation PubMed Central articles Craniospinal Irradiation 2023 articles Craniospinal Irradiation 2024 articles Craniospinal Irradiation Scopus articles Craniospinal Irradiation impact factor journals Craniospinal Irradiation Scopus journals Craniospinal Irradiation PubMed journals Craniospinal Irradiation medical journals Craniospinal Irradiation free journals Craniospinal Irradiation best journals Craniospinal Irradiation top journals Craniospinal Irradiation free medical journals Craniospinal Irradiation famous journals Craniospinal Irradiation Google Scholar indexed journals Ewing Sarcoma articles Ewing Sarcoma Research articles Ewing Sarcoma review articles Ewing Sarcoma PubMed articles Ewing Sarcoma PubMed Central articles Ewing Sarcoma 2023 articles Ewing Sarcoma 2024 articles Ewing Sarcoma Scopus articles Ewing Sarcoma impact factor journals Ewing Sarcoma Scopus journals Ewing Sarcoma PubMed journals Ewing Sarcoma medical journals Ewing Sarcoma free journals Ewing Sarcoma best journals Ewing Sarcoma top journals Ewing Sarcoma free medical journals Ewing Sarcoma famous journals Ewing Sarcoma Google Scholar indexed journals Intramedullary articles Intramedullary Research articles Intramedullary review articles Intramedullary PubMed articles Intramedullary PubMed Central articles Intramedullary 2023 articles Intramedullary 2024 articles Intramedullary Scopus articles Intramedullary impact factor journals Intramedullary Scopus journals Intramedullary PubMed journals Intramedullary medical journals Intramedullary free journals Intramedullary best journals Intramedullary top journals Intramedullary free medical journals Intramedullary famous journals Intramedullary Google Scholar indexed journals Meningeal Carcinomatosis articles Meningeal Carcinomatosis Research articles Meningeal Carcinomatosis review articles Meningeal Carcinomatosis PubMed articles Meningeal Carcinomatosis PubMed Central articles Meningeal Carcinomatosis 2023 articles Meningeal Carcinomatosis 2024 articles Meningeal Carcinomatosis Scopus articles Meningeal Carcinomatosis impact factor journals Meningeal Carcinomatosis Scopus journals Meningeal Carcinomatosis PubMed journals Meningeal Carcinomatosis medical journals Meningeal Carcinomatosis free journals Meningeal Carcinomatosis best journals Meningeal Carcinomatosis top journals Meningeal Carcinomatosis free medical journals Meningeal Carcinomatosis famous journals Meningeal Carcinomatosis Google Scholar indexed journals spinal cord articles spinal cord Research articles spinal cord review articles spinal cord PubMed articles spinal cord PubMed Central articles spinal cord 2023 articles spinal cord 2024 articles spinal cord Scopus articles spinal cord impact factor journals spinal cord Scopus journals spinal cord PubMed journals spinal cord medical journals spinal cord free journals spinal cord best journals spinal cord top journals spinal cord free medical journals spinal cord famous journals spinal cord Google Scholar indexed journals neuroaxis irradiation articles neuroaxis irradiation Research articles neuroaxis irradiation review articles neuroaxis irradiation PubMed articles neuroaxis irradiation PubMed Central articles neuroaxis irradiation 2023 articles neuroaxis irradiation 2024 articles neuroaxis irradiation Scopus articles neuroaxis irradiation impact factor journals neuroaxis irradiation Scopus journals neuroaxis irradiation PubMed journals neuroaxis irradiation medical journals neuroaxis irradiation free journals neuroaxis irradiation best journals neuroaxis irradiation top journals neuroaxis irradiation free medical journals neuroaxis irradiation famous journals neuroaxis irradiation Google Scholar indexed journals leptomeningeal relapse articles leptomeningeal relapse Research articles leptomeningeal relapse review articles leptomeningeal relapse PubMed articles leptomeningeal relapse PubMed Central articles leptomeningeal relapse 2023 articles leptomeningeal relapse 2024 articles leptomeningeal relapse Scopus articles leptomeningeal relapse impact factor journals leptomeningeal relapse Scopus journals leptomeningeal relapse PubMed journals leptomeningeal relapse medical journals leptomeningeal relapse free journals leptomeningeal relapse best journals leptomeningeal relapse top journals leptomeningeal relapse free medical journals leptomeningeal relapse famous journals leptomeningeal relapse Google Scholar indexed journals craniospinal irradiation articles craniospinal irradiation Research articles craniospinal irradiation review articles craniospinal irradiation PubMed articles craniospinal irradiation PubMed Central articles craniospinal irradiation 2023 articles craniospinal irradiation 2024 articles craniospinal irradiation Scopus articles craniospinal irradiation impact factor journals craniospinal irradiation Scopus journals craniospinal irradiation PubMed journals craniospinal irradiation medical journals craniospinal irradiation free journals craniospinal irradiation best journals craniospinal irradiation top journals craniospinal irradiation free medical journals craniospinal irradiation famous journals craniospinal irradiation Google Scholar indexed journals neuroectodermal articles neuroectodermal Research articles neuroectodermal review articles neuroectodermal PubMed articles neuroectodermal PubMed Central articles neuroectodermal 2023 articles neuroectodermal 2024 articles neuroectodermal Scopus articles neuroectodermal impact factor journals neuroectodermal Scopus journals neuroectodermal PubMed journals neuroectodermal medical journals neuroectodermal free journals neuroectodermal best journals neuroectodermal top journals neuroectodermal free medical journals neuroectodermal famous journals neuroectodermal Google Scholar indexed journals Neoplasms articles Neoplasms Research articles Neoplasms review articles Neoplasms PubMed articles Neoplasms PubMed Central articles Neoplasms 2023 articles Neoplasms 2024 articles Neoplasms Scopus articles Neoplasms impact factor journals Neoplasms Scopus journals Neoplasms PubMed journals Neoplasms medical journals Neoplasms free journals Neoplasms best journals Neoplasms top journals Neoplasms free medical journals Neoplasms famous journals Neoplasms Google Scholar indexed journals
Article Details
1. Introduction
The Ewing family of tumors (EFT) is a spectrum of highly aggressive neoplasms with a common histology composed of small blue round cells and a presumably common neuroectodermal origin. The most relevant member is Ewing sarcoma defined by translocations that joins FUS or EWSR1genes to a member of the ETS family. As science advances, new entities are defined inside the EFT such as CIC-rearranged sarcoma and sarcoma with BCOR genetic alterations [1]. Neoplasms that compose this family used to be called peripheral primitive neuroectodermal tumors (PNETs) [2]. The EFT are highly proliferative, poorly differentiated, round-cell tumors with aggressive clinical behavior. Although metastatic disease is present in fewer than 25% of patients at diagnosis, the relapse rate in patients undergoing local therapy alone reaches 80-90%, probably due to the presence of subclinical disseminated disease in nearly all cases [3]. In a compilation of data from 945 patients, from the European Intergroup Cooperative Ewing Sarcoma Studies (EI-CESS), 54% of EFT arose in the axial skeleton, 42% in the appendicular skeleton, and 0.7% in other bones, with just around 3% of extraosseous ES arising in soft tissues [4]. Compared with bone ES, extraosseous ES tends to appear in older and female patients [5]. Intradural ES are considered a subgroup of extraosseous ES, and since most of them are extramedullary, intramedullary ES of the spinal cord makes up an extremely rare entity. Modern multidisciplinary treatment has improved the outcome of ES, reaching long-term survival rates in nearly 70-80% of patients presenting with non-metastatic disease [6]. Nowadays, a multimodal approach is recommended for the treatment of ES, with an overall 10 to 12-month- long treatment of induction chemotherapy (CT), surgery, radiotherapy, and consolidation CT thereafter [7]. Interval-compressed CT with alternating vincristine-doxorubicin-cyclophosphamide and ifosfamide-etoposide (VDC/IE scheme) is currently the upfront option of systemic treatment [8]. Due to the extremely low incidence of spinal intramedullary ES, its management is extrapolated from the recommendations for other EFT, though aspects such as the preferable scheme of CT and the convenience of craniospinal irradiation remain controversial.
2. Case Description
Our patient is an 18 years-old woman, with no relevant previous medical history, who consults over a progressive and insidious non-traumatic low-back pain in September 2019, with a weak response to common analgesia. Physical examination showed left low limb paresia (strength score 4/5) and a sensory level at T1. A magnetic resonance (MRI) of the cervical, thoracic, and lumbar spine revealed an intramedullary solid lesion from T2 to T3 vertebrae, with a maximum diameter of 20.5 mm, with an intense contrast enhancement and infiltration of the subarachnoid space (Figure 1).
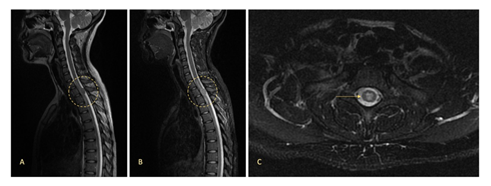
Figure 1: MRI study at diagnosis showing an intramedullary solid lesion with a maximum diameter of 20.5 mm, extending from the medium level of T2 to the medium level of T3 vertebrae, with an extent infiltration of spinal cord and subarachnoid space. Sagittal vision with a T2-weighted sequence (A) and a short-TI inversion recovery (STIR) sequence (B). Axial vision with a T2-weighted sequence (C).
Given the radiological findings, a neuroectodermic primary tumor was suspected and the patient underwent decompressive laminectomy and tumor complete resection, under neurophysiological monitoring, in October 2019. Histological examination found a tumor comprised of small round cells, with a high nuclear- cytoplasmratio, frequent apoptotic forms, 13 mitoses per 10 high-power fields, multiple foci of tumor necrosis and several Homer-Wright rosettes. These findings were conclusive of an intramedullary Ewing sarcoma. An immunohistochemistry study revealed an intense positivity for CD99, and was negative for Lin28A, H3K27M, epithelial, and glial markers (Figure 2). The Ki67 proliferation index was 25%. Gene EWSR1 rearrangement was detected by fluorescence in situ hybridization (FISH) technique. Translocation of genes CIC and BCOR was not detected.
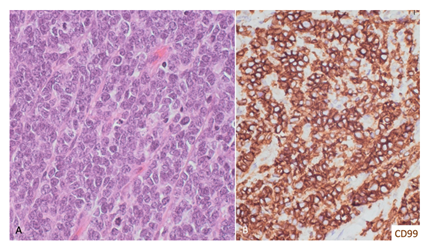
Figure 2: Histopathological study. (A) The tumor is composed of crowed small cells with high nuclear-to-cytoplasm ratio and finely stippled chromatin with inconspicuous nucleoli. Mitosis and apoptosis were frequent (Hematoxylin- eosin, 20x). (B) The neoplastic cells were diffusely membrane positive for CD99 (20x).
Distant metastases were reasonably discarded with a body computerized tomography (CT) scan, a bone scintigraphy, and a positron emission tomography with 18F-fluorodeoxyglucose (FDG- PET). The patient had a good surgical recovery and adjuvant treatment was initiated with alternating vincristine-doxorubicin-cyclophosphamide and ifosfamide-etoposide (VDC/IE protocol).
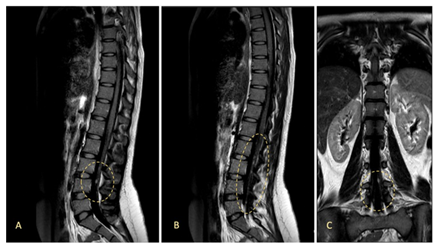
Figure 3: MRI study after one cycle of adjuvant chemotherapy, showing several millimetric nodular lesions with gadolinium contrast enhancement inside the dural sac and the roots of cauda equina, suggesting an early relapse of ES with meningeal carcinomatosis. Sagittal vision (A, B) and coronal vision (C) with a fluid attenuated inversion recovery (FLAIR) sequence.
Two weeks after the first cycle of chemotherapy, the patient was admitted to the emergency department due to right upper limb paresia (strength score 3/5). A MRI study of neuroaxis revealed multiple millimetric focal lesions with gadolinium contrast enhancement inside the dural sac and the cauda equina, as well as hyperintense signal alterations in both internal auditive conducts, all of them suggesting a new onset subarachnoid tumor dissemination (Figure 3). A positive cerebrospinal fluid (CSF) cytology, showing atypical small round cells, confirmed the diagnosis of Ewing sarcoma relapse with meningeal dissemination. Analysis of tumor cells through next generation sequencing (NGS) (Foundation One) detected EWS: FLI1 translocation, without any targetable genetic alterations. Given that she had received just one cycle of VDC, we decided to continue the same chemotherapy scheme, achieving a maintained clinical and radiological response. In December 2020, chemotherapy was paused after a total of 17 cycles and the patientunderwent neuroaxis irradiation, ending in February 2021. She has been followed up for one extra year and a half, remaining asymptomatic and with no evidence of disease relapse in quarterly MRI studies of brain and neuroaxis, last ones being performed in October 2022.
3. Discussion
The involvement of the central nervous system (CNS) in patients with Ewing sarcoma is usually due to brain and meningeal metastasis, which are estimated to appear in 1% to 8% of cases [9]. The infiltration of the spinal cord is rare and, in most cases, due to intradural extramedullary lesions. Purely intramedullary ES is an extraordinarily uncommon entity, with just 37 previous cases reported, including 6 recurrent metastatic cases [10-15] and 31 primary tumors [14-40] (summarized in Table 1). The most relevant retrospective study of primary spinal EFT was published in 2012 by Saeedinia et al. [16], including 106 cases of extradural/extramedullary-intradural tumors and the 21 cases of intramedullary ES published by then [15-35]. The average age of occurrence of primary intraspinal EFT was 22.9 years, confirming the tendency of these tumors to appear in children and young adults, with a similar gender distribution. Lumbosacral location was preponderant (51.5%), followed by thoracic (26.5%) and cervical (22%) locations. In all the reported cases of intramedullary ES, the clinical findings preceding diagnosis were secondary to spinal cord compressions, such as muscle weakness, sensory level, hyperreflexia, and spasticity, with frequent involvement of sphincters. When compared to other spinal tumors, ES tends to show a more acute onset, with quickly progressive myelopathy that may result in severe myeloplegia [12]. Therefore, it is of vital importance to consider this entity among the differential diagnoses of intradural lesions, even in patients with no previous history of the disease, since most of the reported cases were primary tumors. Gadolinium-enhanced spinal MRI remains the preferable technique for radiological diagnosis [17]. EFT usually presents as space-occupying, well-circumscribed lesions with neural compression, mainly hyperintense in T2-weighed images and isointense in T1-weighed images [13]. Solid components usually display segmental and scattered post-contrast enhancement [18]. However, histology remains the gold standard for the diagnosis of EFT, and it must be accompanied by immunohistochemical staining and molecular analysis.
Table 1: Reported cases of spinal intramedullary primary ES. N/A: not available; M: male; F: female; y: years; m: months; d: days; RT: radiotherapy; CT: chemotherapy; NE: not specified; M: methotrexate; V: vincristine; N: nitrosourea; P: cisplatin; MP: methylprednisolone; H: hydroxyurea; PZ: procarbazine; AraC: cytosine arabinoside; C: cyclophosphamide; E: etoposide; CP: carboplatin; I: ifosfamide; L: lomustine; D: doxorubicin; T: temozolomide; CR: complete response; PR: partial response; PD: progressive disease; SD: stable disease; DOD: died of disease; AWD: alive with disease; NED: no evidence of disease; DOC: died of other cause.
*Leptomeningeal carcinomatosis (LMC) has been defined as a positive CSF cytology or clearly suggestive findings on the MRI study.
**All patients underwent decompressive laminectomy and surgical resection of the tumor.
The identification of the fusion protein EWS: FLI1 is one of the most specific markers to confirm the diagnosis of EFT [14]. It is known that the genesis of EWS: FLI1 is caused by the translocation t(11;22)(q24;q12) of the N-terminus of EWSR1 to the C-terminus of FLI1, which is considered the key event in the tumorigenesis of ES. The fusion protein binds RNA helicase A and directly interacts with gene promoters, affecting enhancer elements for oncogenesis and cell proliferation [46]. This event, which is specific of EFT, has been associated with the cellular enrichment with proteins (MMP2, MMP9, MT1-MMP) that promote the proliferation of malignant cells [47]. The detection of CD99 (MIC-2 gene product) on the cell surface is also a sensitive diagnostic marker, though its specificity is relatively lower than EWS-FLI1 [48]. In our patient, CD99 expression was detected by immunohistochemistry techniques in the surgical piece, and EWS translocation was also detected in the CSF sample by cytologic analysis. The combined use of these two markers has been proposed as a powerful diagnostic tool to differentiate EFT from other spinal small round-cell tumors [49]. Moreover, the recent molecular analysis of 323 CNS-PNETs has proven that small round-cell tumors of the neuroaxis are a heterogenous group that should be defined by genetic/epigenetic alterations to differentiate clinically relevant entities [50]. The treatment of spinal cord EFT usually comprises surgical resection, as complete as possible while maintaining the neural anatomy, followed by radiotherapy (RT) and/or chemotherapy (CT). Saeedinia et al. [16] performed a meta-analysis of their series and reported a survival rate of 88% with trimodal therapy (surgery + CT + RT) after 1-year follow-up, versus 70% in patients who received a different treatment. The survival rate after 2 years of follow-up was 59% in the group of trimodal therapy and 44% in the remaining patients. Trimodal therapy also seemed to reduce the risk of distant metastases (32% versus 46%). Though most patients in this series had intradural but extramedullary tumors, given the concordance of these data with the known evidence for the combined treatment in bone ES [51], it seems reasonable to extrapolate the same strategy to the treatment of intramedullary EFT. All the 31 reported cases of intramedullary ES (see Table 1) underwent surgical resection, and adjuvant treatment was delivered in 29 cases (the 2 remaining patients [30,44] could not receive further treatment due to quick clinical deterioration). 17 patients (54.8%) received a combination of RT+CT, 6 patients (19.4%) received CT and other 6 patients (19.4%) received RT alone (mainly due to patients’ unwillingness to receive CT). The prognosis and survival rate for patients with spinal ES are not well known because of the low incidence of this condition. Including the current case, a total of 10 patients with intramedullary ES have been reported to live more than 2 years from diagnosis, and all of them have received a combination of adjuvant RT and CT. In 3 patients, the CT scheme was not specified [20,34,41], but the remaining 7 patients have received vincristine and/or platinum-based CT protocols [22,26,37,38,39,43]. Among long survivors, only one previous case with meningeal carcinomatosis had been published, being the patient alive with disease after 40 months of follow-up (Benesch et al. [37]). The impact of whole neuroaxis or craniospinal (CSI) irradiation on local control and survival of patients with intraspinal ES, in comparison to focal RT into the macroscopically affected area, remains controversial. Troschel et al. [52] have recently published a meta-analysis of 21 studies, including 24 patients with intraspinal ES, who were treated with CSI between 1992 and 2021, at a standard total dose of 36 Gy. They were compared to 55 patients treated with focal spinal RT from five retrospective reviews. The characteristics of both groups were not accurately balanced, since the tumors that received CSI were more likely to be located within the dura (p < 0.001) and were more likely to be multifocal at diagnosis (p < 0.001), two features that were associated with worse clinical outcomes. However, the rates of long-term survival were higher among patients treated with CSI, though without reaching statistical significance (p = 0.58). In this series, CSI led to a rate of radiological complete responses of 67% and 6% of patients developed craniospinal metastases during follow-up (in contrast to 19% among patients receiving focal irradiation). A potential limitation of CSI is the increase of myelotoxicity compared to focal irradiation, given the expanded radiation field, which may limit the concomitant application of systemic therapy. Our patient underwent CSI and concomitant CT, without any significant long-term side effect nor cognitive impairment, and remains free of disease 3 years after diagnosis, despite the confirmed finding of meningeal carcinomatosis at relapse. In the absence of larger, potentially prospective studies, the retrospective data currently available may suggest the use of CSI in patients with spinal ES, at least in those with unfavorable prognosis factors, such as intramedullary location, multifocal presentation, or evidence of meningeal dissemination.
4. Conclusions
Intramedullary Ewing family tumors comprise an extraordinarily infrequent form of extraosseous sarcoma, often mistaken for more common intradural lesions. Gadolinium-enhanced MRI and a complete histologic study are key for an accurate differential diagnosis, especially with the incorporation of molecular and immunohistochemical markers, such as EWS: FLI1 and CD99 detection. Though the scientific evidence is scarce, and many recommendations are extrapolated from bone ES, it is generally accepted that patients with intramedullary EFT should receive trimodal therapy with surgery, radiotherapy, and chemotherapy. Our case highlights the importance of multimodal therapy and supports the positive impact of vincristine and platinum- based schemes, together with craniospinal irradiation, especially in patients with meningeal dissemination or multifocal presentation.
References
- Choi JH, Ro JY. The 2020 WHO Classification of Tumors of Soft Tissue: Selected Changes and New Entities. Adv Anat Pathol 28 (2021): 44-58.
- Jedlicka P. Ewing Sarcoma, an enigmatic malignancy of likely progenitor cell origin, driven by transcription factor oncogenic fusions. Int J Clin Exp Pathol 3 (2010): 338-347.
- Nesbit ME, Gehan EA, Burgert EO, et al. Multimodal therapy for the management of primary, nonmetastatic Ewing’s sarcoma of bone: a long-term follow-up of the First Intergroup study. J Clin Oncol off J Am Soc Clin Oncol 8 (1990): 1664-1674.
- Cotterill SJ, Ahrens S, Paulussen M, et al. Prognostic factors in Ewing’s tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J Clin Oncol Off J Am Soc Clin Oncol 18 (2000): 3108-31014.
- Galyfos G, Karantzikos GA, Kavouras N, et al. Extraosseous Ewing Sarcoma: Diagnosis, Prognosis and Optimal Management. Indian J Surg 78 (2016): 49-53.
- Gatta G, Capocaccia R, Stiller C, et al. Childhood cancer survival trends in Europe: a EUROCARE Working Group study. J Clin Oncol Off J Am Soc Clin Oncol 23 (2005): 3742-3751.
- Casali PG, Bielack S, Abecassis N, et al. Bone sarcomas: ESMO–PaedCan–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29 (2018): iv79-95.
- Womer RB, West DC, Krailo MD, et al. Randomized controlled trial of interval- compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children’s Oncology Group. J Clin Oncol Off J Am Soc Clin Oncol 30 (2012): 4148-4154.
- Chaigneau L, Patrikidou A, Ray-Coquard I, et al. Brain Metastases from Adult Sarcoma: Prognostic Factors and Impact of Treatment. A Retrospective Analysis from the French Sarcoma Group (GSF/GETO). The Oncologist 23 (2018): 948-955.
- Weil RJ, Zhuang Z, Pack S, et al. Intramedullary Ewing sarcoma of the spinal cord: consequences of molecular diagnostics. Case report. J Neurosurg 95 (2001): 270-275.
- Gorgulu A, Albayrak BS, Kose T. Cervical leptomeningeal and intramedullary metastasis of a cerebral PNET in an adult. J Neurooncol 74 (2005): 339-340.
- Jia L, Li G, You C, et al. Intramedullary Ewing’s sarcoma of the spinal cord associated with hydrocephalus. Neurol India 57 (2009): 828-829.
- Yurtsever C, Kafadar C, Sonmez G, et al. Intramedullary spinal cord metastasis from Ewing sarcoma. Spine J Off J North Am Spine Soc 16 (2016): e745-746.
- Fukushima K, Tsuji O, Suzuki S, et al. Cervical intramedullary recurrent Ewing sarcoma after 10-year disease-free survival in an adult: a case report and review of literature. Spinal Cord Ser Cases 7 (2021): 45.
- Mousavi SR, Farrokhi MR, Eghbal K, et al. Metastatic thoracic and lumbar intramedullary and extramedullary Ewing’s sarcoma: a rare case report and literature review. J Int Med Res 50 (2022): 030006052211080.
- Saeedinia S, Nouri M, Alimohammadi M, et al. Primary spinal extradural Ewing’s sarcoma (primitive neuroectodermal tumor): Report of a case and meta- analysis of the reported cases in the literature. Surg Neurol Int 3 (2012): 55.
- Harper K, Sathiadoss P, Saifuddin A, et al. A review of imaging of surface sarcomas of bone. Skeletal Radiol 50 (2021): 9-28.
- Jiang S, Wang G, Chen J, et al. Comparison of clinical features and outcomes in patients with extraskeletal vs skeletal Ewing sarcoma: an SEER database analysis of 3,178 cases. Cancer Manag Res 10 (2018): 6227-6236.
- Kosnik EJ, Boesel CP, Bay J, et al. Primitive neuroectodermal tumors of the central nervous system in children. J Neurosurg 48 (1978): 741-746.
- Jaksche H, Wöckel W, Wernert N. Primary spinal medulloblastomas? Neurosurg Rev 11 (1988): 259-265.
- Freyer DR, Hutchinson RJ, McKeever PE. Primary primitive neuroectodermal tumor of the spinal cord associated with neural tube defect. Pediatr Neurosci 15 (1989): 181-187.
- Ogasawara H, Kiya K, Kurisu K, et al. Intracranial metastasis from a spinal cord primitive neuroectodermal tumor: case report. Surg Neurol 37 (1992): 307-312.
- Kwon OK, Wang KC, Kim CJ, et al. Primary intramedullary spinal cord primitive neuroectodermal tumor with intracranial seeding in an infant. Childs Nerv Syst ChNS off J Int Soc Pediatr Neurosurg 12 (1996): 633-636.
- Deme S, Ang LC, Skaf G, et al. Primary intramedullary primitive neuroectodermal tumor of the spinal cord: case report and review of the literature. Neurosurgery 41 (1997): 1417-1420.
- Mottl H, Koutecky J. Treatment of spinal cord tumors in children. Med Pediatr Oncol 29 (1997): 293-295.
- Meltzer CC, Townsend DW, Kottapally S, et al. FDG imaging of spinal cord primitive neuroectodermal tumor. J Nucl Med Off Publ Soc Nucl Med 39 (1998): 1207-1209.
- Mawrin C, Synowitz HJ, Kirches E, et al. Primary primitive neuroectodermal tumor of the spinal cord: case report and review of the literature. Clin Neurol Neurosurg 104 (2002): 36-40.
- Albrecht CF, Weiss E, Schulz-Schaeffer WJ, et al. Primary intraspinal primitive neuroectodermal tumor: report of two cases and review of the literature. J Neurooncol 61 (2003): 113-120.
- Kim YW, Jin BH, Kim TS, et al. Primary intraspinal primitive neuroectodermal tumor at conus medullaris. Yonsei Med J 45 (2004): 533-538.
- Kampman WA, Kros JM, De Jong THR, et al. Primitive neuroectodermal tumours (PNETs) located in the spinal canal; the relevance of classification as central or peripheral PNET: case report of a primary spinal PNET occurrence with a critical literature review. J Neurooncol 77 (2006): 65-72.
- Jain A, Jalali R, Nadkarni TD, et al. Primary intramedullary primitive neuroectodermal tumor of the cervical spinal cord. Case report. J Neurosurg Spine 4 (2006): 497-502.
- De Tommasi A, De Tommasi C, Occhiogrosso G, et al. Primary intramedullary primitive neuroectodermal tumor (PNET)--case report and review of the literature. Eur J Neurol 13 (2006): 240-243.
- Kumar R, Reddy SJ, Wani AA, et al. Primary spinal primitive neuroectodermal tumor: case series and review of the literature. Pediatr Neurosurg 43 (2007): 1-6.
- Han IH, Kuh SU, Chin DK, et al. Surgical treatment of primary spinal tumors in the conus medullaris. J Korean Neurosurg Soc 44 (2008): 72-77.
- Otero-Rodríguez A, Hinojosa J, Esparza J, et al. Purely intramedullary spinal cord primitive neuroectodermal tumor: case report and review of the literature. Neurocir Astur Spain 20 (2009): 381-386; discussion 386-387.
- Tsutsumi S, Nonaka Y, Abe Y, et al. Intramedullary primitive neuroectodermal tumor presenting with rapidly-progressive cauda equina syndrome. Neurol Med Chir (Tokyo) 50 (2010): 1031-1035.
- Benesch M, Sperl D, von Bueren AO, et al. Primary central nervous system primitive neuroectodermal tumors (CNS-PNETs) of the spinal cord in children: four cases from the German HIT database with a critical review of the literature. J Neurooncol 104 (2011): 279-286.
- Ellis JA, Rothrock RJ, Moise G, et al. Primitive neuroectodermal tumors of the spine: a comprehensive review with illustrative clinical cases. Neurosurg Focus 30 (2011): E1.
- Gollard RP, Rosen L, Anson J, et al. Intramedullary PNET of the spine: long-term survival after combined modality therapy and subsequent relapse. J Pediatr Hematol Oncol 33 (2011): 107-112.
- Alexiou GA, Siozos G, Stefanaki K, et al. Intramedullary Spinal Cord Primitive Neuroectodermal Tumor Presenting With Hydrocephalus. J Child Neurol 28 (2013): 246-250.
- Coulibaly O, Gana R, Sogoba Y, et al. Primary Intramedullary Ewing’s Sarcoma: A Case Report and Review of the Literature. Case Rep Clin Med 04 (2015): 110-113.
- Wang G, Guo F. Primary intramedullary primitive neuroectodermal tumor: A case report and review of the literature. Medicine (Baltimore) 96 (2017): e9001.
- Khwaja R, Mantilla E, Fink K, et al. Adult Primary Peripheral PNET/Ewing’s Sarcoma of the Cervical and Thoracic Spine. Anticancer Res 39 (2019): 4463-4465.
- Chen X, Zhang G. Multiple Spinal Intramedullary Primitive Neuroectodermal Tumors Mimicking Acute Myelitis. World Neurosurg 126 (2019): 72-75.
- Yamada S, Muto J, De Leon JCA, et al. Primary spinal intramedullary Ewing-like sarcoma harboring CIC-DUX4 translocation: a similar cytological appearance as its soft tissue counterpart but no lobulation in association with desmoplastic stroma. Brain Tumor Pathol 37 (2020): 111-117.
- Riggi N, Knoechel B, Gillespie SM, et al. EWS-FLI1 utilizes divergent chromatin remodeling mechanisms to directly activate or repress enhancer elements in Ewing sarcoma. Cancer Cell 26 (2014): 668-681.
- Grünewald TGP, Cidre-Aranaz F, Surdez D, et al. Ewing sarcoma. Nat Rev Dis Primer 4 (2018): 5.
- Ambros IM, Ambros PF, Strehl S, et al. MIC2 is a specific marker for Ewing’s sarcoma and peripheral primitive neuroectodermal tumors. Evidence for a common histogenesis of Ewing’s sarcoma and peripheral primitive neuroectodermal tumors from MIC2 expression and specific chromosome aberration. Cancer 67 (1991): 1886-1893.
- Shibuya R, Matsuyama A, Nakamoto M, et al. The combination of CD99 and NKX2.2, a transcriptional target of EWSR1-FLI1, is highly specific for the diagnosis of Ewing sarcoma. Virchows Arch Int J Pathol 465 (2014): 599-605.
- Sturm D, Orr BA, Toprak UH, et al. New Brain Tumor Entities Emerge from Molecular Classification of CNS-PNETs. Cell 164 (2016): 1060-1072.
- Eaton BR, Claude L, Indelicato DJ, et al. Ewing sarcoma. Pediatr Blood Cancer [Internet] 68 (2021).
- Troschel FM, Kröger K, Siats JJ, et al. The Role of Neuroaxis Irradiation in the Treatment of Intraspinal Ewing Sarcoma: A Review and Meta-Analysis. Cancers 14 (2022): 1209.
