Specific Composite Biomarker Profiles for β-Amyloid Accumulation in Peripheral Blood in Healthy Subjects Relevant to Biological Factors: Comparison with Patients with Dementia
Article Information
Kouji Satoh1, Maremi Sato-Ueshima1, Hiroyo Kagami-Katsuyama1, Masakazu Nakamura2, Akihiko Ogata2, Mari Maeda-Yamamoto3, Jun Nishihira1*
1Department of Medical Management and Informatics, Hokkaido Information University, Ebetsu, Hokkaido, Japan
2Department of Neurology, Hokkaido Neurosurgical Memorial Hospital, Sapporo, Hokkaido, Japan
3National Agriculture and Food Research Organization, Tsukuba, Ibaraki, Japan
*Corresponding author: Jun Nishihira, Department of Medical Management and Informatics, Hokkaido Information University, Ebetsu, Hokkaido, Japan.
Received: 10 January 2024; Accepted: 17 January 2024; Published: 25 January 2024
Citation: Kouji Satoh, Maremi Sato-Ueshima, Hiroyo Kagami-Katsuyama, Masakazu Nakamura, Akihiko Ogata, Mari Maeda-Yamamoto, Jun Nishihira. Specific Composite Biomarker Profiles for β-Amyloid Accumulation in Peripheral Blood in Healthy Subjects Relevant to Biological Factors: Comparison with Patients with Dementia. Journal of Biotechnology and Biomedicine 7 (2024): 33-46.
View / Download Pdf Share at FacebookAbstract
Measurement of amyloid concentration in blood using time-of-flight mass spectrometry was developed, and the combined normalized score of amyloid precursor proteins (composite biomarker: CM) in plasma was found to be related to the accumulation of β-amyloid (Aβ) in the brain. Therefore, we measured the CM value in healthy subjects aged 30-79 years and investigated the association between the CM value and clinical characteristics using multiple regression and decision tree analyses. The results indicated that the CM value was associated with age, sex, ApoE genotype, blood glucose, and Mg in healthy subjects. Factors affecting CM values differed by age. The CM values of patients with central nervous system disorders were higher than those of the healthy subjects. Moreover, the relationship between CM and Mg concentrations differed between the patients and healthy subjects. These results suggest that the CM value can be used as a biomarker for the onset of dementia, including Alzheimer’s disease. The combination of CM values and blood component results may allow for more accurate diagnosis.
Keywords
β-amyloid; Aging; Sex; ApoE; Magnesium; Central nervous disorders
β-amyloid articles; Aging articles; Sex articles; ApoE articles; Magnesium articles; Central nervous disorders articles
Article Details
Introduction
The World Health Organization reports that dementia affects more than 55 million people worldwide [1], 60–70% of whom have Alzheimer’s Disease (AD). AD can also be complicated by other central nervous system (CNS) disorders; thus, early detection of AD is crucial for all CNS disorders [2,3]. AD induces the following neuropathological changes: (1) accumulation of neurofibrillary tangles, amyloid plaques, and other deposits; (2) atrophy caused by neural and synaptic loss; and (3) neurodegeneration, such as neuroinflammation [2,4]. In the brain, an early symptom of AD is the accumulation of β-amyloid (Aβ), which is produced from amyloid precursor protein (APP) by β- and δ-secretase [2,4-6]. Aβ accumulation triggers pathological changes in AD, including amyloid plaques and NFTs. Aβ, composed of 42 amino acids (Aβ1-42), is more likely to form amyloid plaques, and Aβ accumulation causes neuropathological changes such as loss of synapses. NFTs are filaments of hyperphosphorylated tau proteins and their aggregation causes the loss of cytoskeletal components, leading neuronal loss. Tau hyperphosphorylation occurs downstream of Aβ accumulation and activates Aβ synthesis. Thus, AD causes accumulation of Aβ in the early stages, which results in cognitive decline. The concentration of Aβ in cerebrospinal fluid (CSF) was positively correlated with whole brain volume in non-demented individuals. Therefore, Aβ accumulation in brain may not be sufficient to induce dementia or may be associated with brain atrophy during the preclinical phase of AD [7,8]. Various factors influence AD development, including sex and ApoE genotype [6,9]. Sex differences are among the factors that affect the development of AD. Their effects are broad and include brain structure, stress response, and sex hormones. Serum and brain testosterone concentrations were significantly lower in the patients than in healthy individuals. In addition, endogenous testosterone deficiency caused by orchiectomy leads to an increase in soluble Aβ concentrations in the rat brains, and androgen administration reduces Aβ concentrations [6,10]. The female hormone, estrogen, is also involved in the risk of developing AD. Ovariectomized mice show elevated levels of soluble Aβ in the brain, followed by accelerated accumulation of Aβ and rapid worsening of cognitive behaviors [6,10]. Decreased estrogen levels in adulthood increase the risk of AD. Individuals with one copy of the E4-allele in the ApoE gene have a three- to four-fold higher risk of developing AD, and those with two copies have an 8-12 times higher risk than those without the copy. Furthermore, with regard to sex differences, females with E4-allele have faster cognitive decline than males [6,11].
Although Aβ is a typical biomarker of AD, its accumulation has also been observed in other CNS disorders. It has been reported that patients having vascular dementia (VaD) and dementia with Lewy bodies (DLB) with cognitive decline have more Aβ accumulation in the brain than in subjects with normal cognitive function [12,13]. Patients with Parkinson’s disease (PD) patients without cognitive decline have Aβ concentrations in the CSF that are similar to those in normal subjects; however, patients with PD with cognitive decline have significantly lower Aβ concentrations in the CSF than normal subjects [13,14]. Thus, we speculate that Aβ accumulation in the brain occur prior to the onset of cognitive decline. Therefore, measuring Aβ accumulation in the brain using CSF or positron emission tomography (PET) is critical. However, these specialized techniques are unfavorable owing their high cost, invasiveness, and limited availability in routine clinical practice.
In 2018, Nakamura et al. measured Aβ concentration in plasma using mass spectrometry and showed a 90% diagnostic accuracy for AD [15]. They demonstrated that Aβ1-42 concentrations decreased in the plasma of patients with AD, while Aβ1-40 and APP669-711 concentrations remained unchanged. Furthermore, the combined normalized scores of APP669-711/Aβ1-42 and Aβ1-40/Aβ1-42 (composite biomarker value: CM value) positively correlated with Aβ accumulation in the brain and negatively correlated with Aβ1-42 concentration in CSF [15,16]. Managing many samples in a limited period with reduced invasiveness by measuring Aβ in peripheral blood and predicting the accumulation of Aβ in the brain in now possible.
In this study, we hypothesized that the transition from healthy individuals to patients with dementia, including AD, is accompanied by a change in Aβ concentration in the brain. We measured the change in CM value with clinical characteristic factors in healthy subjects (n=603) using multiple regression analysis and decision tree analysis. Based on the regression equation obtained from the healthy subjects, the estimated CM values of patients with CNS disorders (n=112) using the regression equation obtained from healthy subjects were compared with the measured CM values. Here, we showed that CM could be a valuable biomarker for the detection of CNS disorders, particularly AD.
Materials and Methods
Healthy subjects
The participants were healthy volunteers registered at the Health Information Science Research Center of Hokkaido Information University (Ebetsu, Hokkaido, Japan). They thoroughly understood the significance, contents, and purposes of this study, “Accumulation and analysis of biomarkers involved in the prevention and improvement of dementia in healthy subjects,” and provided written consent. These participants had not been diagnosed or examined for cognitive decline or dementia in their clinical history as confirmed by medical inversions at the Health Information Science Research Center at Hokkaido Information University. Healthy Japanese adult males and females aged 30-79 years (n = 603) participated in this study. The data included age, sex, family history of dementia, blood chemistry results, and other clinical data.
Patients with CNS disorders
We recruited patients who have been clinically diagnosed as AD, PD with dementia (PDD), or DLB at the outpatient clinic of the Hokkaido Neurosurgical Memorial Hospital (Sapporo, Hokkaido, Japan) and who consented to participate in this study. Patients with AD, who satisfied the criteria of probable AD of the National Institute on Aging-Alzheimer’s Association (NIA/AA) Workgroup 2011 [17] were included in this study. Among patients with clinically diagnosed PDD or DLB, we limited the objectives to those who satisfied the criteria for clinically established or probable PD according to the clinical diagnostic criteria for PD by the Movement Disorder Society (MDS) 2015 [18] or the criteria for probable DLB in the revised clinical diagnosis for DLB by the DLB Consortium 2017 [19]. Furthermore, PDD or DLB was re-discriminated according to the one-year rule; we considered a patient to have DLB when dementia preceded more than one year before the establishment of Parkinsonism [19]. Finally, in these re-categorized patients with PDD or DLB, we extracted those who showed atrophy of medial temporal lobes on MRI scans [20,21] and hypoperfusion in the parietal lobes, precuneus, and posterior cingulate gyrus on iodine-123-labeled N-isopropyl-p-iodoamphetamine (123I-IMP) single-photon emission tomography (SPECT) scans [22,23], and defined them to be AD-PD or AD-DLB, combining the AD pathology.
Laboratory tests
Blood samples were collected from healthy subjects and patients and sent to the Sapporo Clinical Laboratory, Inc. (Sapporo, Hokkaido, Japan). Complete blood count (white blood cell [WBC], red blood cell [RBC], and platelet [Plt]), liver function (aspartate aminotransferase [AST], alanine aminotransferase [ALT], gamma-glutamyl transpeptidase [γGTP], total protein [TP], and albumin [Alb]), kidney function (blood urea nitrogen [BUN], creatinine [Cre], and uric acid [UA]), lipid metabolism (low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], and triglyceride [TG]), glucose metabolism (blood glucose [BG] and hemoglobin A1c [HbA1c]), thyroid-stimulating hormone [TSH], serum electrolyte (sodium [Na], chloride [Cl], calcium [Ca], iron [Fe], magnesium [Mg], zinc [Zn]), and 25-hydroxyvitamin D (25OH.VD) was then performed.
Aβ measurement
Peripheral blood Aβ levels were measured as described by Nakamura et al. [15]. Whole blood was collected in 7 mL EDTA-2Na tubes (Venoject II, TERUMO, Shibuya, Tokyo, Japan) and centrifuged at 2400×g for 5 min at 25°C within 60 min after blood collection. Plasma (500 µL) was transferred to storage tubes (96 Jacket Tubes 0.7 mL, FCR&Bio, Kobe, Hyogo, Japan) and frozen at a -80°C freezer. After the isolation and enrichment of abundant plasma proteins by immunoprecipitation, the specific affinity of the antibody plasma Aβ levels was measured using immunoprecipitation-mass spectrometry (IP–MS; Shimadzu Corporation, Kyoto, Kyoto, Japan). Furthermore, they used an analytical technique to quantify Aβ-related peptides of different masses using matrix-assisted laser desorption/ionization TOF–MS (Shimadzu Corporation, Kyoto, Kyoto, Japan). Shimadzu Corporation blindly performed the IP–MS analysis.
Genotyping of ApoE gene
Whole blood was collected in a 4 mL EDTA-2K tube (Venoject II, TERUMO), and DNA was isolated from 400 μL whole blood using a QIAamp DNA Blood Mini Kit (QIAGEN, Hilden Germany). The DNA content of the concentrated solution was quantified using NanoDrop spectrophotometer (Thermo Fisher, MA, USA). The genotype of the ApoE gene was analyzed using Probe PCR with two TaqMan probe sets (C___3084793_20, C____904973_10; ThermoFisher, MA, USA). The reaction solution was mixed with 1 ng/μL DNA solution. ApoE was analyzed using predesigned TaqMan genotyping assays (Applied Biosystems, CA, USA). Approximately 1 ng of genomic DNA was amplified in a 5 μL reaction mixture in a 96-well plate containing 1 × Probe PCR ExTaq master mix (Takara Bio, Shiga, Japan) and 1 × Taqman probe mix containing the respective primers and probes. QuantStudio 3 was used with the standard endpoint genotyping program (Thermo Fisher Scientific, MA, USA). According to the manufacturer’s protocol, PCR was performed with an initial denaturation at 94°C for 5 min, followed by 40 two-step cycles (denaturation for 30 s at 94°C, primer annealing, and extension for 60 s at 60°C).
Statistical analysis
All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS® Statistics version 25; IBM Corp, NY, USA) with a significance level of p<0.05. A three-step analysis was performed to investigate the relationship between CM values and clinical characteristics in healthy subjects. First, a single regression analysis was performed to select factors associated with the CM value. Second, a multiple regression analysis was performed to investigate the effects of these factors on the CM value. Third, a classification and regression tree (CART) was used for the analysis of CM-related clinical characteristic factors and the classification of subject groups according to these factors. Because the residuals of the multiple regression analysis using the original blood test values were not normally distributed, the blood test values and age were transformed to log10 value, and the CM values were transformed to log10 of (CM+3). Student’s t test (t-test), Mann-Whitney U test (U test), or analysis of variance (ANOVA) were also used.
Results
Effects of biological factors (age, sex, and ApoE genotype) on composite biomarker values in healthy subjects
Healthy subjects were 603 Japanese individuals, ranging in age from 30-79 years (average 54.5 years); 70% of the participants were female. Subjects were genotyped for ApoE, and 20.4% were E4 carriers (Table 1). We then confirmed that the CM values were associated with subject characteristics (age, sex, and ApoE genotype) using single regression analysis (Supplementary Table S1). Next, multiple regression analysis was performed on the relationship between CM values and biological factors (age, sex, and ApoE4 carrier status) (Table 2). From the analysis, we found that the residuals with the regression equation were normally distributed (Shapiro-Wilk test p=0.588) and confirmed that the multiple regression equation was a significant equation with a significance of p<0.001. With R2=0.140, we concluded that the prediction accuracy of the multiple regression equation was satisfactory. The coefficients of the regression equation showed that the CM value was positively correlated with age, and its influence was strongest among the three characteristics. In brief, the CM values were lower in females, whereas they were higher in both sexes with the E4 allele of ApoE (Table 2).
|
Healthy subjects (n=603) |
Patients (n=112) |
|||||
|
Ave |
Std |
Ave |
Std |
U-test (p)* |
||
|
Demographic data |
Age |
54.49 |
11.35 |
77.87 |
8.4 |
<0.001 |
|
Woman (%) |
70 |
56.3 |
||||
|
ApoE4 carrier (%) |
20.4 |
18.8 |
||||
|
Laboratory data |
CM |
-0.467 |
0.509 |
0.647 |
0.842 |
<0.001 |
|
WBC |
5.71 |
1.44 |
5.46 |
1.45 |
0.106 |
|
|
RBC |
456.9 |
43.3 |
407.1 |
49.3 |
<0.001 |
|
|
Plt |
25.43 |
5.44 |
22.4 |
7.22 |
<0.001 |
|
|
AST |
22.56 |
7.24 |
20.59 |
6.23 |
<0.001 |
|
|
ALT |
19.84 |
12.51 |
15.32 |
7.77 |
<0.001 |
|
|
γGTP |
30.94 |
35.71 |
31.95 |
76.07 |
0.015 |
|
|
TP |
7.39 |
0.35 |
7.49 |
0.85 |
<0.001 |
|
|
Alb |
4.64 |
0.25 |
4.41 |
0.51 |
0.041 |
|
|
ChE |
341.6 |
76.1 |
301.4 |
86 |
0.767 |
|
|
BUN |
14.03 |
3.59 |
17.49 |
5.87 |
<0.001 |
|
|
CRE |
0.77 |
0.14 |
0.77 |
0.25 |
<0.001 |
|
|
UA |
4.94 |
1.27 |
4.93 |
1.35 |
<0.001 |
|
|
TG |
95.69 |
78.6 |
110.9 |
62.24 |
<0.001 |
|
|
HDL-C |
76.59 |
19.85 |
58.3 |
16.66 |
<0.001 |
|
|
LDL-C |
128 |
32.8 |
100.8 |
33.4 |
0.023 |
|
|
BG |
88.9 |
11.3 |
111.6 |
34.3 |
0.071 |
|
|
HbA1c |
5.37 |
0.34 |
5.73 |
0.65 |
<0.001 |
|
|
TSH |
1.58 |
0.96 |
2.16 |
2.18 |
<0.001 |
|
|
Na |
142.2 |
1.7 |
141.1 |
3.1 |
0.003 |
|
|
Cl |
102.9 |
1.9 |
105.6 |
4 |
<0.001 |
|
|
Mg |
2.11 |
0.14 |
1.99 |
0.23 |
<0.001 |
|
|
Ca |
9.47 |
0.38 |
9.14 |
0.46 |
<0.001 |
|
|
Fe |
97.9 |
34.88 |
81.53 |
33.54 |
<0.001 |
|
|
Zn |
86.74 |
12.56 |
65.32 |
10.72 |
<0.001 |
|
|
25OH-VD |
17.41 |
7.02 |
14.92 |
5.04 |
<0.001 |
|
|
*: Significant difference between healthy subjects and patients was analyzed by Mann-Whitney U test. |
||||||
Table 1: Laboratory data of healthy subjects and patients with CNS disorders.
|
Coefficients |
Standardized Coefficients |
Significance (p) |
95.0% Confidence Interval |
||
|
Lower |
Upper |
||||
|
(Constant) |
-0.074 |
0.223 |
-0.193 |
0.045 |
|
|
Sex |
-0.023 |
-0.125 |
0.001 |
-0.037 |
-0.009 |
|
ApoE4 |
0.024 |
0.115 |
0.003 |
0.008 |
0.04 |
|
logAge |
0.278 |
0.314 |
<0.001 |
0.21 |
0.346 |
|
R2=0.140 ANOVA p<0.001 |
|||||
Table 2: Multiple linear regression analysis for CM value for healthy subjects of different attributes
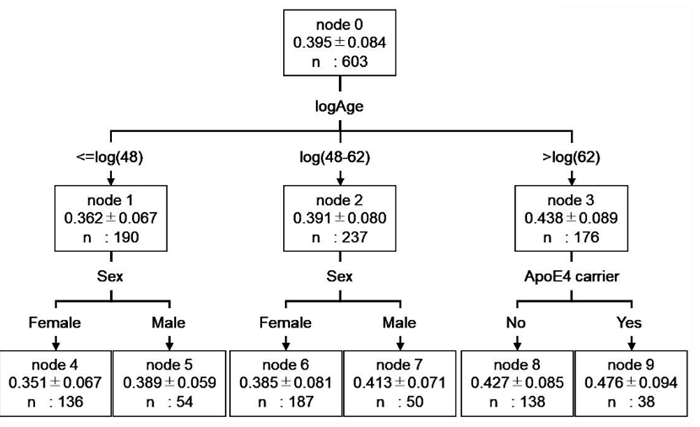
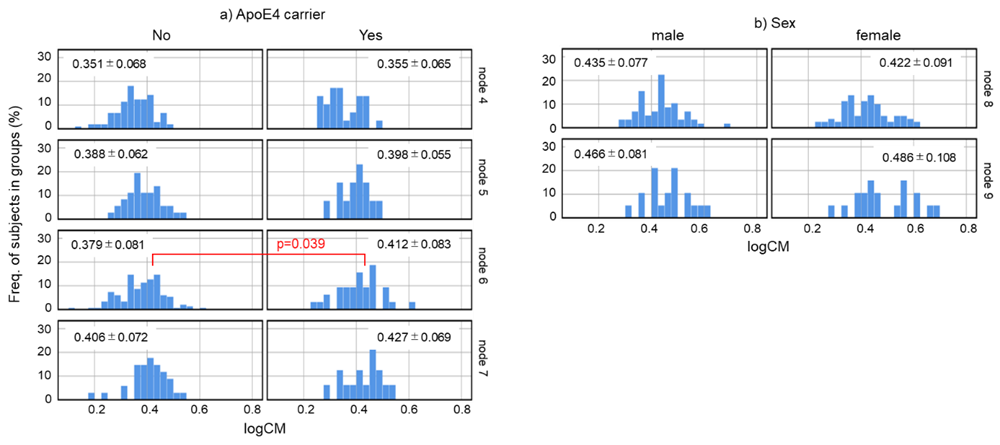
Decision tree analysis was used to investigate the relationship between CM values and biological factors. The results showed that the CM value of healthy subjects could be classified into six groups according to their biological factors. However, the factors affecting the CM values varied by age (Figure 1). The groups were classified by age: ≤48 years, 48-62 years, and >62 years. The two groups, ≤48 years and 48-62 years, were further classified into two groups by gender (node 4, 5, 6, 7). These >62 years were divided into two groups according to the presence or absence of the E4 allele (node 8, 9). We further investigated the distribution of CM in node 4-9 to study the influence of sex and ApoE genotype on CM (Supplementary Figure S1). Nodes 4 (female subjects ≤48 years of age) and 5 (male subjects ≤48 years of age) had no difference in CM values due to ApoE genotype. In subjects aged 48-62 years, node 7 (male subjects) showed no difference in CM values by ApoE genotype, while node 6 (female subjects) showed significantly higher CM values in E4 carriers (p=0.039, Mann-Whitney U test). In subjects aged 62 years, neither node 8 (subjects without E4 allele) nor node 9 (subjects with E4 allele) differed in CM values by sex.
Figure 1: Decision tree model for predicting CM values based on demographic data of healthy subjects. The root and leaf nodes are represented by squares, which contain the average logCM±standard division and the number of subjects in each group. Each branch represents the decision rules and each leaf node represents the classification outcome. Node 0 (healthy subjects) was classified into three subgroups according to age: node 1 (≤48 years), node 2 (48–62 years), and node 3 (>62 years). Node 1 was classified into two subgroups according to gender: node 4 (female) and node 5 (male). Node 2 was classified into two subgroups according to sex: node 6 (female) and node 7 (male). Node 3 was classified into two subgroups according to ApoE genotype: node 8 (non ApoE4 carrier) and node 9 (ApoE4 carrier). logCM, log10 of (CM value+3); logAge, log10 of age; ApoE4 carrier, No = subject without E4 allele; Yes = subject with E4 allele.
Effects of blood components on composite biomarker values in healthy subjects
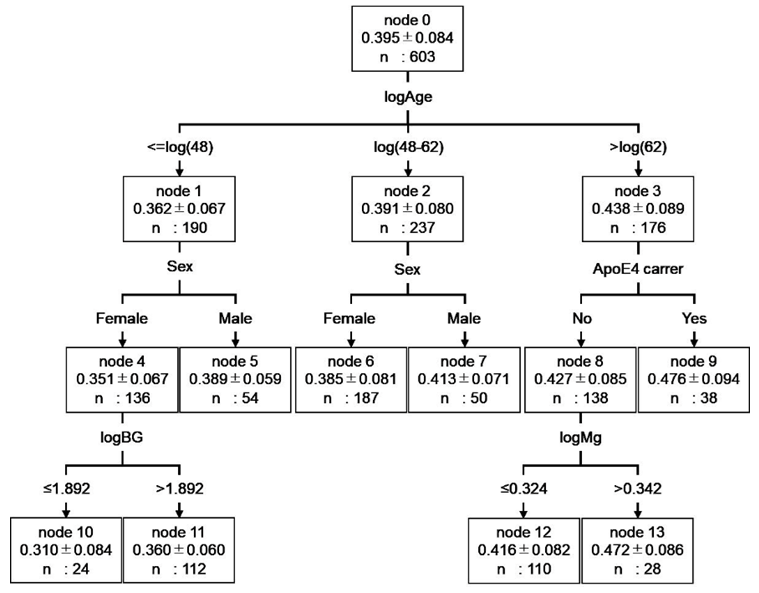
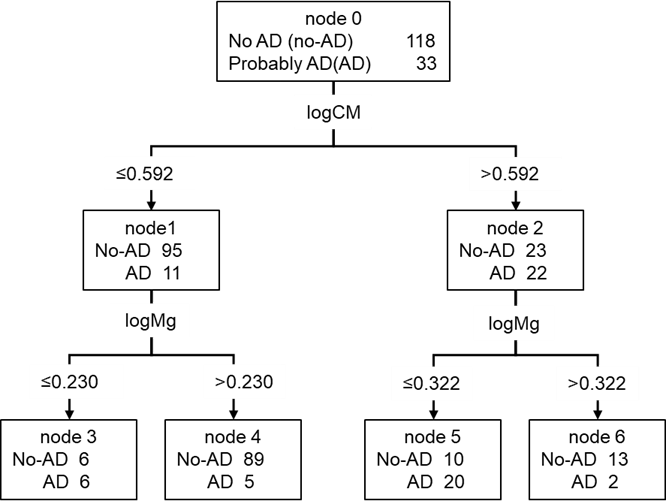
The relationship between CM values and blood components was investigated using hierarchical multiple regression analysis. Seventeen blood components were significantly correlated with the CM value in a single regression analysis (Supplementary Table S1). Demographic factors were analyzed using the forced entry method and 17 blood components were analyzed using the stepwise method. The results showed that the residuals with the regression equation were normally distributed (Shapiro-Wilk test p=0.428) and correlated with R2=0.172 (Table 3). Based on the coefficients of the regression equation, multiple regression analysis, even with the addition of blood constituents, also showed correlations with age, sex, and ApoE genotype. Furthermore, the blood components ALT, BG, HbA1c, and Mg correlated well with CM values. Moreover, ALT, BG, and Mg concentrations positively correlated with CM values, whereas CM values negatively correlated with HbA1c levels. Decision tree analysis was used to investigate the relationship between CM values and the seven factors obtained from the multiple regression analysis. The results showed that HbA1c and ALT levels did not result in decision trees. The subject groups (nodes 4-9) were the same as those in Fig. 1, up to the second tree. The BG resulted in a decision tree for women aged ≤48 years (Figure 2). Mg resulted in a decision tree for subjects aged >62 years without E4 allele (Figure 2). Based on these results, we concluded that CM values are significantly related to BG and Mg in the blood components.
|
Method |
Coefficients |
Standardized Coefficients |
Significance (p) |
95.0% Confidence Interval |
||
|
Lower |
Upper |
|||||
|
(Constant) |
-0.437 |
0.002 |
-0.713 |
-0.161 |
||
|
forced |
Sex |
-0.015 |
-0.081 |
0.045 |
-0.03 |
0 |
|
ApoE4 |
0.021 |
0.103 |
0.007 |
0.006 |
0.037 |
|
|
logAge |
0.25 |
0.283 |
<0.001 |
0.176 |
0.324 |
|
|
stepwise |
logBG |
0.272 |
0.154 |
0.001 |
0.113 |
0.431 |
|
logALT |
0.044 |
0.096 |
0.02 |
0.007 |
0.08 |
|
|
logHbA1c |
-0.344 |
-0.105 |
0.028 |
-0.651 |
-0.037 |
|
|
logMg |
0.227 |
0.079 |
0.044 |
0.006 |
0.449 |
|
|
R2=0.172 ANOVA p<0.001 |
||||||
Table 3: Summary of hierarchical multiple linear regression analysis predicting CM value using data of healthy subjects.
Figure 2: Decision tree model for predicting CM values based on data of healthy subjects. The root node and leaf nodes are represented by squares, which contain the average logCM±standard division and the number of subjects in each group. Each branch represents the decision rules, and each leaf node represents the classification outcome. node 0 (healthy subjects) was classified into three subgroups according to age: node 1 (≤48 years), node 2 (48–62 years), and node 3 (>62 years). Node 1 was classified into two subgroups according to gender: node 4 (female) and node 5 (male). Node 4 was classified into two subgroups according to logBG: node10 (≤1.892) and node 11 (>1.892). Node 2 was classified into two subgroups according to gender: node 6 (female) and node 7 (male). Node 3 was classified into two subgroups according to ApoE genotype: node 8 (non ApoE4 carrier) and node 9 (ApoE4 carrier). Node 8 was classified into two subgroups according to logMg: node 12 (≤1.892) and node 13 (>1.892). Abbreviation: logCM, log10 of (CM+3); logAge, log10 of age; logBG, log10 of BG; logMg, log10 of Mg.
Comparison of CM values between healthy subjects and patients with CNS disorder
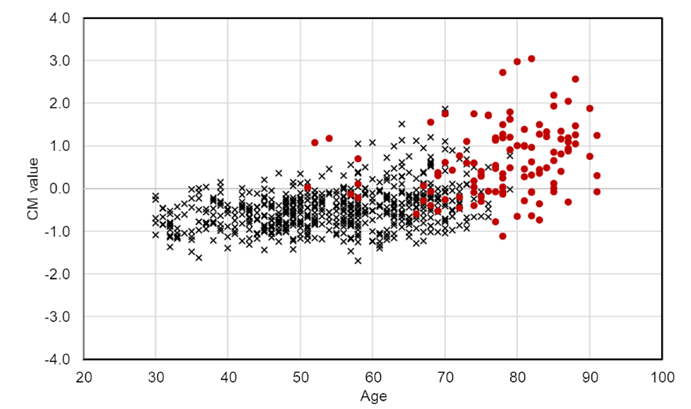
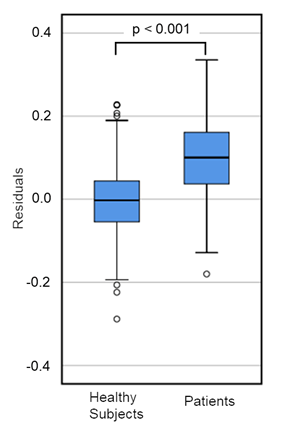
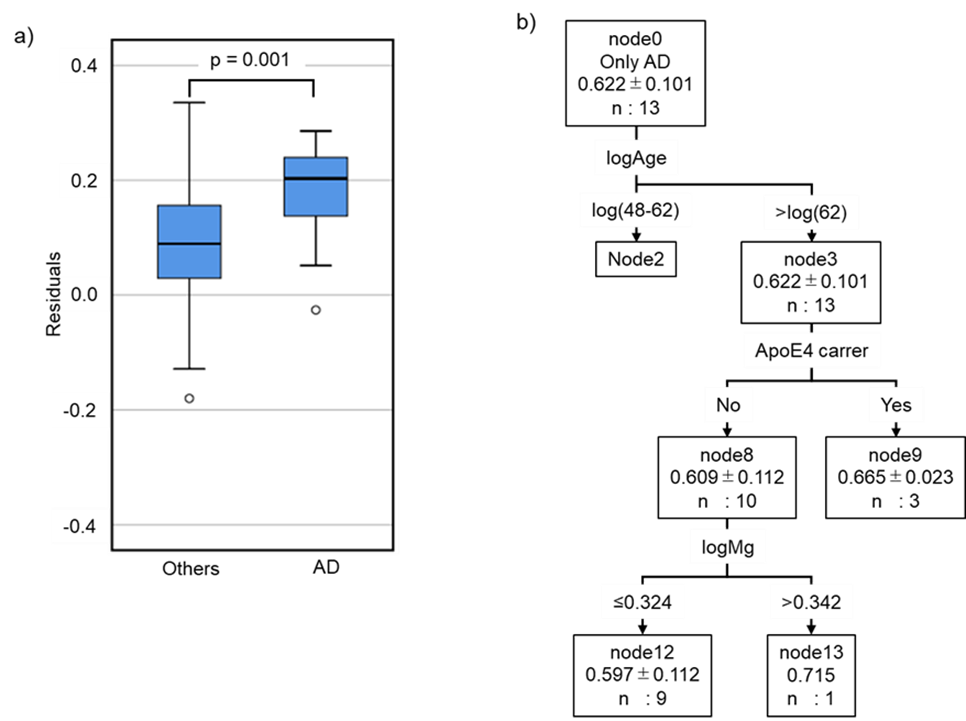
Patients with neurological diseases (n=112) showed higher CM than healthy subjects (Figure 3); however, their age and blood concentration values were significantly different from those of healthy subjects (Table 1). These findings suggest that clinical characteristics should be considered when compared CM values between patients and healthy subjects. Accordingly, the CM values of the patients were estimated using the regression equation obtained from the data of the healthy subject, and the results were compared with the measured values. The results showed that the residuals of the patients were normally distributed but were significantly higher than those of the healthy subjects (Figure 4). Moreover, the residuals in patients with AD (n=13) were significantly higher than those in patients with other neurological diseases, although the sample size was small (Supplementary Figure S2). In addition, a decision tree created using data from healthy subjects was used to classify patients. Patients with E4 allele (node 9) had significantly higher CM values than those without E4 allele (node 8). These results were consistent with those of healthy subjects. In patients without the E4 allele, there was no significant difference in the CM values between the group of patients with a low Mg concentration (node 12) and a high Mg concentration (node 13) (node12=0.539 node13=0.532, t-test: p=0.814) (Table 4). This suggests that Mg plays an important role in regulation of the absence of the E4 allele.
Figure 4: The difference of residuals between healthy subjects and patients with CNS disorders. The residuals of healthy subjects (n=603) and patients with CNS disorders (n=105) were calculated by the regression equation in Table3. Both residuals were normally distributed (healthy subjects: Shapiro-Wilk test p=0.428, patients: p=0.718). the difference in residuals between healthy subjects and patients was calculated with student t-test (p).
Discussions
In 2019, Nakamura et al. measured APP669-711, Aβ1-40, and Aβ1-42 in peripheral blood and calculated the CM value by combining normalized scores of APP669-711/ Aβ1-42 and Aβ1-40/Aβ1-42. Because the CM value is 0.376 or higher and is present approximately 90% of patients with AD [15], it is possible to detect the accumulation of Aβ in the brain with high sensitivity. Furthermore, this method uses plasma from available blood collection, with a minimal burden on patients. In this study, we performed CM measurements on a large number of healthy subjects and investigated the correlation between CM values and clinical characteristics. Furthermore, we hypothesized that the transition from healthy to a patient with CNS disorders might be related to changes in CM values, and analyzed the diversity of CM value between them.
Influence of clinical characteristic factors on CM value in healthy subjects
In this study, we showed that the CM values increased with age; the value in females was lower than that in males, and the value in ApoE4 carrier was higher by multiple regression analysis (Table 2). In addition, using decision tree analysis, we demonstrated that demographic factors affecting CM values varied with age (Figure 1). Among the subjects younger than 62 years, the male group had significantly higher CM values than those of female group. In subjects >62 years of age, the CM value is higher in ApoE4 carriers. We found that the effects of sex and ApoE on CM values varied with age, which may be due to the opposing effects of sex hormones and ApoE4 on Aβ accumulation. Sex hormones are known to have inhibitory effects on Aβ formation [9,10,24-27]. In males, blood testosterone concentrations and the risk of developing AD have been correlated based on a report that male patients with AD have lower serum testosterone levels [25,26]. In addition, there is an inverse correlation between testosterone and Aβ concentrations in the brain [27]. In females, reduced estrogen levels appear to be associated with an increased risk of AD [9,10]. Treatment with ovarian sex steroid hormones significantly increased the expression of Aβ clearance factors and decreased the soluble Aβ level in in rat brain [10].
In contract, ApoE4 is known to increase Aβ oligomer formation and Aβ deposition. Therefore, E4 allele is a genetic risk factor for Aβ accumulation in the brain [4,6,11]. Taken together, the results of the current study indicate that Aβ concentrations in the brain are largely influenced by sex hormones at age 48 and younger, but by ApoE genotype at age 62 and older. In the 48-62 age group, the influence of ApoE genotype was also observed in female, which is a variable label in female sex hormone concentrations. In males, testosterone levels decreased linearly with the age ≥20 years [28,29]. However, in females, estrogen levels decreased sharply with the onset of menopause [29]. Therefore, the influence of ApoE genotype was also observed in females in the 48-62 age group and may imply that the Aβ clearance mechanisms of sex hormones in brain differ between males and females. We used multiple regression and decision tree analyses to analyze the blood components affecting the CM values. These results suggest a relationship between the CM values and ALT, BG, HbA1c, and Mg (Table 3). BG and Mg also showed a relationship with CM values in both analyses (Table 3, Figure 2).
In this study, subjects with higher BG levels had higher CM values. Cognitive decline is a complication of diabetes associated with hyperglycemia [30-33]. These reports are consistent with the results of this study in that the BG and HbA1c levels of patients with CNS disorders were significantly higher than those of healthy subjects (Table 1). In the present study, HbA1c was inversely related to CM values associated with Aβ accumulation in brain, which differ from previous reports. This difference may be due to the fact that the subjects in this analysis are healthy subjects and the HbA1c changes are at the level of healthy subjects, which may be different from that of patients with hyperglycemia. The current study showed that CM values were positively correlated with Mg concentrations, which was expected because Mg is related to the accumulation of Aβ. In healthy individuals, the serum Mg concentration is physiologically constant, independent of age, and depends on Mg intake [34,35]. However, serum Mg concentrations are decreased in diabetes, cardiac diseases, and cognitive decline, including AD. Mg is known to inhibit Aβ formation and tau phosphorylation [35-37]. The role of Mg may vary depending on health and disease conditions. However, these reports are inconsistent with the results of the present study. This inconsistency should be discussed from various perspectives, including age, gender, and clinical history of healthy individuals and patients.
Difference in CM values between healthy subjects and patients with CNS disorder.
We showed that the regression equation for CM values estimated from data of healthy subjects could not be adapted for patients with CNS disorder (Figure 3). Moreover, the decision tree created from the data of the healthy subjects was applied to the patients and yielded results different from those of the healthy subjects (Table 4). These results may be linked to the effect of the disease onset on clinical characteristics of the patients. Lower blood Mg concentrations are linked to lower cognitive function (Alzheimer-like disease) [35-37]. It has also known that patients with CNS disorders have lower Mg concentrations [36]. In the current study, the Mg concentrations in patients of CNS disorders were significantly lower than those in healthy subjects (Table 1). In addition, some blood component concentrations were significantly different between the patients and healthy subjects. This indicates that the relationship between clinical characteristics and CM values may differ between healthy subjects and patients due to pathophysiological factors, regardless of the type of CNS disorders, and that patients may have a greater accumulation of Aβ in the brain than in healthy individuals. PD and DLB are categorized into a group of diseases called α-synucleinopathy, in which toxic oligomeric α-synuclein accumulates in nerve cells. The concentration of α-synuclein in the CSF of patients with PD is lower than that in healthy individuals [38]. It is positively correlated with the concentration of Aβ1-42 in the CSF [39]. These reports imply that the concentration of Aβ1-42 in the blood is lower in patients with PD than in healthy individuals. Aβ accumulation in brain has also been detected in patients with VaD and PDD [12-14]. Therefore, patients with CNS disorders may exhibit higher CM values than those of healthy individuals. Since patients with CNS disorders have higher CM values, which are significantly higher in patients with AD (Table 4, Supplementary Figure S2), CM values would be a useful marker for the diagnosis of CNS disorders, including AD. However, as CM values are affected by clinical characteristics, a combination of CM values and other clinical characteristics may be desirable for diagnosis of CNS disorders. In this study, we identified and characterized patients with AD by decision tree analysis using the clinical characteristics of subjects aged ≥70 years. The first decision rule was the CM, followed by the Mg concentration (Supplementary Figure S3). This result suggests that CM is an effective biomarker for AD determination and Mg as a biomarker makes it a more accurate for diagnosis of AD.
|
Healthy subjects |
Patients |
|||||
|
n |
mean |
std |
n |
mean |
std |
|
|
▪node 1 |
190 |
0.362 |
0.067 |
|||
|
▫node 4 |
136 |
0.351 |
0.067 |
|||
|
node 10 |
24 |
0.31 |
0.084 |
|||
|
node 11 |
112 |
0.36 |
0.06 |
|||
|
▫node 5 |
54 |
0.389 |
0.059 |
|||
|
▪node 2 |
237 |
0.391 |
0.08 |
7 |
0.524 |
0.073 |
|
▫node 6 |
187 |
0.385 |
0.081 |
4 |
0.509 |
0.077 |
|
▫node 7 |
50 |
0.413 |
0.071 |
3 |
0.546 |
0.078 |
|
▪node 3 |
176 |
0.438 |
0.089 |
105 |
0.552 |
0.102 |
|
▫node 8 |
138 |
0.427 |
0.085 |
85 |
0.538 |
0.105 |
|
node 12 |
110 |
0.416 |
0.082 |
66 |
0.539 |
0.102 |
|
node 13 |
28 |
0.472 |
0.086 |
19 |
0.532 |
0.119 |
|
▫node 9 |
38 |
0.476 |
0.094 |
20 |
0.613 |
0.053 |
|
Distribution of healthy subjects and patients in each subgroup. N = number of subjects; mean = average logCM value in each subgroup; std = standard deviation of the logCM value in each subgroup. The subgroups were classified using decision tree analysis, as shown in Figure 2. |
||||||
Table 4: The difference of logCM values between healthy subjects and patients with CNS disorders in each subgroup classified by decision tree analysis.
Conclusions
We demonstrated that the CM value calculated from the Aβ concentration in the blood was influenced by clinical characteristic factors. Moreover, the difference of CM value between healthy subjects and patients has been suggested to be caused by the onset of CNS disorders included AD. The measurement of Aβ in the peripheral blood used in this study has a significant advantage, with little burden on the subjects, and it is possible to screen and check a number of patients with CNS disorders and healthy individuals. In future studies, measurements over time can be used to assess changes in the rate of Aβ accumulation, which will help determine the severity and stage of dementia in various cognitive disorders, including AD.
Acknowledgments
This work was supported by Bio-oriented Technology Research Advancement Institution [grant number SIP2B]; Ministry of Health, Labour and Welfare [grant numbers JPMH19AC5003, JPMH20AC5002, JPMH21AC5002]. We thank members of the Health information Science Research Center of Hokkaido Information University and Hokkaido Neurosurgical Memorial Hospital for their technical assistance with the clinical trial and data treatment.
Authors contributions
KS: Investigation, Formal analysis, Visualization, Writing- original draft. MSU: Data curation. Formal analysis. HKK: Formal analysis. Writing - Original Draft. MN: Investigation, Acquisition and interpretation of the data. AO: Conceptualization, Supervision. MMY: Project administration, Funding acquisition. JN: Conceptualization, Project administration, Funding acquisition.
Conflicts of interest statements
The authors have no actual or potential conflicts of interest.
Data availability
The data used in this study are available from the corresponding author (JN), upon reasonable request.
Ethics approval
This study was conducted in accordance with the Declaration of Helsinki. The Ethics Committee approved all procedures involving human subjects at Hokkaido Information University (approved on May 26, 2021; approval number: 2021-22). This study complied with the ethical guidelines for human medical research of the Ministry of Education, Culture, Sports, Science, and Technology and the Ministry of Health, Labour, and Welfare of Japan.
Funding
Mari Maeda-Yamamoto and Jun Nishihira received funding from the cross-ministerial Strategic Innovation Promotion Program (SIP), “Technologies for Smart Bio-industry and Agriculture” (funding agency: Bio-oriented Technology Research Advancement Institution, Japan), grant number: SIP2B, and MHLW KAKENHI Program Grant Number JPMH19AC5003, JPMH20AC5002, and JPMH21AC5002. These funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
References
- World Health Organization. 2023. Dementia. On line (2023).
- Briggs R, Kennelly SP, O'Neill D. Drug treatments in Alzheimer's disease. Clin Med (Lond) 16 (2016): 247-253.
- Venkat P, Chopp M, Chen J. Models and mechanisms of vascular dementia. Exp Neurol 272 (2015): 97-108.
- Breijyeh Z, Karaman R. Comprehensive Review on Alzheimer's Disease: Causes and Treatment. Molecules 25 (2020): 5789.
- Avila J, Perry G. A Multilevel View of the Development of Alzheimer's Disease. Neuroscience 457 (2021): 283-293.
- Zhu D, Montagne A, Zhao Z. Alzheimer's pathogenic mechanisms and underlying sex difference. Cell Mol Life Sci 78 (2021): 4907-4920.
- Fagan AM, Head D, Shah AR, et al. Decreased cerebrospinal fluid Abeta (42) correlates with brain atrophy in cognitively normal elderly. Ann Neurol 65 (2009): 176-183.
- Lloret A, Esteve D, Lloret MA, et al. When Does Alzheimer's Disease Really Start? The Role of Biomarkers. Int J Mol Sci 20 (2019): 5536.
- Mielke MM. Sex and Gender Differences in Alzheimer's Disease Dementia. Psychiatr Times 35 (2018): 14-17.
- Jayaraman A, Carroll JC, Morgan TE, et al. 17β-estradiol and progesterone regulate expression of β-amyloid clearance factors in primary neuron cultures and female rat brain. Endocrinology 153 (2012): 5467-5479.
- Rocca WA, Mielke MM, Vemuri P, et al. Sex and gender differences in the causes of dementia: a narrative review. Maturitas 79 (2014): 196-201.
- Chiasserini D, Biscetti L, Eusebi P, et al. Differential role of CSF fatty acid binding protein 3, α-synuclein, and Alzheimer's disease core biomarkers in Lewy body disorders and Alzheimer's dementia. Alzheimers Res Ther 9 (2017): 52.
- Lewis H, Beher D, Cookson N, et al. Quantification of Alzheimer pathology in ageing and dementia: age-related accumulation of amyloid-beta (42) peptide in vascular dementia. Neuropathol Appl Neurobiol 32 (2006): 103-118.
- Myers PS, O'Donnell JL, Jackson JJ, et al. Proteinopathy and Longitudinal Cognitive Decline in Parkinson Disease. Neurology 99 (2022): e66-e76.
- Nakamura A, Kaneko N, Villemagne VL, et al. High-performance plasma amyloid-β biomarkers for Alzheimer's disease. Nature 554 (2018): 249-254.
- Hanon O, Vidal JS, Lehmann S. et al. Plasma amyloid levels within the Alzheimer's process and correlations with central biomarkers. Alzheimers Dement 14 (2018): 858-868.
- McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7 (2011): 263-269.
- Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 30 (2015): 1591-1601.
- McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89 (2017): 88-100.
- Burton EJ, Barber R, Mukaetova-Ladinska EB, et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer's disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain 132 (2009): 195-203.
- Duara R, Loewenstein DA, Potter E, et al. Medial temporal lobe atrophy on MRI scans and the diagnosis of Alzheimer disease. Neurology 71 (2008): 1986-1992.
- Minoshima S, Foster NL, Kufl DE. Posterior cingulate cortex in Alzheimer's disease. Lancet 344 (1994): 895.
- Nihashi T, Yatsuya H, Hayasaka K, et al. Direct comparison study between FDG-PET and IMP-SPECT for diagnosing Alzheimer's disease using 3D-SSP analysis in the same patients. Radiat Med 25 (2007): 255-262.
- Bianchi VE. Impact of Testosterone on Alzheimer's Disease. World J. Mens Health 40 (2022): 243-256.
- Moffat SD, Zonderman AB, Metter EJ, et al. Free testosterone and risk for Alzheimer disease in older men. Neurology 62 (2004): 188-193.
- Paoletti AM, Congia S, Lello S, et al. Low androgenization index in elderly women and elderly men with Alzheimer's disease. Neurology 62 (2004): 301-303.
- Rosario ER, Chang L, Head EH, et al. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer's disease. Neurobiol Aging 32 (2011): 604-613.
- Iwamoto T, Yanase T, Koh E, et al. Reference ranges of total serum and free testosterone in Japanese male adults. Nihon Hinyokika Gakkai Zasshi 95 (2004): 751-760. (In Japanese).
- Lamberts SW, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science 278 (1997): 419-424.
- Anstey KJ, Sargent-Cox K, Eramudugolla R, et al. Association of cognitive function with glucose tolerance and trajectories of glucose tolerance over 12 years in the AusDiab study. Alzheimers Res Ther 7 (2015): 48.
- Liu Z, Zaid M, Hisamatsu T, et al. Elevated Fasting Blood Glucose Levels Are Associated with Lower Cognitive Function, With a Threshold in Non-Diabetic Individuals: A Population-Based Study. J Epidemiol 30 (2020): 121-127.
- Ortiz GG, Huerta M, González-Usigli HA, et al. Cognitive disorder and dementia in type 2 diabetes mellitus. World J Diabetes 13 (2022): 319-337.
- Sanz CM, Ruidavets JB, Bongard V, et al. Relationship between markers of insulin resistance, markers of adiposity, HbA1c, and cognitive functions in a middle-aged population-based sample: the MONA LISA study. Diabetes Care 36 (2013): 1512-1521.
- Takahashi S, Takahashi J, Osawa N, et al. Trace elements analysis of serum and cerebrospinal fluid with PIXE--effect of age and changes in parkinsonian patients. Nihon Ronen Igakkai Zasshi 31 (1994): 865-871.
- Barbagallo M, Veronese N, Dominguez LJ. Magnesium in Aging, Health and Diseases. Nutrients 13 (2021): 463.
- Xu ZP, Li L, Bao J, et al. Magnesium protects cognitive functions and synaptic plasticity in streptozotocin-induced sporadic Alzheimer's model. PLoS One 9 (2014): e108645.
- Yu J, Sun M, Chen Z, et al. Magnesium modulates amyloid-beta protein precursor trafficking and processing. J Alzheimers Dis 20 (2010): 1091-1106.
- Tokuda T, Salem SA, Allsop D, et al. Decreased alpha-synuclein in cerebrospinal fluid of aged individuals and subjects with Parkinson's disease. Biochem Biophys Res Commun 349 (2006): 162-166.
- Murakami H, Tokuda T, El-Agnaf OMA, et al. Correlated levels of cerebrospinal fluid pathogenic proteins in drug-naïve Parkinson's disease. BMC Neurol 19 (2019): 113.
Supplementary content
Figure S1: Frequency of logCM value. a) As shown in Fig 1, nodes 4 and 6 are female subject groups, and nodes 5 and 7 are male subject groups. The subjects in each group were divided into subgroups according to whether they were ApoE4 carriers. b) Node 8 is the non ApoE4 carrier group, and node 9 is the ApoE4 carrier group. The participants in each group were divided into subgroups according to sex. Histograms show the distribution of logCM in each subgroup. The Mann–Whitney U test was used to compare subgroups derived from the same node. Abbreviation: logCM = log10 of (CM+3).
Figure S2: Characteristics of patients with Alzheimer’s disease without complications. Patients with AD uncomplicated by other CNS disorders were categorized into “AD patients.” a) We compared the residuals between patients with AD (n=13) and other patients (n=92). The difference in residuals was calculated using the Mann–Whitney U test. b) Classification of AD patients based on decision trees using healthy subject data in Figure 2.
Figure S3: Decision tree model for predicting CM values based on data of subjects aged ≥70 years. The root and leaf nodes are represented by squares that indicate the number of subjects with probable AD. Each branch represents decision rules, and each leaf node represents a classification outcome. Node 0 (subjects aged ≥70 years including healthy subjects and patients) was classified into two subgroups according to logCM: node 1 (≤0.592) and node 2 (>0.592). Node 1 was classified into two subgroups according to logMg: node 3 (≤0.230) and node 4 (>0.230). Node 2 was classified into two subgroups according to logMg: node 5 (≤0.322) and node 6 (>0.332).
Single regression analysis was performed to select the characteristic clinical factors correlated with logCM values. logCM: log10 (CM value+3). *logxx = log10 transformed value.
Table S1: Results of single regression analysis.