Sleep analysis results of portable polysomnography in patients with acute and chronic temporomandibular disorder
Article Information
Yeon-Hee Lee* and Q-Schick Auh
Department of Orofacial Pain and Oral Medicine, Kyung Hee University Dental Hospital, Kyung Hee Medical center, Kyung Hee University, Seoul, Korea
*Corresponding Author: Yeon-Hee Lee, Department of Orofacial Pain and Oral Medicine, Kyung Hee University Dental Hospital, Kyung Hee Medical center, Kyung Hee University, Seoul, Korea
Received: 01 October 2022; Accepted 06 October 2022; Published: 07 October 2022
Citation:
Yeon-Hee Lee and Q-Schick Auh. Sleep analysis results of portable polysomnography in patients with acute and chronic temporomandibular disorder. Dental Research and Oral Health 5 (2022): 083-093.
View / Download Pdf Share at FacebookAbstract
Objective: This study aimed to investigate portable polysomnography (PSG)-based 'sleep' and pre-diagnosis of obstructive sleep apnoea (OSA) in acute temporomandibular disorder (TMD) and patients with chronic TMD.
Methods: Randomly selected 25 patients with acute TMD (mean age, 42.58 ± 18.77 years; 14 females) and 26 age-and sex-matched patients with chronic TMD (mean age, 49.24 ± 17.52 years, 19 females) were enrolled.
Results: The eight psychological subscales of SCL-90R had significantly higher values in the chronic TMD group than in the acute TMD group (all p < 0.05). There was no significant group difference in the respiratory event index examined using a portable PSG. OSA was observed in 57.7% in acute TMD, and 68.0% in chronic TMD, respectively. From the multiple regression analysis, palpation index was the strongest predictor of pre-diagnosis of OSA (OR = 17.550). Among the contributing factors for TMD, psychological stress (OR = 12.226), self-reported sleep problems (OR = 10.222), and above-average value of DEP (OR = 1.443) were followed.
Conclusion: Patients with chronic TMD were psychologically more vulnerable than those with acute TMD, and the existence of subjectively perceived sleep problems or objective sleep indices examined by portable PSG could affect TMD symptom severity in different ways.
Keywords
Biopsychosocial, Chronic, Depression, Polysomnography, Sleep, Temporomandibular disorder
Biopsychosocial articles; Chronic articles; Depression articles; Polysomnography articles; Sleep articles; Temporomandibular disorder articles
Biopsychosocial articles Biopsychosocial Research articles Biopsychosocial review articles Biopsychosocial PubMed articles Biopsychosocial PubMed Central articles Biopsychosocial 2023 articles Biopsychosocial 2024 articles Biopsychosocial Scopus articles Biopsychosocial impact factor journals Biopsychosocial Scopus journals Biopsychosocial PubMed journals Biopsychosocial medical journals Biopsychosocial free journals Biopsychosocial best journals Biopsychosocial top journals Biopsychosocial free medical journals Biopsychosocial famous journals Biopsychosocial Google Scholar indexed journals Chronic articles Chronic Research articles Chronic review articles Chronic PubMed articles Chronic PubMed Central articles Chronic 2023 articles Chronic 2024 articles Chronic Scopus articles Chronic impact factor journals Chronic Scopus journals Chronic PubMed journals Chronic medical journals Chronic free journals Chronic best journals Chronic top journals Chronic free medical journals Chronic famous journals Chronic Google Scholar indexed journals Depression articles Depression Research articles Depression review articles Depression PubMed articles Depression PubMed Central articles Depression 2023 articles Depression 2024 articles Depression Scopus articles Depression impact factor journals Depression Scopus journals Depression PubMed journals Depression medical journals Depression free journals Depression best journals Depression top journals Depression free medical journals Depression famous journals Depression Google Scholar indexed journals Polysomnography articles Polysomnography Research articles Polysomnography review articles Polysomnography PubMed articles Polysomnography PubMed Central articles Polysomnography 2023 articles Polysomnography 2024 articles Polysomnography Scopus articles Polysomnography impact factor journals Polysomnography Scopus journals Polysomnography PubMed journals Polysomnography medical journals Polysomnography free journals Polysomnography best journals Polysomnography top journals Polysomnography free medical journals Polysomnography famous journals Polysomnography Google Scholar indexed journals Sleep articles Sleep Research articles Sleep review articles Sleep PubMed articles Sleep PubMed Central articles Sleep 2023 articles Sleep 2024 articles Sleep Scopus articles Sleep impact factor journals Sleep Scopus journals Sleep PubMed journals Sleep medical journals Sleep free journals Sleep best journals Sleep top journals Sleep free medical journals Sleep famous journals Sleep Google Scholar indexed journals Temporomandibular disorder articles Temporomandibular disorder Research articles Temporomandibular disorder review articles Temporomandibular disorder PubMed articles Temporomandibular disorder PubMed Central articles Temporomandibular disorder 2023 articles Temporomandibular disorder 2024 articles Temporomandibular disorder Scopus articles Temporomandibular disorder impact factor journals Temporomandibular disorder Scopus journals Temporomandibular disorder PubMed journals Temporomandibular disorder medical journals Temporomandibular disorder free journals Temporomandibular disorder best journals Temporomandibular disorder top journals Temporomandibular disorder free medical journals Temporomandibular disorder famous journals Temporomandibular disorder Google Scholar indexed journals sleep problems articles sleep problems Research articles sleep problems review articles sleep problems PubMed articles sleep problems PubMed Central articles sleep problems 2023 articles sleep problems 2024 articles sleep problems Scopus articles sleep problems impact factor journals sleep problems Scopus journals sleep problems PubMed journals sleep problems medical journals sleep problems free journals sleep problems best journals sleep problems top journals sleep problems free medical journals sleep problems famous journals sleep problems Google Scholar indexed journals macrotrauma articles macrotrauma Research articles macrotrauma review articles macrotrauma PubMed articles macrotrauma PubMed Central articles macrotrauma 2023 articles macrotrauma 2024 articles macrotrauma Scopus articles macrotrauma impact factor journals macrotrauma Scopus journals macrotrauma PubMed journals macrotrauma medical journals macrotrauma free journals macrotrauma best journals macrotrauma top journals macrotrauma free medical journals macrotrauma famous journals macrotrauma Google Scholar indexed journals microtrauma articles microtrauma Research articles microtrauma review articles microtrauma PubMed articles microtrauma PubMed Central articles microtrauma 2023 articles microtrauma 2024 articles microtrauma Scopus articles microtrauma impact factor journals microtrauma Scopus journals microtrauma PubMed journals microtrauma medical journals microtrauma free journals microtrauma best journals microtrauma top journals microtrauma free medical journals microtrauma famous journals microtrauma Google Scholar indexed journals
Article Details
Introduction
Temporomandibular disorder (TMD) is a broad term characterised by pain and dysfunction in the temporomandibular joint (TMJ), masticatory muscles, and surrounding structures. Among the general population, 60–70% have at least one sign of TMD, 12% have TMD-related pain, and 5% seek treatment [1]. TMD occurs 1.5-2 times more often in females than in males, with 80% of patients treated for TMD are females [2]. The aetiology of TMD is multifactorial, with the two main factors being physical and psychological. Therefore, the world's most widely used diagnostic criteria for TMD (DC/TMD) involve the assessment of two axes: physical factors (Axis I) and possible psychological factors (Axis II) [3]. Typical and frequent symptoms of TMD include TMJ noise and TMD-related pain, followed by restricted mandibular movement, headache, neck pain, ear pain, and tinnitus. Although the aetiology of TMD is multifactorial, it shifts from a mechanically based phenomenon to a biopsychosocial model of chronic TMD pain [4]. Plausible risk factors for TMD include recurrent microtrauma, macrotrauma, other body pain conditions, psychological status, sleep problems, and genetic factors [5].
The mechanism of the transition from acute to chronic TMD is unclear. However, the underlying mechanisms related to the clinical features of patients with acute TMD and chronic TMD may be different [6,7]. As pain becomes chronic, psychological aspects become more vulnerable and sleep problems accompany it, which is also the case in TMD [8]. Unresolved psychological disorders (e.g., depression and anxiety) can cause muscle tension that can lead to clenching and grinding of teeth, which can lead to TMD [9]. Acute TMD pain occurs suddenly and usually responds to traditional management and treatment. Pain can persist for a long time beyond what the patient can cope with, and underlying biological and psychological processes may contribute [10]. Therefore, chronic TMD can present persistent, recurrent, or chronic pain associated with TMJ and/or muscles involved in the masticatory system [11]. Although access to chronic TMD is challenging, the clinician must prevent the chronicity of TMD signs and symptoms through comparative studies of patients with acute and chronic TMD.
Studies are still lacking on whether obstructive sleep apnoea (OSA), a classic sleep-disordered respiration, is closely related to the onset and chronicity of TMD. OSA is characterised by intermittent obstruction of the upper airways during sleep and recurrent apnoea and hypopnea, leading to intermittent hypoxia and sleep fragmentation and impaired sleep quality [12]. OSA is very common in adults, with one in five adults having at least mild OSA and one in 15 adults having moderate or more OSA [13]. OSA-related symptoms include daytime sleepiness, decreased cognitive ability, decreased quality of life, increased risk of accidents, and cardiovascular side effects, which cause social and personal burdens. In a previous prospective cohort study, a high probability of OSA was associated with a higher incidence of first-onset and chronic TMD [14]. OSA causes the airway to collapse, causing the body to push the lower jaw forward and lift the airway, and this constant mandibular movement overnight can cause stress and strain on the TMJ and adjacent muscles. However, the downside of this study is that OSA risk was measured using a sleep questionnaire rather than an objective sleep evaluation. Although there have been expert opinions on the relationship between TMD, orofacial pain, and OSA [15], objective, evidence-based studies remain weak.
The gold standard diagnostic route for patients with suspected sleep apnoea is sleep laboratory referral for overnight polysomnography (PSG). The PSG procedure involves continuous recording of various physiological data, including airflow, chest/abdominal movement, electroencephalography, electro-ophthalmoscopy, electromyography, electrocardiography, and oxyhemoglobin saturation [16]. Apnoea and hypopnea were detected with the monitoring equipment during the sleep period, and the number of events per hour was summed to generate an apnoea–hypopnea index (AHI). However, PSG is not readily available in sleep laboratories in many areas because it is expensive, cumbersome, and difficult to perform repeatedly. Evaluating a patient’s sleep and OSA with a portable monitoring device based on a limited number of channels in a home-like environment may reduce costs and improve access to treatment. Self-administered patient testing may be the preferred method for obtaining the necessary physiological data. The American Sleep Disorders Association (ASDA) classified the diagnostic system into four categories according to the test environment, technician presence, and number of parameters recorded [17]. ASDA level III, for use in the present study, was reserved for devices that monitor airflow, chest/abdominal movement, heart rate/pulse rate, and oxyhemoglobin saturation.
To date, research in this field has focused mainly on chronic TMD. However, to better understand the predispositions that may contribute to the transition to chronic TMD, factors that influence the symptoms of acute TMD must also be investigated. Therefore, we investigated and compared the demographics, clinical characteristics, psychological factors, and contributing factors of TMD in patients with acute and chronic TMD. We also investigated sleep and pre-diagnosis of OSA using a level III portable PSG. We hypothesized that the clinical characteristics of acute and chronic TMD may be different and that psychological and sleep factors will be more impaired in patients with chronic TMD. Finally, we examined factors associated with the severity of TMD symptoms.
Materials and Methods
Participants
The research protocol was reviewed in accordance with the Helsinki Declaration and approved by the Institutional Review Board of Kyung Hee University Dental Hospital (KHD IRB no.1804–2). Written informed consent was obtained from all participants. Informed consent was obtained to publish de-identified images of one participant who performed portable PSG in an online open–access publication.
To investigate the research purpose, the authors designed and conducted a randomised controlled study. We identified patients with TMD who visited the Department of Orofacial Pain and Oral Medicine at Kyung Hee University Dental Hospital of Seoul from 1 June 2018 to 31 March 2022. The patients were diagnosed with TMD using DC/TMD for TMD Axis I [3]. When pain persists for >3–6 months, individuals are usually considered in a chronic state [18]. We assigned patients who experienced TMD signs and symptoms lasting <6 months for ‘acute TMD’ and those after >6 months of onset as ‘chronic TMD.’ Among the 83 patients with TMD with sleep reports, 25 patients with acute TMD and 26 age-and sex-matched patients with chronic TMD were randomly selected. We retrospectively reviewed all sleep and TMJ reports and patients with any level of data loss were excluded from the study.
The study sample consisted of patients with TMD according to the inclusion and exclusion criteria. The inclusion criteria for patients with TMD were as follows: completed a set of routine TMJ assessments, patients who performed portable PSG and completed sleep reports, as well as constructive questionnaires, and no treatment of the current episode other than medication. The exclusion criteria were as follows: serious injuries, such as facial fracture and unstable multiple traumas, previous injury, neurological disorder not related to the trauma, musculoskeletal disorder before the injury, rheumatism, psychological problems, and pregnancy. To assess the impact of TMD chronicity on the distribution of demographics, clinical factors for signs and symptoms of TMD, psychological factors, and presence of OSA, all variables were compared by group.
TMD classification and clinical evaluation
Clinical evaluation procedures included an oral examination, interview, panoramic radiography, and a comprehensive questionnaire in DC/TMD Axis I diagnostic algorithms for the diagnosis of TMD. Diagnostic algorithms for myofascial pain, disc displacement, arthralgia, and headache attributed to TMD are based on DC/TMD Axis I diagnostic algorithms.
Characteristics of pain
Patients reported the duration of symptoms in the masticatory muscles, TMJ, and adjacent structures regarding the number of days elapsed since the patient first experienced symptoms related to TMD. TMD-related pain was scored subjectively by the patients, ranging from 0 (no pain at all) to 10 (the worst pain imaginable) using the visual analogue scale (VAS).
Palpation index (PI), neck PI, and dysfunction index (DI)
PI is a reliable scoring system that analyses the severity of myofascial pain, allowing an objective evaluation of TMD symptoms. In each patient, we palpated 20 intraoral and extraoral muscle sites and three sites in the neck. The index-finger palpation pressure was 1 kg/cm2and was applied for 5 s. To calibrate the palpation pressures, we regularly pretested our index-finger pressure using a hand-held pressure algometer. A binary answer (yes/no) was provided for each site. To calculate the PI score, we added all positive responses and divided the sum by the number of events, as described in a previous study [5]. To further investigate the intensity of neck pain, we calculated the neck PI, which was defined as the number of positive responses to the palpation of the neck muscles, including the sternocleidomastoid, splenius capitis, and trapezius muscles, divided by the number of events. Using these two indices, we quantified clinical myofascial pain. DI was used to quantify TMD-related mandibular dysfunction and pain in the TMJ region. DI was defined as the number of positive answers regarding mandibular movements, joint noise, and joint capsule sensitivity divided by the number of events.
Contributing factors for TMD
We investigated self-reported parafunctional activities using the Oral Behaviour Checklist, including bruxism [19]. Self-assessment of sleep problems, headaches, psychological stress, and tinnitus has also been reported. Self-reported macrotrauma experience was evaluated using the dichotomous question, ‘Do you have any macrotrauma experience associated with current TMD?’ Each parameter was recorded as a binary answer (yes or no) for all patients.
Psychological evaluation with SCL-90R
Psychological characteristics were evaluated using the Symptom Checklist-90-Revised (SCL-90R) scale. SCL-90R comprises nine symptom subscales: somatisation (SOM), obsessive-compulsiveness (O-C), interpersonal sensitivity (I-S), depression (DEP), anxiety (ANX), hostility (HOS), phobic anxiety (PHOB), paranoid ideation (PAR), and psychosis (PSY), as well as three global indices of functioning, including the global severity index (GSI), positive symptom distress index (PSDI), and positive symptom total (PST).
Portable PSG index and pre-diagnosis of OSA
We identified and diagnosed OSA using The Alice OneNight (Philips, Amsterdam, The Netherlands). Alice OneNight home sleep testing is a portable level 3 polysomnography device used to test sleep apnoea at home. It includes oxygen saturation (SpO2, finger probe, and oximetry board Nonin), pulse rate (from oximeter probe), airflow (pressure-based airflow with detection of snore through a nasal cannula and thermistor), thoracic and abdominal effort (inductance plethysmography), and body position (Figure 1). The patient installed and operated this device on his or her body for more than one day, and the operator selected representative data for one day without data loss and used it for analysis.
To pre-diagnose OSA, the respiratory event index (REI) was used. The REI represents the number of apnoeas and hypopneas detected by the portable monitoring device per hour of elapsed recording time. The central apnoea index (CAI), obstructive apnoea index (OAI), and mixed apnoea index (MAI) were added, and each index calculated the number of events per hour. Apnoea was defined as a 90% reduction in airflow for at least 10 s, and hypopnea was defined as a ≥30% reduction in airflow for at least 10 s, associated with a ≥3% reduction in oxygen saturation. OSA was defined as an REI ≥5 /hour and classified as mild (REI 5.0–14.9 /hour), moderate (REI 15.0–29.9 /hour), severe (REI ≥30 /hour), and normal (REI <5 /hour) [20]. Sleep studies were considered acceptable for data analysis if none of the following rejection criteria occurred: (1) a portable monitoring device elapsed time less than 2 h or (2) poor quality PSG recording (defined as a substantial portion of the PSG not interpretable for the scoring of sleep and respiratory events).
Data analysis
The descriptive statistics presented percentages, means and standard deviations (SDs) for continuous variables. Student’s t-test for nonnormally distributed variables was used to compare the acute and chronic TMD groups. Differences in the means of continuous variables between the independent groups were examined using the Student’s t-test. The chi-square test and Fisher’s exact test with Bonferroni correction were used to determine the equality of proportions. The chi-square test and Pearson’s correlation test were used to analyze bivariate correlations between categorical and continuous variables. Kappa statistics were used to measure the degree of agreement (Kappa coefficient) between the two examiners who evaluated and rated the same subjects. Multiple regression analysis was performed with OSA as a dependent variable and the presence of demographics, clinical factors, psychological factors, and contributing factors for TMD as an independent variable. We also investigated the increased risk of OSA in patients with chronic versus acute TMD. Odds ratios (ORs) with 95% confidence intervals (CIs) and p-values were investigated. Spearman’s correlation analysis was performed to investigate the relationship between TMD indices and other variables. Statistical significance was set at a two-tailed p-value < 0.05. Data were analysed using IBM SPSS Statistics for Windows (version 20.0; IBM Corp., Armonk, NY, USA).
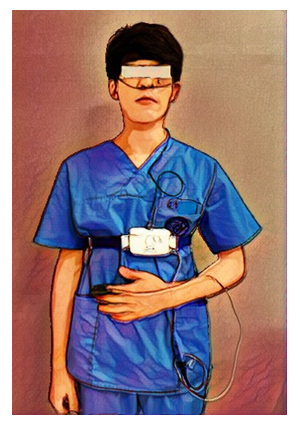
Figure 1: Portable PSG including nasal cannula, thoracic and abdominal respiratory effort, body position sensor, and pulse oximetry
Results
Demographics and TMD index
The average age of all patients with TMD was 45.84 ± 18.29 years old, and the female (n = 33) to male (n = 18) ratio was 1.83:1, with a high female ratio. Table 1 compares the demographics and TMD indices between the acute and chronic TMD groups. There was no significant difference in age difference and male and female composition of the acute TMD and chronic TMD groups with age-and sex-matched composition. The mean TMD symptom duration was significantly longer in the chronic TMD group than in the acute TMD group (40.77 ± 48.85 days vs. 1261.60 ± 1590.11 days, p < 0.001).
Regarding the TMD index for the quantification of TMD symptoms, VAS, neck PI, and DI did not differ significantly. In the case of PI, the incidence of chronic TMD was significantly higher than that of acute TMD (0.147 ± 0.094 vs. 0.253 ± 0.107, p < 0.001).
|
Acute TMD (n = 26) |
Chronic TMD (n = 25) |
p-value |
|
|
mean ± SD or n (%) |
mean ± SD or n (%) |
||
|
Demographics |
|||
|
Age (years)a |
42.58 ± 18.77 |
49.24 ± 17.52 |
0.197 |
|
Sexb |
|||
|
Female |
14 (53.8%) |
19 (76.0%) |
0.086 |
|
Male |
12 (46.2%) |
6 (24.0%) |
|
|
Symptom duration (days) a |
40.77 ± 48.85 |
1261.60 ± 1590.11 |
<0.001*** |
|
TMD index |
|||
|
VAS (0-10) a |
5.73 ± 2.01 |
5.52 ± 2.14 |
0.719 |
|
Neck PI (0-1) a |
0.331 ± 0.211 |
0.404 ± 0.226 |
0.238 |
|
PI (0-1) a |
0.147 ± 0.094 |
0.253 ± 0.107 |
<0.001*** |
|
DI (0-1) a |
0.459 ± 0.245 |
0.523 ± 0.208 |
0.316 |
TMD, temporomandibular disorder; VAS, visual analogue scale; PI, palpation index; DI, dysfunction index; SD, standard deviation.
Statistical significance was set at p < 0.05; ***: p < 0.001
a: The results were obtained using t-test; b: The results are obtained using the chi-square test.
Table 1: Epidemiology and clinical characteristics of patients with TMD
Contributing factors for TMD
Among the contributing factors to TMD that we investigated, there was no factor with a difference in distribution between the two groups. When the presence of each factor was investigated, headache was the most frequent contributing factor (n = 28, 54.9%), psychological stress (n = 27, 52.9%), sleep problem (n = 17, 33.3%), bruxism (n = 11, 21.6%), tinnitus (n = 10, 19.6%), and macrotrauma history (n = 9, 17.6%) were followed.
Psychological factors using SCL-90R
Among the nine SCL-90R subscales, the mean value of eight items, excluding PAR, was significantly higher in chronic TMD than in acute TMD. That is, the mean value of including SOM (46.50 ± 5.13 vs. 53.72 ± 10.45, p < 0.01); O-C (43.04 ± 6.64 vs. 53.32 ± 7.82, p < 0.001); I-S (45.65 ± 7.28 vs. 54.40 ± 10.07, p < 0.001); DEP (44.19 ± 7.34 vs. 54.68 ± 9.56, p < 0.001); ANX (46.15 ± 7.66 vs. 55.04 ± 10.19, p < 0.01); HOS (44.92 ± 5.30 vs. 52.48 ± 9.11, p < 0.01); PHOB (44.85 ± 2.87 vs. 52.88 ± 15.04, p < 0.05); PSY (44.23 ± 3.66 vs. 52.20 ± 8.71, p < 0.001) was significantly higher than in chronic TMD compared to acute TMD. All three composite indicators, GSI, PDSI, and PST, were significantly higher in chronic TMD than in acute TMD (Table 2).
|
Acute TMD (n = 26) |
Chronic TMD (n = 25) |
p-value |
|
|
mean ± SD or n (%) |
mean ± SD or n (%) |
||
|
Contributing factor |
|||
|
Bruxism a |
5 (19.2%) |
6 (24.0%) |
0.743 |
|
Self-reported sleep problem a |
10 (38.5%) |
7 (28.0%) |
0.555 |
|
Headache a |
15 (57.7%) |
13 (52.0%) |
0.781 |
|
Psychological distress a |
14 (53.8%) |
13 (52.0%) |
1 |
|
Tinnitus a |
6 (23.1%) |
4 (16.0%) |
0.726 |
|
Macrotrauma b |
3 (11.5%) |
6 (24.0%) |
0.291 |
|
SCL-90R |
|
|
|
|
SOM c |
46.50 ± 5.13 |
53.72 ± 10.45 |
0.003** |
|
O-C c |
43.04 ± 6.64 |
53.32 ± 7.82 |
<0.001*** |
|
I-S c |
45.65 ± 7.28 |
54.40 ± 10.07 |
<0.001*** |
|
DEP c |
44.19 ± 7.34 |
54.68 ± 9.56 |
<0.001*** |
|
ANX c |
46.15 ± 7.66 |
55.04 ± 10.19 |
0.001** |
|
HOS c |
44.92 ± 5.30 |
52.48 ± 9.11 |
0.001** |
|
PHOB c |
44.85 ± 2.87 |
52.88 ± 15.04 |
0.010* |
|
PAR c |
44.69 ± 4.84 |
47.44 ± 8.56 |
0.162 |
|
PSY c |
44.23 ± 3.66 |
52.20 ± 8.71 |
<0.001*** |
|
GSI c |
43.35 ± 5.80 |
54.16 ± 9.70 |
<0.001*** |
|
PDSI c |
46.54 ± 5.78 |
50.32 ± 8.25 |
0.005** |
|
PST c |
42.23 ± 9.25 |
57.01 ± 7.03 |
<0.001*** |
TMD, temporomandibular disorder; SOM, somatisation; O-C, obsessive-compulsiveness; I-S, interpersonal sensitivity; DEP, depression; ANX, anxiety; HOS, hostility; PHOB, phobic anxiety; PAR, paranoid ideation; PSY, psychosis; GSI, global severity index; PSDI, positive symptom distress index; PST, positive symptom total.
Statistical significance was set at p < 0.05; *: p < 0.05; **: p < 0.01; ***: p < 0.001
a: The results are obtained using the chi-square test; b: The results are obtained using Fisher’s exact test and Bonferroni correction; c: The results are obtained using t-test.
Table 2: Distribution of contributing factors and psychological profiles
Results with portable PSG
Table 3 shows the objective sleep and pre-diagnosis of OSA using portable PSG. Regarding the portable PSG index, the mean values of OAI, CAI, MAI, hypopnea, and REI were not significantly different between the two groups (Figure 2). For all patients with TMD, the mean value of REI was 10.63 ± 9.65. The REI values of acute TMD were 10.31 ± 10.50, and that of chronic TMD was 10.95 ± 8.89, respectively. The lowest SpO2 values were not significantly different between the acute and chronic TMD groups (86.35 ± 4.66 vs. 84.04 ± 14.13, p = 0.404).
A pre-diagnosis of OSA was observed in 32 patients with TMD (72.7%). OSA was diagnosed in 68.0% of the patients in the chronic TMD group, which was higher than that of the acute TMD group (57.7%), but the difference was not statistically significant (p = 0.605). In both the acute and chronic TMD groups, moderate OSA (30.8% and 44.0%, respectively) was observed with greater frequency than mild OSA (26.9% and 24.0%, respectively). Severe OSA was not observed in any case of the two TMD groups.
aTMD, acute TMD group; cTMD, chronic TMD group; REI, respiratory event index; OAI, obstructive apnoea index; CAI, central apnoea index.
The results were obtained using a t-test. Statistical significance was set at P < 0.05. A p-value ³ 0.05 indicated by “ns” for not significant.
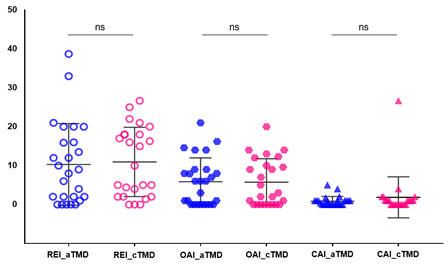
Figure 2: Comparison between TMD groups in portable PSG index
|
Acute TMD (n = 26) |
Chronic TMD (n = 25) |
p-value |
|
|
mean ± SD or n (%) |
mean ± SD or n (%) |
||
|
Total recording time (min) a |
265.86 ± 182.56 |
281.24 ± 183.30 |
0.765 |
|
Portable PSG index |
|||
|
Lowest SpO2 (%) a |
86.35 ± 4.66 |
84.04 ± 14.13 |
0.404 |
|
OAI (events/hour) a |
5.87 ± 6.11 |
5.76 ± 6.04 |
0.945 |
|
CAI (events/hour) a |
0.85 ± 1.24 |
1.84 ± 5.27 |
0.358 |
|
MAI (events/hour) a |
0.62 ± 0.89 |
0.72 ± 0.75 |
0.64 |
|
Hypopnea (events/hour) a |
3.09 ± 4.62 |
2.52 ± 2.64 |
0.594 |
|
REI (events/hour) a |
10.31 ± 10.50 |
10.95 ± 8.89 |
0.815 |
|
OSA severity |
|||
|
Normal (REI <5) b |
11 (42.3%) |
8 (32.0%) |
0.605 |
|
OSA (REI ≥5) |
15 (57.7%) |
17 (68.0%) |
|
|
1) Mild OSA (REI 5.0–14.9) |
7 (26.9%) |
6 (24.0%) |
|
|
2) Moderate OSA (REI 15.0–29.9) |
8 (30.8%) |
11 (44.0%) |
TMD, temporomandibular disorder; PSG, polysomnography; SpO2, oxygen saturation; OAI, obstructive apnoea index; CAI, central apnoea index; MAI, mixed apnoea index; REI, respiratory event index; OSA, obstructive sleep apnoea
a: The results are obtained using t-test; b: The results are obtained using the chi-square test. Statistical significance was set at p < 0.05.
Table 3: Objective sleep and pre-diagnosis of OSA investigation with portable PSG
Regression analysis for predicting OSA
Multiple regression analysis was performed to analyse predictive factors, and the pre-diagnosis of OSA was assumed as a dependent variable (Table 4). The pre-diagnosis of OSA was confirmed based on the REI score (³5) obtained from the portable PSG test. The previously investigated demographics, TMD index, psychological factors, and contributing factors for TMD were included as independent variables. From the results of the multiple regression analysis, PI (above-average value) was the strongest predictor of pre-diagnosis of OSA (OR = 17.550). Among the contributing factors for TMD, psychological stress (OR = 12.226), self-reported sleep problems (OR = 10.222), and above-average value of DEP (OR = 1.443) were followed.
|
Pre-diagnosis of OSA in patients with TMD (n = 51) |
||||
|
OR |
95% CI lower |
95% CI upper |
p-value |
|
|
Demographics |
||||
|
Age [ref.=under average value] |
1.293 |
0.158 |
10.565 |
0.81 |
|
Female [ref.=Male] |
0.469 |
0.061 |
3.622 |
0.468 |
|
Chronic TMD [ref.=Acute TMD] |
1.425 |
0.187 |
10.868 |
0.733 |
|
TMD index |
||||
|
VAS |
0.794 |
0.255 |
0.476 |
0.691 |
|
Neck PI [ref.=under average value] |
7.185 |
0.623 |
82.888 |
0.114 |
|
PI [ref.=under average value] |
17.55 |
0.582 |
528.929 |
0.009** |
|
DI [ref.=under average value] |
0.449 |
0.081 |
2.505 |
0.361 |
|
SCL-90R |
||||
|
SOM [ref.=under average value] |
0.964 |
0.774 |
1.201 |
0.743 |
|
OC [ref.=under average value] |
0.889 |
0.726 |
1.088 |
0.254 |
|
IS [ref.=under average value] |
1.114 |
0.899 |
1.381 |
0.323 |
|
DEP [ref.=under average value] |
1.443 |
0.989 |
2.106 |
0.048* |
|
ANX [ref.=under average value] |
0.831 |
0.641 |
1.079 |
0.164 |
|
HOS [ref.=under average value] |
0.646 |
0.453 |
0.921 |
0.056 |
|
PHOB [ref.=under average value] |
0.991 |
0.825 |
1.19 |
0.924 |
|
PAR [ref.=under average value] |
0.79 |
0.573 |
1.09 |
0.151 |
|
PSY [ref.=under average value] |
1.204 |
0.919 |
1.579 |
0.178 |
|
Contributing factors |
||||
|
Bruxism [ref.=none] |
0.649 |
0.092 |
4.564 |
0.664 |
|
Self-reported sleep problem [ref.=none] |
10.222 |
1.481 |
70.564 |
0.018* |
|
Headache [ref.=none] |
0.354 |
0.068 |
1.856 |
0.219 |
|
Psychological distress [ref.=none] |
12.226 |
1.277 |
117.084 |
0.017* |
|
Tinnitus [ref.=none] |
0.528 |
0.066 |
4.209 |
0.547 |
|
Macrotrauma [ref.=none] |
0.547 |
0.065 |
4.618 |
0.579 |
OSA, obstructive sleep apnoea; OR, odds ratio; CI, confidence interval; TMD, temporomandibular disorder; VAS, visual analogue scale; PI, palpation index; DI, dysfunction index; SOM, somatisation; O-C, obsessive-compulsiveness; I-S, interpersonal sensitivity; DEP, depression; ANX, anxiety; HOS, hostility; PHOB, phobic anxiety; PAR, paranoid ideation; PSY, psychosis
The results were obtained using multiple regression analysis. Statistical significance was set at p < 0.05; *: p < 0.05; **: p < 0.01
Table 4: Multiple regression analysis to predict OSA in patients with TMD
Correlations among the TMD indices
Significant positive correlations were observed between neck PI, PI, and DI in both the acute TMD and chronic TMD groups. In the acute TMD group, neck PI and PI had a strong positive correlation (r = 0.566, p < 0.01), and PI and DI also had a positive correlation (r = 0.441, p < 0.05). Similarly, positive correlations were observed between neck PI and PI (r = 0.623, p < 0.01) and between PI and DI (r = 0.513, p < 0.01) in the chronic TMD group. In both the acute and chronic TMD groups, the higher the PI, the higher the neck PI and DI. In both groups, there was no correlation between neck PI, which indicates the degree of myofascial pain in the neck, and DI, which indicates TMJ dysfunction and pain (Table 5).
|
Spearman's r |
Acute TMD (n = 26) |
Chronic TMD (n = 25) |
|||||||
|
VAS |
Neck PI |
PI |
DI |
VAS |
Neck PI |
PI |
DI |
||
|
TMD index |
Neck PI |
-0.081 |
0.566** |
0.031 |
-0.206 |
0.623** |
0.299 |
||
|
PI |
0 |
0.566** |
0.441* |
0.045 |
0.623** |
0.513** |
|||
|
DI |
-0.078 |
0.031 |
0.441* |
0.045 |
0.299 |
0.513** |
|||
|
Contributing factor |
Bruxism |
0.293 |
0.055 |
-0.114 |
0.418* |
0.540** |
0.257 |
0.309 |
0.121 |
|
Sleep problem |
0.158 |
0.089 |
-0.013 |
-0.197 |
0.471* |
-0.014 |
0.014 |
0.194 |
|
|
Headache |
0.078 |
-0.031 |
0.065 |
-0.055 |
0.199 |
0.045 |
0.045 |
0.045 |
|
|
Stress |
0.309 |
0.187 |
0.283 |
0.012 |
0.359 |
0.368 |
0.116 |
0.116 |
|
|
Tinnitus |
0 |
0.177 |
0.228 |
0.27 |
0.017 |
0.167 |
0.053 |
0.053 |
|
|
Macrotrauma |
0.12 |
0.263 |
0.241 |
0.309 |
0.022 |
0.121 |
0.257 |
0.257 |
|
|
SCL-90R |
SOM |
0.109 |
0.207 |
0.062 |
0.037 |
0.329 |
0.449* |
0.623** |
0.219 |
|
O-C |
0.216 |
0.276 |
0.34 |
0.141 |
0.184 |
0.409 |
0.476* |
0.14 |
|
|
I-S |
0.047 |
0.196 |
0.292 |
0.183 |
0.1 |
0.354 |
0.590** |
0.23 |
|
|
DEP |
0.211 |
0.27 |
0.406* |
0.146 |
0.25 |
0.347 |
0.566** |
0.073 |
|
|
ANX |
0.263 |
0.341 |
0.436* |
0.23 |
0.05 |
0.414* |
0.571** |
0.073 |
|
|
HOS |
0.338 |
0.082 |
0.384 |
0.29 |
0.184 |
0.191 |
0.37 |
0.034 |
|
|
PHOB |
0.054 |
0.062 |
0.064 |
0.103 |
0.223 |
0.421* |
0.730** |
0.247 |
|
|
PAR |
0.199 |
-0.011 |
0.21 |
-0.065 |
0.142 |
0.434* |
0.326 |
0.051 |
|
|
PSY |
0.282 |
0.33 |
0.414* |
0.058 |
0.028 |
0.281 |
0.298 |
0.101 |
|
|
Portable PSG index |
Lowest SpO2 |
0.026 |
-0.271 |
-0.525** |
-0.125 |
0.206 |
-0.017 |
-0.129 |
-0.062 |
|
OAI |
0.036 |
-0.268 |
-0.068 |
0.317 |
0.023 |
-0.148 |
0.176 |
0.119 |
|
|
CAI |
-0.216 |
0.076 |
0.533** |
0.459* |
0.212 |
-0.101 |
0.296 |
0.053 |
|
|
MAI |
-0.022 |
-0.098 |
0.268 |
0.279 |
-0.179 |
-0.18 |
0.041 |
-0.174 |
|
|
Hypopnea |
-0.026 |
-0.148 |
0.102 |
0.511** |
-0.135 |
0.04 |
0.141 |
0.017 |
|
|
REI |
-0.046 |
-0.228 |
0.078 |
0.454* |
-0.089 |
0.022 |
0.336 |
0.146 |
TMD, temporomandibular disorder; VAS, visual analogue scale; PI, palpation index; DI, dysfunction index; SOM, somatisation; O-C, obsessive-compulsiveness; I-S, interpersonal sensitivity; DEP, depression; ANX, anxiety; HOS, hostility; PHOB, phobic anxiety; PAR, paranoid ideation; PSY, psychosis; PSG, polysomnography; SpO2, oxygen saturation; OAI, obstructive apnoea index; CAI, central apnoea index; MAI, mixed apnoea index; REI, respiratory event index.
The results are obtained using Spearman’s correlation analysis. Spearman's r indicates the correlation between two factors (Spearman's r: -1 to 1). The larger the absolute value, the stronger the correlation. In this table, red indicates a positive correlation, and green indicates a negative correlation. Statistical significance was set at p < 0.05; *: p < 0.05; **: p < 0.01
Table 5: Factors that correlate with TMD symptom severity
Correlation between clinical characteristics and the TMD index
In the acute TMD group, bruxism was positively correlated with the DI score (r = 0.418, p < 0.05). In the chronic TMD group, bruxism (r = 0.540, p < 0.01) and self-reported sleep problems (r = 0.471, p <0.05) were positively correlated with the VAS score. Interestingly, psychological factors examined with SCL-90R showed a correlation with PI and neck PI. In the acute TMD group, DEP (r = 0.406), ANX (r = 0.436), and PSY (r=0.414) were positively correlated with an increase in PI. In chronic TMD, the factors correlated with neck PI were SOM (r = 0.449, p < 0.05), ANX (r = 0.414, p < 0.05), PHOB (r = 0.421, p < 0.05), and PAR (r=0.434, p <0.05). The factors positively correlated with PI were SOM (r = 0.623, p < 0.01), O-C (r = 0.476, p < 0.05), I-S (r = 0.590, p < 0.01), DEP (r = 0.566, p < 0.01), ANX (r = 0.571, p < 0.01), and PHOB (r = 0.730, p < 0.01). The nine psychological subscales of SCL-90R were not significantly correlated with VAS and DI. Thus, it is inferred that muscular pain and psychological factors are more closely related than joint pain (Table 5).
Correlation between the portable PSG index and the TMD index
A significant correlation was observed in the portable PSG index in the acute TMD group. In the acute TMD group, the lowest SpO2 was significantly negatively correlated with PI (r = -0.525, p < 0.01). CAI was positively correlated with PI (r = 0.553, p < 0.01) and neck PI (r = 0.459, p < 0.05). Hypopnea was positively correlated with REI (r = 0.511, p < 0.01) and DI (r = 0.454, p < 0.05) (Table 5).
Discussion
Psychological aspects, the existence of subjectively perceived sleep problems, or objective sleep indices examined by portable PSG could affect TMD symptom severity in different ways in acute and chronic TMD. In our study, PI was significantly higher in patients with chronic TMD than in those with acute TMD. Interestingly, patients with chronic TMD were psychologically more vulnerable than those with acute TMD. The eight psychological subscales of SCL-90R, except for PAR, including SOM, O-C, I-S, DEP, ANX, HOS, PHOB, and PSY, had significantly higher values in chronic TMD than in acute TMD. Furthermore, OSA was observed in many patients with TMD (n = 32, 62.7%). In the multiple regression analysis, the 'TMD group' was not a significant predictor of OSA. PI was the strongest predictor of OSA (OR = 17.550), followed by psychological stress (OR = 12.226), self-reported sleep problems (OR = 10.222), and DEP (OR = 1.443). In acute TMD, the decrease in the lowest SpO2 value and an increase in CAI were correlated with an increase in the PI score. In chronic TMD, an increase in REI was correlated with an increase in DI.
The severity of temporomandibular myofascial pain, measured by PI, was significantly higher in the chronic TMD group than in the acute TMD group. Conversely, DI, which quantifies TMJ pain and dysfunction, did not differ between the acute and chronic TMD groups. As TMD pain becomes chronic, the effect of pain of myogenous origin rather than arthrogenous origin may increase. TMD pain of myofascial origin is difficult to diagnose and treat and is considered a chronic pain disorder [21]. The overall prevalence of myofascial TMD pain is up to 45.3%, and TMD itself is also commonly considered a chronic pain condition [22]. General treatment of temporomandibular myofascial pain in our clinical environment combines pharmacological therapy, stabilisation splint therapy, cognitive therapy, and meditation, which produces relief of symptoms. However, no consensus protocol for the treatment of temporomandibular myofascial pain has been established. In our previous study, myofascial pain was associated with poor sleep quality in patients with chronic TMD [8]. More approaches to sleep and biopsychosocial aspects are needed to manage TMD pain of myogenous origin.
The pathophysiological mechanism of TMD has shifted from a mechanistic-based theory to a biopsychosocial model, particularly in patients with chronic TMD [23,24]. In the present study, the distribution of TMD contributing factors, such as bruxism and history of macrotrauma, did not differ significantly between acute and chronic TMD. According to the International Classification of Sleep Disorders, the classification criteria for sleep bruxism include the objective signs and symptoms observed by clinicians [25]. However, this study was conducted based on self-reported bruxism and its interpretation was limited. Unsolved psychological problems such as anxiety and depression can cause an increase in muscle tension that can lead to tooth clenching or bruxism, which in turn can lead to the onset and persistence of TMD [9]. In addition, macrotrauma, such as whiplash injury, may be associated with the chronicity of TMD pain because a series of peripheral and central sensitisation and psychological damage from macrotrauma occur [26,27]. In this study, a macrotrauma history did not increase the risk of OSA and did not significantly affect the symptom severity of TMD.
OSA is a common sleep disorder that leads to hypoxemia and sleep fragmentation. Interrupted breathing attributed to OSA leads to a reduced level of oxygen in the blood, which can trigger inflammation [28]. OSA has been associated with many medical conditions, such as cardiovascular diseases, juvenile idiopathic arthritis, and neuromuscular disorders [29,30]. OSA patients with chronic pain also have higher pain levels, higher disability levels, and lower quality of life [31]. OSA more than tripled the incidence of chronic TMD in a previous OPPERA study [32]. Sleep disturbances due to hypoxia and sleep fragmentation reliably predict new occurrences and exacerbations of chronic pain [33]. In this study, the prevalence of OSA in patients with TMD was 62.7%, and it occurred more frequently in patients with chronic TMD (68.0%) than in those with acute TMD (57.7%). Regarding the occurrence of OSA in patients with TMD, abnormal movement of the lower jaw can increase the myofascial pain intensity and tightness of the surrounding muscles [34]. Only in chronic TMD was the increase in REI correlated with an increase in DI. Poor sleep quality is very common in patients with TMD and has been observed in up to 90% of patients [35]. OSA is a common comorbidity in the general population with chronic pain, with an overall prevalence of approximately 37%, and is reported to be approximately 29% in patients with TMD [36]. Poor sleep quality was strongly associated with chronic TMD pain [8]. However, the relationship between OSA and TMD chronicity was unclear in this study.
Furthermore, the PI score was the strongest predictor of OSA (OR = 17.550) in patients with TMD, followed by psychological stress (OR = 12.226), self-reported sleep problems (OR = 10.222), and DEP (OR = 1.443). Myofascial pain in TMD is associated with a mild increase in sleep fragmentation [37]. In acute TMD, the decrease in the lowest SpO2 value and an increase in CAI were correlated with an increase in the PI score. Repeated intermittent hypoxia or ischaemia ultimately leads to muscle damage [38]. Decreased sleep quality, pain, and psychological vulnerability interact inseparably with each other. Compared with healthy controls, patients with OSA had more perceived psychological stress, and stress was closely related to anxiety and depressive symptoms [39]. Furthermore, psychological stress contributes to the formation of trigger points for pain-causing muscle tension and myofascial pain [40]. Although somewhat complicated, clinicians should always consider the effects of nocturnal hypoxemia and fragmented sleep during OSA on pain intensity in patients with TMD inpatient screening. Furthermore, based on the biopsychosocial model, factors such as psychological stress, depression, and myofascial pain should be considered in patients with chronic pain. More studies are needed to determine how each of the various factors affects acute and chronic TMD and how they have complex correlations.
This study had several limitations. Although the portable SPG device has a simpler configuration than the general PSG, patients still feel uncomfortable even when they fall asleep with the device installed on their body in a comfortable environment at home. This discomfort caused a decrease in the total sleep time and monitoring time. Additionally, since this study was conducted on patients who agreed to perform portable PSG among those who visited during the study period, bias may have occurred in selecting patients with TMD.
Conclusion
When comparing the acute and chronic TMD groups, the most striking difference was that psychological parameters were more impaired in the chronic TMD group than in the acute TMD group. Psychological factors, such as SOM, O-C, I-S, DEP, ANX, and PHOB, increase myofascial pain intensity in patients with chronic TMD and negatively affect them. There was no direct difference between the TMD groups in the portable PSG index, and there was no significant difference in OSA incidence and REI scores. However, in the acute TMD group, a decrease in the lowest SpO2 and an increase in CAI were associated with an increase in myofascial pain intensity, while an increase in REI and CAI was associated with an increase in TMJ pain and dysfunction. Therefore, when screening patients with TMD, clinicians must consider that the biopsychosocial profile may differ depending on signs and symptoms, and the underlying mechanisms or related body systems may be different.
Acknowledgments
None.
Funding details
This work was supported by the National Research Foundation of Korea funded by the Korean government under Grant number NRF/2020R1F1A1070072 to (Y.-H.L) and Kyung Hee University in 2021 (KHU-20211863).
Declaration of interest
The authors report that they have no conflict of interest.
Authors contributions
Y.-H.L. conceptualised and prepared the original draft and wrote the manuscript; Y.-H.L. and Q.-S.A. performed data acquisition; Y.-H.L. and Q.-S.A. performed data analysis and interpretation; Y.-H.L. prepared the figures; Y.-H.L. revised the manuscript; Y.-H.L. acquired funding. All authors read and agreed to the final version of the manuscript.
Data availability
Data supporting the findings of this study are available upon request from the corresponding author [Y.-H.L.].
Ethics declarations
The requirement for informed consent was waived by the IRB of Kyung Hee University Dental Hospital owing to the study design.
Consent to publish
The authors consent to the publication of this study, including their data.
References
- Lim PF, Smith S, Bhalang K, et al. Development of temporomandibular disorders is associated with greater bodily pain experience. Clin J Pain 26 (2010): 116-120.
- Warren MP, Fried JL. Temporomandibular disorders and hormones in women. Cells Tissues Organs 169 (2001): 187-192.
- Schiffman E. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group†. J Oral Facial Pain Headache 28 (2014): 6-27.
- Lee YH, Lee KM, Auh QS, et al. Sex-related differences in symptoms of temporomandibular disorders and structural changes in the lateral pterygoid muscle after whiplash injury. J Oral Rehabil 46 (2019): 1107-1120.
- Lee YH, Lee KM, Auh QS, et al. Magnetic Resonance Imaging-Based Prediction of the Relationship between Whiplash Injury and Temporomandibular Disorders. Frontiers in Neurology 8 (2018): 12635.
- Sabsoob O. Acute and Chronic Temporomandibular Disorder Pain: A critical review of differentiating factors and predictors of acute to chronic pain transition. J Oral Rehabil 49 (2022): 362-372.
- Gatchel RJ, Garofalo JP, Ellis E, et al. Major psychological disorders in acute and chronic TMD: an initial examination. J Am Dent Assoc 127 (1996): 1365-1370.
- Lee YH, Auh QS, An JS, et al. Poorer sleep quality in patients with chronic temporomandibular disorders compared to healthy controls. BMC Musculoskeletal Disorders 23 (2022): 246.
- Emodi-Perlman A. Temporomandibular Disorders and Bruxism Outbreak as a Possible Factor of Orofacial Pain Worsening during the COVID-19 Pandemic-Concomitant Research in Two Countries. J Clin Med 9 (2020): 3250.
- Fillingim RB. Individual differences in pain: understanding the mosaic that makes pain personal. Pain 158 (2017): S11-S18.
- Furquim BDA, Flamengui LMSP, Conti PCR. TMD and chronic pain: a current view. Dental Press J Orthod 20 (2015): 127-133.
- Sforza E, Roche F. Chronic intermittent hypoxia and obstructive sleep apnea: an experimental and clinical approach. Hypoxia (Auckl) 4 (2016): 99-108.
- Motamedi KK, McClary AC, Amedee RG. Obstructive sleep apnea: a growing problem. Ochsner J 9 (2009): 149-153.
- Sanders AE. Sleep apnea symptoms and risk of temporomandibular disorder: OPPERA cohort. J Dent Res 92 (2013): 70S-77S.
- Talley RL. CRANIO® 37 (2019): 273-274.
- Yilmaz B, Asyali MH, Arikan E, et al. Sleep stage and obstructive apneaic epoch classification using single-lead ECG. Biomed Eng Online 9 (2010): 39-39.
- Kapur VK. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 13 (2017): 479-504.
- Treede RD. A classification of chronic pain for ICD-11. Pain 156 (2015): 1003-1007.
- Markiewicz MR, Ohrbach R, McCall WD, et al. Oral behaviors checklist: reliability of performance in targeted waking-state behaviors. J Orofac Pain 20 (2006): 306-316.
- Goyal M, Johnson J. Obstructive Sleep Apnea Diagnosis and Management. Mo Med 114 (2017): 120-124.
- Gonzalez-Perez LM, Infante-Cossio P, Granados-Nuñez M, et al. Treatment of temporomandibular myofascial pain with deep dry needling. Med Oral Patol Oral Cir Bucal 17 (2012): e781-e785.
- Al-Khotani A. Prevalence of diagnosed temporomandibular disorders among Saudi Arabian children and adolescents. J Headache Pain 17 (2016): 41.
- Lee YH, Lee KM, Auh QS. MRI-Based Assessment of Masticatory Muscle Changes in TMD Patients after Whiplash Injury. J Clin Med 10 (2021): 36-54.
- Gatchel RJ, Peng YB, Peters ML, et al. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull 133 (2007): 581-624.
- Sateia MJ. International classification of sleep disorders (3rd edition): highlights and modifications. Chest 146 (2014): 1387-1394.
- Curatolo M. et al. Central hypersensitivity in chronic pain after whiplash injury. Clin J Pain 17 (2001): 306-315.
- Lee YH, Lee KM, Auh QS, et al. Magnetic Resonance Imaging-Based Prediction of the Relationship between Whiplash Injury and Temporomandibular Disorders. Frontiers in neurology 8 (2018): 725-725.
- Unnikrishnan D, Jun J, Polotsky V. Inflammation in sleep apnea: an update. Rev Endocr Metab Disord 16 (2015): 25-34.
- Selva-O'Callaghan A. Obstructive sleep apnea in patients with inflammatory myopathies. Muscle Nerve 39 (2009): 144-149.
- Culebras, A. Sleep and neuromuscular disorders. Neurol Clin 23 (2005): 1209-1223.
- Aytekin E. Chronic widespread musculoskeletal pain in patients with obstructive sleep apnea syndrome and the relationship between sleep disorder and pain level, quality of life, and disability. J Phys Ther Sci 27 (2015): 2951-2954.
- Sanders AE. Sleep apnea symptoms and risk of temporomandibular disorder: OPPERA cohort. J Dent Res 92 (2013): 70s-77s.
- Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain 14 (2013): 1539-1552.
- Cunali PA. Prevalence of temporomandibular disorders in obstructive sleep apnea patients referred for oral appliance therapy. J Orofac Pain 23 (2009): 339-344.
- Yatani H, Studts J, Cordova M, et al. Comparison of sleep quality and clinical and psychologic characteristics in patients with temporomandibular disorders. J Orofac Pain 16 (2002): 221-228.
- Smith MT. Sleep disorders and their association with laboratory pain sensitivity in temporomandibular joint disorder. Sleep 32 (2009): 779-790.
- Dubrovsky B. Polysomnographic investigation of sleep and respiratory parameters in women with temporomandibular pain disorders. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine 10 (2014): 195-201.
- Valle-Tenney R, Rebolledo D, Acuña MJ, et al. HIF-hypoxia signaling in skeletal muscle physiology and fibrosis. J Cell Commun Signal 14 (2020): 147-158.
- Wong JL. Stress in obstructive sleep apnea. Scientific Reports 11 (2021): 12631.
- Jafri MS. Mechanisms of Myofascial Pain. Int Sch Res Notices 12 (2014): 523924.
